Genetic and Physiological Factors Affecting Human Milk Production and Composition
Abstract
1. Introduction
1.1. Hormones Inducing and Sustaining Human Milk Production: Prolactin and Oxytocin
1.2. Physiological Changes in Breast Milk Composition
2. Materials and Methods
3. Genetic Variations that Affect Human Milk Composition
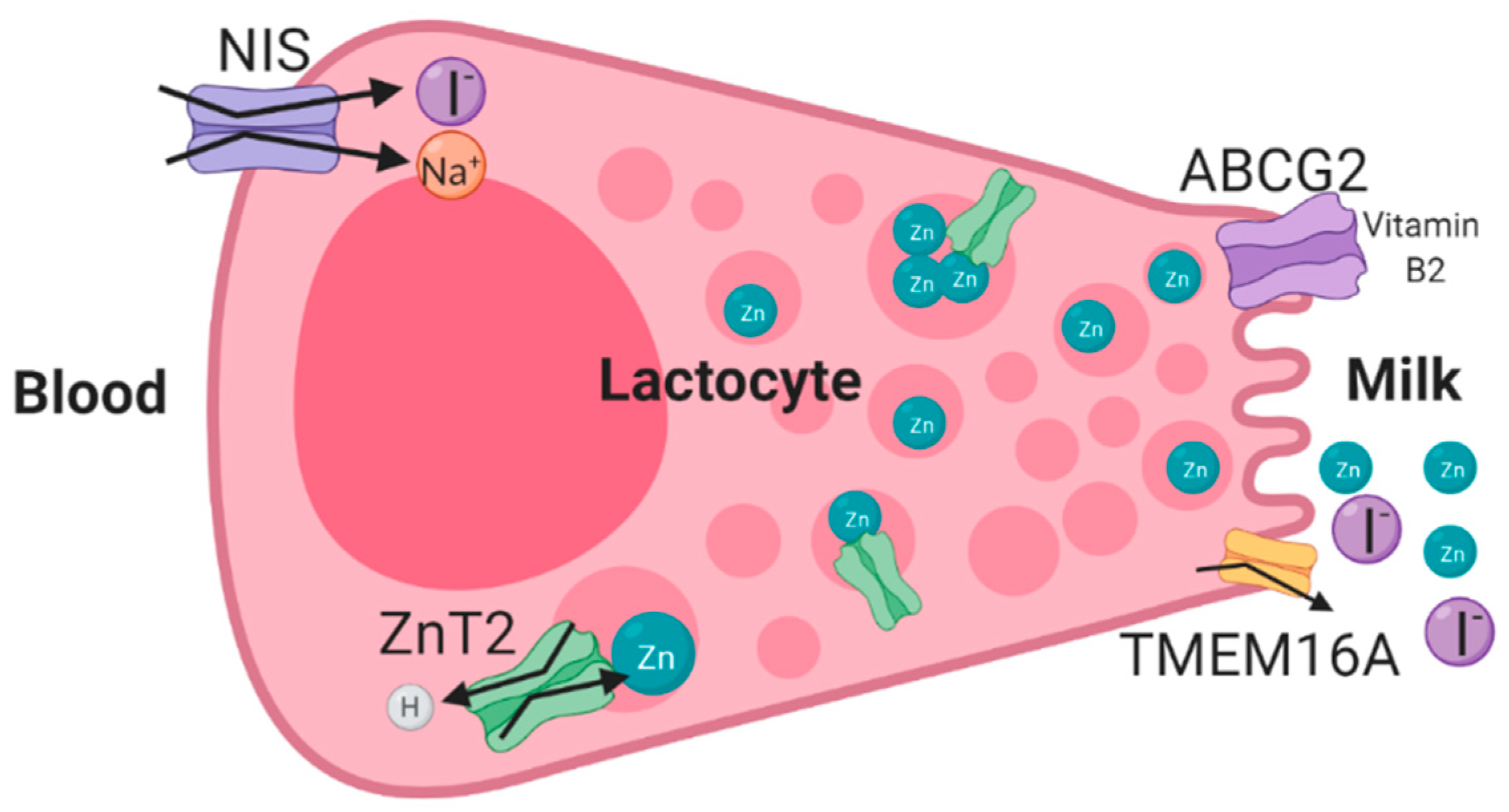
3.1. ZnT2 (SLC30A2) Mutations and Transient Neonatal Zinc Deficiency (TNZD)
3.2. ABCG2 (BCRP)—Riboflavin Secretion and Milk Volume
3.3. Genetic Variations in the Sodium Iodide Symporter (NIS/SLC5A5) and Iodine Deficiency in Human Milk
3.4. Human Milk Choline Concentrations
3.5. Folate Content in Human Milk
3.6. Fat Percentage in Human Milk
3.7. Fatty Acid Desaturases (FADS) and Fatty Acid Composition in Human Milk
3.8. Human Milk Protein Content
3.9. Human Milk Oligosaccharides (HMOs)
3.10. Other Genes
4. Genetic Variations that Affect Human Milk Supply
4.1. Prolactin
4.2. Prolactin Receptor (PRLR)
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, N.Y.; Nolan, E.; Lindeman, G.J.; Visvader, J.E. Stem Cells and the Differentiation Hierarchy in Mammary Gland Development. Physiol. Rev. 2020, 100, 489–523. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Sahu, J.; Grewal, S.; Aggarwal, A.; Ghosh, S. Role of hormones in persistency of lactation: A review. J. Entomol. Zool. Stud. 2019, 7, 677–686. [Google Scholar]
- Wang, Q.A.; Scherer, P.E. Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution. J. Mammary Gland Biol. Neoplasia 2019, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Anderson, S.M.; McManaman, J.L.; Badger, T.M.; Bunik, M.; Contractor, N.; Crume, T.; Dabelea, D.; Donovan, S.M.; Forman, N.; et al. Lactation and Neonatal Nutrition: Defining and Refining the Critical Questions. J. Mammary Gland Biol. Neoplasia 2012, 17, 167–188. [Google Scholar] [CrossRef]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef]
- Pang, W.W.; Hartmann, P.E. Initiation of Human Lactation: Secretory Differentiation and Secretory Activation. J. Mammary Gland Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef]
- Crowley, W.R. Neuroendocrine regulation of lactation and milk production. Compr. Physiol. 2015, 5, 255–291. [Google Scholar] [CrossRef]
- Leng, G.; Caquineau, C.; Sabatier, N. Regulation of Oxytocin Secretion. Vitam. Horm. 2005, 71, 27–58. [Google Scholar]
- Feldman, R.; Bakermans-Kranenburg, M.J. Oxytocin: A parenting hormone. Curr. Opin. Psychol. 2017, 15, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher, A.E.; Collins, F.S.; Burke, W. Genomic medicine: Genetic testing. N. Engl. J. Med. 2002, 347, 1867–1875. [Google Scholar]
- Bardanzellu, F.; Fanos, V.; Reali, A. “Omics” in Human Colostrum and Mature Milk: Looking to Old Data with New Eyes. Nutrients 2017, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Palhière, I.; Maroteau, C.; Bardou, P.; Canale-Tabet, K.; Sarry, J.; Woloszyn, F.; Bertrand-Michel, J.; Racke, I.; Besir, H.; et al. A genome scan for milk production traits in dairy goats reveals two new mutations in Dgat1 reducing milk fat content. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.D.; Breakey, A.A.; Scelza, B.; Valeggia, C.; Jasienska, G.; Hinde, K. Concentrations of trace elements in human milk: Comparisons among women in Argentina, Namibia, Poland, and the United States. PLoS ONE 2017, 12, e0183367. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Gibson, R.S.; Diana, A.; Haszard, J.J.; Rahmannia, S.; Luftimas, D.E.; Hampel, D.; Shahab-Ferdows, S.; Reid, M.; Melo, L.; et al. Micronutrient intakes of lactating mothers and their association with breast milk concentrations and micronutrient adequacy of exclusively breastfed Indonesian infants. Am. J. Clin. Nutr. 2019, 110, 391–400. [Google Scholar] [CrossRef]
- Specker, B.L.; Black, A.; Allen, L.; Morrow, F. Vitamin B-12: Low milk concentrations are related to low serum concentrations in vegetarian women and to methylmalonic aciduria in their infants1-3. Am. J. Clin. Nutr. 1990, 52, 1073–1076. [Google Scholar] [CrossRef]
- Valentine, C.J.; Wagner, C.L. Nutritional Management of the Breastfeeding Dyad. Pediatr. Clin. N. Am. 2013, 60, 261–274. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Overview of nutrients in humanmilk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Hampel, D.; Dror, D.K.; Allen, L.H. Micronutrients in Human Milk: Analytical Methods. Adv. Nutr. 2018, 9, 313S–331S. [Google Scholar] [CrossRef]
- Golan, Y.; Kambe, T.; Assaraf, Y.G. The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency. Metallomics 2017, 9, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; da Costa, K.A.; Galanko, J.; Sha, W.; Stephenson, B.; Vick, J.; Zeisel, S.H. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am. J. Clin. Nutr. 2010, 92, 336–346. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Plat, J.; Mensink, R.P.; Müller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Lönnerdal, B.; Kelleher, S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Juan, S. Zinc Transporter SLC30A2 Genetic Variations and Health Implications. Master’s Thesis, University of Manitobain, Winnipeg, MB, Canada, 2014. [Google Scholar]
- Itsumura, N.; Kibihara, Y.; Fukue, K.; Miyata, A.; Fukushima, K.; Tamagawa-Mineoka, R.; Katoh, N.; Nishito, Y.; Ishida, R.; Narita, H.; et al. Novel mutations in SLC30A2 involved in the pathogenesis of transient neonatal zinc deficiency. Pediatr. Res. 2016, 80, 586–594. [Google Scholar] [CrossRef]
- Miletta, M.C.; Bieri, A.; Kernland, K.; Schöni, M.H.; Petkovic, V.; Flück, C.E.; Eblé, A.; Mullis, P.E. Transient Neonatal Zinc Deficiency Caused by a Heterozygous G87R Mutation in the Zinc Transporter ZnT-2 (SLC30A2) Gene in the Mother Highlighting the Importance of Zn2+ for Normal Growth and Development. Int. J. Endocrinol. 2013, 2013, 259189. [Google Scholar] [CrossRef]
- Liew, H.M.; Tan, C.W.; Ho, C.K.M.; Chee, J.N.; Koh, M.J.A. Transient Neonatal Zinc Deficiency Caused by a Novel Mutation in the SLC30A2 Gene. Pediatr. Dermatol. 2017, 1–2. [Google Scholar] [CrossRef]
- Lasry, I.; Seo, Y.A.; Ityel, H.; Shalva, N.; Pode-Shakked, B.; Glaser, F.; Berman, B.; Berezovsky, I.; Goncearenco, A.; Klar, A.; et al. A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J. Biol. Chem. 2012, 287, 29348–29361. [Google Scholar] [CrossRef]
- Lova Navarro, M.; Vera Casano, A.; Benito Lopez, C.; Fernandez Ballesteros, M.D.; Godoy Diaz, D.J.; Crespo Erchiga, A.; Romero Brufau, S. Transient neonatal zinc deficiency due to a new autosomal dominant mutation in gene SLC30A2 (ZnT-2). Pediatr. Dermatol. 2014, 31, 251–252. [Google Scholar] [CrossRef]
- Golan, Y.; Itsumura, N.; Glaser, F.; Berman, B.; Kambe, T.; Assaraf, Y.G. Molecular Basis of Transient Neonatal Zinc Deficiency: Novel ZnT2 Mutations Disrupting Zinc Binding and Permeation. J. Biol. Chem. 2016, 291, 13546–13559. [Google Scholar] [CrossRef]
- Golan, Y.; Yerushalmi, B.; Efrati, E.; Assaraf, Y.G. Identification of Genetic Diseases Using Breast Milk Cell Analysis: The Case of Transient Neonatal Zinc Deficiency (TNZD). Cell. Mol. Med. 2017, 3. [Google Scholar] [CrossRef]
- Itsumura, N.; Inamo, Y.; Okazaki, F.; Teranishi, F.; Narita, H.; Kambe, T.; Kodama, H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: A novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE 2013, 8, e64045. [Google Scholar] [CrossRef]
- Golan, Y.; Lehvy, A.; Horev, G.; Assaraf, Y.G. High proportion of transient neonatal zinc deficiency causing alleles in the general population. J. Cell. Mol. Med. 2018, 23. [Google Scholar] [CrossRef]
- Lasry, I.; Golan, Y.; Berman, B.; Amram, N.; Glaser, F.; Assaraf, Y.G. In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J. Biol. Chem. 2014, 289, 7275–7292. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hennigar, S.R.; Alam, S.; Nishida, K.; Kelleher, S.L. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015, 290, 13064–13078. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wang, B.; Tang, N.; Zhang, W.; Cai, W. Polymorphisms of SLC30A2 and selected perinatal factors associated with low milk zinc in Chinese breastfeeding women. Early Hum. Dev. 2012, 88, 663–668. [Google Scholar] [CrossRef]
- van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef]
- Yue, W.; Fang, X.; Zhang, C.; Pang, Y.; Xu, H.; Gu, C.; Shao, R.; Lei, C.; Chen, H. Two novel SNPs of the ABCG2 gene and its associations with milk traits in Chinese Holsteins. Mol. Biol. Rep. 2011, 38, 2927–2932. [Google Scholar] [CrossRef]
- Cohen-Zinder, M.; Seroussi, E.; Larkin, D.M.; Loor, J.J.; Everts-Van Der Wind, A.; Lee, J.H.; Drackley, J.K.; Band, M.R.; Hernandez, A.G.; Shani, M.; et al. Identification of a missense mutation in the bovine ABCG2 gene with a major effect on the QTL on chromosome 6 affecting milk yield and composition in Holstein cattle. Genome Res. 2005, 15, 936–944. [Google Scholar] [CrossRef]
- Malfará, B.N.; de Lima Benzi, J.R.; de Oliveira Filgueira, G.C.; Zanelli, C.F.; Duarte, G.; de Carvalho Cavalli, R.; de Moraes, N.V. ABCG2 c.421C>A polymorphism alters nifedipine transport to breast milk in hypertensive breastfeeding women. Reprod. Toxicol. 2019, 85, 1–5. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Iodine in Human Milk: A Systematic Review. Adv. Nutr. 2018, 9, 347S–357S. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Meyer, F. Neuronal effects of thyroid hormone metabolites. Mol. Cell. Endocrinol. 2017, 458, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Heuer, H. Thyroid hormone action during brain development: More questions than answers. Mol. Cell. Endocrinol. 2010, 315, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J. Thyroid Hormones and Brain Development. Vitam. Horm. 2005, 71, 95–122. [Google Scholar] [PubMed]
- Semba, R.D.; Delange, F. Iodine in Human Milk: Perspectives for Infant Health. Nutr. Rev. 2009, 59, 269–278. [Google Scholar] [CrossRef]
- Kamikawa, A.; Ichii, O.; Sakazaki, J.; Ishikawa, T. Ca 2+ -activated Cl − channel currents in mammary secretory cells from lactating mouse. Am. J. Physiol. Physiol. 2016, 311, C808–C819. [Google Scholar] [CrossRef]
- De La Vieja, A.; Santisteban, P. Role of iodide metabolism in physiology and cancer. Endocr. Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef]
- Lemay, D.G.; Ballard, O.A.; Hughes, M.A.; Morrow, A.L.; Horseman, N.D.; Nommsen-Rivers, L.A. RNA Sequencing of the Human Milk Fat Layer Transcriptome Reveals Distinct Gene Expression Profiles at Three Stages of Lactation. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878. [Google Scholar] [CrossRef]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef]
- Nicola, J.P.; Nazar, M.; Serrano-Nascimento, C.; Goulart-Silva, F.; Sobrero, G.; Testa, G.; Nunes, M.T.; Muñoz, L.; Miras, M.; Masini-Repiso, A.M. Iodide transport defect: Functional characterization of a novel mutation in the Na+/I− symporter 5′-untranslated region in a patient with congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2011, 96, E1100–E1107. [Google Scholar] [CrossRef]
- Mizokami, T.; Fukata, S.; Hishinuma, A.; Kogai, T.; Hamada, K.; Maruta, T.; Higashi, K.; Tajiri, J. Iodide Transport Defect and Breast Milk Iodine. Eur. Thyroid J. 2016, 5, 145–148. [Google Scholar] [CrossRef]
- Reed-Tsur, M.D.; De La Vieja, A.; Ginter, C.S.; Carrasco, N. Molecular characterization of V59E NIS, a Na+/I− symporter mutant that causes congenital I− transport defect. Endocrinology 2008, 149, 3077–3084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paroder, V.; Nicola, J.P.; Ginter, C.S.; Carrasco, N. The iodide-transport-defect-causing mutation R124H: A δ-amino group at position 124 is critical for maturation and trafficking of the Na+/I− symporter. J. Cell Sci. 2013, 126, 3305–3313. [Google Scholar] [CrossRef]
- Kosugi, S.; Okamoto, H.; Tamada, A.; Sanchez-Franco, F. A novel peculiar mutation in the sodium/iodide symporter gene in Spanish siblings with iodide transport defect. J. Clin. Endocrinol. Metab. 2002, 87, 3830–3836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De la Vieja, A.; Ginter, C.S.; Carrasco, N. The Q267E mutation in the sodium/iodide symporter (NIS) causes congenital iodide transport defect (ITD) by decreasing the NIS turnover number. J. Cell Sci. 2004, 117, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Reyna-Neyra, A.; Saenger, P.; Rodriguez-Buritica, D.F.; Godoy, J.D.G.; Muzumdar, R.; Amzel, L.M.; Carrasco, N. Sodium/iodide symporter mutant V270E causes stunted growth but no cognitive deficiency. J. Clin. Endocrinol. Metab. 2015, 100, E1353–E1361. [Google Scholar] [CrossRef]
- Pohlenz, J.; Medeiros-Neto, G.; Gross, J.L.; Silveiro, S.P.; Knobel, M.; Refetoff, S. Hypothyroidism in a Brazilian kindred due to iodide trapping defect caused by a homozygous mutation in the sodium/iodide symporter gene. Biochem. Biophys. Res. Commun. 1997, 240, 488–491. [Google Scholar] [CrossRef]
- Montanelli, L.; Agretti, P.; De Marco, G.; Bagattini, B.; Ceccarelli, C.; Brozzi, F.; Lettiero, T.; Cerbone, M.; Vitti, P.; Salerno, M.; et al. Congenital hypothyroidism and late-onset goiter: Identification and characterization of a novel mutation in the sodium/iodide symporter of the proband and family members. Thyroid 2009, 19, 1419–1425. [Google Scholar] [CrossRef]
- Levy, O.; Ginter, C.S.; De la Vieja, A.; Levy, D.; Carrasco, N. Identification of a structural requirement for thyroid Na+/I- symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Lett. 1998, 429, 36–40. [Google Scholar] [CrossRef]
- Dohán, O.; Verónica Gavrielides, M.; Ginter, C.; Mario Amzel, L.; Carrasco, N. Na+/i- symporter activity requires a small and uncharged amino acid residue at position 395. Mol. Endocrinol. 2002, 16, 1893–1902. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Nicola, J.P.; Amzel, L.M.; Carrasco, N. Asn441 plays a key role in folding and function of the Na+/I− symporter (NIS). FASEB J. 2013, 27, 3229–3238. [Google Scholar] [CrossRef]
- De La Vieja, A.; Ginter, C.S.; Carrasco, N. Molecular analysis of a congenital iodide transport defect: G543E impairs maturation and trafficking of the Na+/I- symporter. Mol. Endocrinol. 2005, 19, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, J.; Rosenthal, I.M.; Weiss, R.E.; Jhiang, S.M.; Burant, C.; Refetoff, S. Congenital hypothyroidism due to mutations in the sodium/iodide symporter. Identification of a nonsense mutation producing a downstream cryptic 3’ splice site. J. Clin. Investig. 1998, 101, 1028–1035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, L.; Innis, S.M. Genetic Variants of the FADS1 FADS2 Gene Cluster Are Associated with Altered (n-6) and (n-3) Essential Fatty Acids in Plasma and Erythrocyte Phospholipids in Women during Pregnancy and in Breast Milk during Lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Atakora, F.; Miliku, K.; Sepehri, S.; Robertson, B.; Duan, Q.L.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Integrated Analysis of Human Milk Microbiota With Oligosaccharides and Fatty Acids in the CHILD Cohort. Front. Nutr. 2019, 6, 58. [Google Scholar] [CrossRef]
- Smith-Brown, P.; Morrison, M.; Krause, L.; Davies, P.S.W. Mothers secretor status affects development of childrens microbiota composition and function: A pilot study. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’donnell, D.; Blanton, L.V.; Lebrilla, C.; Mills, D.A.; Correspondence, J.I.G.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition Article Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Kunz, C.; Rudloff, S.; García-Mantrana, I.; Crehuá-Gaudiza, E.; Martínez-Costa, C.; Collado, M.C. Association of Maternal Secretor Status and Human Milk Oligosaccharides With Milk Microbiota. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 256–263. [Google Scholar] [CrossRef]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.S.; German, J.B.; et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Zhang, P.; Gonzalez-Freire, M.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Ferrucci, L.; Manary, M.J. The association of serum choline with linear growth failure in young children from rural Malawi. Am. J. Clin. Nutr. 2016, 104, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Holmes-McNary, M.Q.; Cheng, W.L.; Mar, M.H.; Fussell, S.; Zeisel, S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996, 64, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef]
- MTHFD1 Gene Genetics Home Reference NIH. Available online: https://ghr.nlm.nih.gov/gene/MTHFD1#normalfunction (accessed on 22 April 2020).
- Rillema, J.A. Hormone Regulation of Choline Uptake and Incorporation in Mouse Mammary Gland Explants. Exp. Biol. Med. 2004, 229, 323–326. [Google Scholar] [CrossRef]
- Sharp, J.A.; Lefèvre, C.; Watt, A.; Nicholas, K.R. Analysis of human breast milk cells: Gene expression profiles during pregnancy, lactation, involution, and mastitic infection. Funct. Integr. Genom. 2016, 16, 297–321. [Google Scholar] [CrossRef]
- Hou, J.; Fang, F.; An, X.; Yan, Y.; Ma, T.; Han, P.; Meng, F.; Song, Y.; Wang, J.; Cao, B. Polymorphisms of PRLR and FOLR1 genes and association with milk production traits in goats. Genet. Mol. Res. 2014, 13, 2555–2562. [Google Scholar] [CrossRef]
- Ford, J.E. Some observations on the possible nutritional significance of vitamin B 12-and folate-binding proteins in milk. Br. J. Nutr. 1974, 31, 243–257. [Google Scholar] [CrossRef][Green Version]
- Mason, J.B.; Selhub, J. Folate-binding protein and the absorption of folic acid in the small intestine of the suckling rat. Am. J. Clin. Nutr. 1988, 48, 620–625. [Google Scholar] [CrossRef]
- Visentin, M.; Diop-Bove, N.; Zhao, R.; Goldman, I.D. The Intestinal Absorption of Folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Goldman, I.D. The proton-coupled folate transporter: Physiological and pharmacological roles. Curr. Opin. Pharmacol. 2013, 13, 875–880. [Google Scholar] [CrossRef]
- Page, R.; Wong, A.; Arbuckle, T.E.; Macfarlane, A.J. The MTHFR 677C>T polymorphism is associated with unmetabolized folic acid in breast milk in a cohort of Canadian women. Am. J. Clin. Nutr. 2019, 110, 401–409. [Google Scholar] [CrossRef]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef]
- Martin, C.; Ling, P.-R.; Blackburn, G. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Pappenheim, S.; Yener, S.; van Valenberg, H.J.F.; Tzompa-Sosa, D.A.; Bovenhuis, H. The DGAT1 K232A polymorphism and feeding modify milk fat triacylglycerol composition. J. Dairy Sci. 2019, 102, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Tăbăran, A.; Balteanu, V.A.; Gal, E.; Pusta, D.; Mihaiu, R.; Dan, S.D.; Tăbăran, A.F.; Mihaiu, M. Influence of DGAT1 K232A polymorphism on milk fat percentage and fatty acid profiles in Romanian holstein cattle. Anim. Biotechnol. 2015, 26, 105–111. [Google Scholar] [CrossRef]
- Rincon, G.; Islas-Trejo, A.; Castillo, A.R.; Bauman, D.E.; German, B.J.; Medrano, J.F. Polymorphisms in genes in the SREBP1 signalling pathway and SCD are associated with milk fatty acid composition in Holstein cattle. J. Dairy Res. 2012, 79, 66–75. [Google Scholar] [CrossRef]
- Cruz, V.A.R.; Oliveira, H.R.; Brito, L.F.; Fleming, A.; Larmer, S.; Miglior, F.; Schenkel, F.S. Genome-Wide Association Study for Milk Fatty Acids in Holstein Cattle Accounting for the DGAT1 Gene Effect. Animals 2019, 9, 997. [Google Scholar] [CrossRef]
- Zheng, X.R.; Jiang, L.; Ning, C.; Hu, Z.Z.; Zhou, L.; Yu, Y.; Zhang, S.L.; Liu, J.F. A novel mutation in the promoter region of RPL8 regulates milk fat traits in dairy cattle by binding transcription factor Pax6. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Scheper, C.; Brügemann, K.; Swalve, H.H.; König, S. Phenotypic relationships, genetic parameters, genome-wide associations, and identification of potential candidate genes for ketosis and fat-to-protein ratio in German Holstein cows. J. Dairy Sci. 2019, 102, 6276–6287. [Google Scholar] [CrossRef] [PubMed]
- Zielke, L.G.; Bortfeldt, R.H.; Reissmann, M.; Tetens, J.; Thaller, G.; Brockmann, G.A. Impact of Variation at the FTO Locus on Milk Fat Yield in Holstein Dairy Cattle. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Krämer, W.; Werner, F.A.O.; Kollers, S.; Kata, S.; Durstewitz, G.; Buitkamp, J.; Womack, J.E.; Thaller, G.; Fries, R. Association of a lysine-232/alanine polymorphism in a bovine gene encoding acyl-CoA:Diacylglycerol acyltransferase (DGAT1) with variation at a quantitative trait locus for milk fat content. Proc. Natl. Acad. Sci. USA 2002, 99, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Grisart, B.; Coppieters, W.; Farnir, F.; Karim, L.; Ford, C.; Berzi, P.; Cambisano, N.; Mni, M.; Reid, S.; Simon, P.; et al. Positional candidate cloning of a QTL in dairy cattle: Identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 2002, 12, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Spelman, R.J.; Ford, C.A.; McElhinney, P.; Gregory, G.C.; Snell, R.G. Characterization of the DGAT1 gene in the New Zealand dairy population. J. Dairy Sci. 2002, 85, 3514–3517. [Google Scholar] [CrossRef]
- Thaller, G.; Kramer, W.; Winter, A.; Kaupe, B.; Erhardt, G.; Fries, R. Effects of DGAT1 variants on milk production traits in German cattle breeds1. J. Anim. Sci. 2003, 81, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.I.; Golik, M.; Seroussi, E.; Ezra, E.; Ron, M. Population-wide analysis of a QTL affecting milk-fat production in the Israeli Holstein population. J. Dairy Sci. 2003, 86, 2219–2227. [Google Scholar] [CrossRef]
- Gautier, M.; Capitan, A.; Fritz, S.; Eggen, A.; Boichard, D.; Druet, T. Characterization of the DGAT1 K232A and variable number of tandem repeat polymorphisms in French dairy cattle. J. Dairy Sci. 2007, 90, 2980–2988. [Google Scholar] [CrossRef]
- Fürbass, R.; Winter, A.; Fries, R.; Kühn, C. Alleles of the bovine DGAT1 variable number of tandem repeat associated with a milk fat QTL at chromosome 14 can stimulate gene expression. Physiol. Genom. 2006, 25, 116–120. [Google Scholar] [CrossRef]
- Berry, D.P.; Howard, D.; O’Boyle, P.; Waters, S.; Kearney, J.F.; McCabe, M. Associations between the K232A polymorphism in the diacylglycerol-O-transferase 1 (DGAT1) gene and performance in Irish Holstein-Friesian dairy cattle. Irish J. Agric. Food Res. 2010, 49, 1–9. [Google Scholar]
- Gluchowski, N.L.; Chitraju, C.; Picoraro, J.A.; Mejhert, N.; Pinto, S.; Xin, W.; Kamin, D.S.; Winter, H.S.; Chung, W.K.; Walther, T.C.; et al. Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. J. Lipid Res. 2017, 58, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.T.; Winter, H.S.; Lim, E.; Kirby, A.; Blumenstiel, B.; DeFelice, M.; Gabriel, S.; Branski, D.; Grueter, C.A.; Toporovski, M.S.; et al. DGAT1 mutation is linked to a congenital diarrheal disorder. J. Clin. Investig. 2012, 122, 4680–4684. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, G.L.; Li, X.; Chen, X.Y.; Wu, Y.X.; Cui, C.C.; Zhang, X.; Yang, G.; Xie, L. Association of polyunsaturated fatty acids in breast milk with fatty acid desaturase gene polymorphisms among Chinese lactating mothers. Prostaglandins Leukot. Essent. Fat. Acids 2016, 109, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Rzehak, P.; Szabó, É.; Jakobik, V.; Weck, M.; Weyermann, M.; Grallert, H.; Rothenbacher, D.; Heinrich, J.; Brenner, H.; et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 2011, 93, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Morales, E.; Bustamante, M.; Gonzalez, J.R.; Guxens, M.; Torrent, M.; Mendez, M.; Garcia-Esteban, R.; Julvez, J.; Forns, J.; Vrijheid, M.; et al. Genetic Variants of the FADS Gene Cluster and ELOVL Gene Family, Colostrums LC-PUFA Levels, Breastfeeding, and Child Cognition. PLoS ONE 2011, 6, e17181. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Tian, H.; Lu, T.; Yu, M.; Xu, W.; Liu, G.; Xie, L. DHA intake interacts with ELOVL2 and ELOVL5 genetic variants to influence polyunsaturated fatty acids in human milk. J. Lipid Res. 2019, 60, 1043–1049. [Google Scholar] [CrossRef]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.E.P.; Huber, M.; Van Ree, R.; Simões-Wüst, A.P.; Dagnelie, P.C.; Van Den Brandt, P.A. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy 2011, 66, 58–67. [Google Scholar] [CrossRef]
- Chowanadisai, W.; Kelleher, S.L.; Nemeth, J.F.; Yachetti, S.; Kuhlman, C.F.; Jackson, J.G.; Davis, A.M.; Lien, E.L.; Lönnerdal, B. Detection of a single nucleotide polymorphism in the human α-lactalbumin gene: Implications for human milk proteins. J. Nutr. Biochem. 2005, 16, 272–278. [Google Scholar] [CrossRef]
- Nilsen, H.; Olsen, H.G.; Hayes, B.; Sehested, E.; Svendsen, M.; Nome, T.; Meuwissen, T.; Lien, S. Casein haplotypes and their association with milk production traits in Norwegian Red cattle. Genet. Sel. Evol. 2009, 41. [Google Scholar] [CrossRef] [PubMed]
- Alim, M.A.; Dong, T.; Xie, Y.; Wu, X.P.; Zhang, Y.; Zhang, S.; Sun, D.X. Effect of polymorphisms in the CSN3 (κ-casein) gene on milk production traits in Chinese Holstein Cattle. Mol. Biol. Rep. 2014, 41, 7585–7593. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Raman, A.S.; Murch, S.H.; Rollins, N.C.; Gordon, J.I. Understanding the mother-breastmilk-infant “triad”. Science 2020, 367, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Lagström, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Mäkelä, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 2020, 11, 769–778. [Google Scholar] [CrossRef]
- Borewicz, K.; Gu, F.; Saccenti, E.; Hechler, C.; Beijers, R.; de Weerth, C.; van Leeuwen, S.S.; Schols, H.A.; Smidt, H. The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci. Rep. 2020, 10, 4270. [Google Scholar] [CrossRef]
- Bai, Y.; Tao, J.; Zhou, J.; Fan, Q.; Liu, M.; Hu, Y.; Xu, Y.; Zhang, L.; Yuan, J.; Li, W.; et al. Fucosylated Human Milk Oligosaccharides and N-Glycans in the Milk of Chinese Mothers Regulate the Gut Microbiome of Their Breast-Fed Infants during Different Lactation Stages. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Wacklin, P.; Mä, H.; Alakulppi, N.; Nikkilä, J.; Tenkanen, H.; Rä, J.; Partanen, J.; Aranko, K.; Mä, J. Secretor Genotype (FUT2 gene) Is Strongly Associated with the Composition of Bifidobacteria in the Human Intestine. PLoS ONE 2011, 6, e20113. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Hickman, B.; Kunz, C.; Sprenger, N.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; de Vos, W.M. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Blanton, L.V.; DiGiulio, D.B.; Relman, D.A.; Lebrilla, C.B.; Mills, D.A.; Gordon, J.I. Human developmental biology viewed from a microbial perspective. Nature 2016, 535, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.M.; Bode, L.; Sharma, A.K.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Azad, M.B. Maternal Factors and Human Milk Oligosaccharide Composition in the CHILD Cohort. FASEB J. 2017, 31. [Google Scholar]
- Cui, X.; Hou, Y.; Yang, S.; Xie, Y.; Zhang, S.; Zhang, Y.; Zhang, Q.; Lu, X.; Liu, G.E.; Sun, D. Transcriptional profiling of mammary gland in Holstein cows with extremely different milk protein and fat percentage using RNA sequencing. BMC Genom. 2014, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.J. Isolated prolactin deficiency: A case report. Fertil. Steril. 1992, 58, 1060–1062. [Google Scholar] [CrossRef]
- Berens, P.D.; Villanueva, M.; Nader, S.; Swaim, L.S. Isolated prolactin deficiency: A possible culprit in lactation failure. AACE Clin. Case Rep. 2018, 4, e509–e512. [Google Scholar] [CrossRef]
- Iwama, S.; Welt, C.K.; Romero, C.J.; Radovick, S.; Caturegli, P. Isolated Prolactin Deficiency Associated With Serum Autoantibodies Against Prolactin-Secreting Cells. J. Clin. Endocrinol. Metab. 2013, 98, 3920–3925. [Google Scholar] [CrossRef]
- Saito, T.; Tojo, K.; Oki, Y.; Sakamoto, N.; Matsudaira, T.; Sasaki, T.; Tajima, N. A Case of Prolactin Deficiency with Familial Puerperal Alactogenesis Accompanying Impaired ACTH Secretion. Endocr. J. 2006, 54, 59–62. [Google Scholar] [CrossRef]
- Moriwaki, M.; Welt, C. MON-434 Familial Alactogenesis Associated with a Prolactin Mutation. J. Endocr. Soc. 2019, 3. [Google Scholar] [CrossRef]
- Brym, P.; Kamiński, S.; Wójcik, E. Polymorphism within the Bovine Prolactin Receptor Gene (PRLR). Anim. Sci. Pap. Rep. 2005, 23, 61–66. [Google Scholar]
- Lü, A.; Hu, X.; Chen, H.; Dong, Y.; Zhang, Y.; Wang, X. Novel SNPs of the bovine PRLR gene associated with milk production traits. Biochem. Genet. 2011, 49, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zan, L.; Fang, P.; Zhang, F.; Shen, G.; Tian, W. Genetic variation of PRLR gene and association with milk performance traits in dairy cattle. Can. J. Anim. Sci. 2008, 88, 33–39. [Google Scholar] [CrossRef]
- Kelly, P.A.; Binart, N.; Lucas, B.; Bouchard, B.; Goffin, V. Implications of Multiple Phenotypes Observed in Prolactin Receptor Knockout Mice. Front. Neuroendocr. 2001, 22, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Newey, P.J.; Gorvin, C.M.; Cleland, S.J.; Willberg, C.B.; Bridge, M.; Azharuddin, M.; Drummond, R.S.; van der Merwe, P.A.; Klenerman, P.; Bountra, C.; et al. Mutant Prolactin Receptor and Familial Hyperprolactinemia. N. Engl. J. Med. 2013, 369, 2012–2020. [Google Scholar] [CrossRef]
- Majumdar, A.; Mangal, N.S. Hyperprolactinemia. J. Hum. Reprod. Sci. 2013, 6, 168–175. [Google Scholar] [CrossRef]
- Kobayashi, T.; Usui, H.; Tanaka, H.; Shozu, M. Variant Prolactin Receptor in Agalactia and Hyperprolactinemia. N. Engl. J. Med. 2018, 379, 2230–2236. [Google Scholar] [CrossRef]
- Hlusko, L.J.; Carlson, J.P.; Chaplin, G.; Elias, S.A.; Hoffecker, J.F.; Huffman, M.; Jablonski, N.G.; Monson, T.A.; O’Rourke, D.H.; Pilloud, M.A.; et al. Environmental selection during the last ice age on the mother-to-infant transmission of vitamin D and fatty acids through breast milk. Proc. Natl. Acad. Sci. USA 2018, 115, E4426–E4432. [Google Scholar] [CrossRef]
- De Amici, D.; Gasparoni, A.; Guala, A.; Klersy, C. Does ethnicity predict lactation? A study of four ethnic communities. Eur. J. Epidemiol. 2001, 17, 357–362. [Google Scholar] [CrossRef]
- Seo, Y.A.; Kelleher, S.L. Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): Implications for mammary gland function and breast disease in women. Physiol. Genom. 2010, 42A, 219–227. [Google Scholar] [CrossRef][Green Version]
- Mathias, R.A.; Pani, V.; Chilton, F.H. Genetic Variants in the FADS Gene: Implications for Dietary Recommendations for Fatty Acid Intake. Curr. Nutr. Rep. 2014, 3, 139–148. [Google Scholar] [CrossRef]
- Ekmekci, A.; Cirak, M.Y. Nutrigenomics and nutrigenetics. In Advances in Food Biochemistry; CRC Press: Boca Raton, FL, USA, 2009; Chapter 14; pp. 457–475. ISBN 9781420007695. [Google Scholar]
- Sales, N.M.R.; Pelegrini, P.B.; Goersch, M.C. Nutrigenomics: Definitions and advances of this new science. J. Nutr. Metab. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Hickman, J.A. Limits to Personalized Cancer Medicine. N. Engl. J. Med. 2016, 375, 1289–1295. [Google Scholar] [CrossRef]
- Turnbull, A.K. Personalized medicine in cancer: Where are we today? Future Oncol. 2015, 11, 2795–2798. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Abdeen, S.K.; Elinav, E. Personalized Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Hietaranta-Luoma, H.L.; Tahvonen, R.; Iso-Touru, T.; Puolijoki, H.; Hopia, A. An intervention study of individual, apoe genotype-based dietary and physical-activity advice: Impact on health behavior. J. Nutr. Nutr. 2014, 7, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Dhar, S.; Mitchell, L.M.; Fu, B.; Tyson, J.; Shwan, N.A.A.; Yang, F.; Thomas, M.G.; Armour, J.A.L. Obesity, starch digestion and amylase: Association between copy number variants at human salivary (AMY1) and pancreatic (AMY2) amylase genes. Hum. Mol. Genet. 2015, 24, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Knott, C.D.; Conklin-Brittain, N. Infant sex predicts breast milk energy content. Am. J. Hum. Biol. 2010, 22, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Almog, M.; Peri, R.; Halasz, K.; Srugo, I.; Kessel, A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr. Res. 2012, 71, 220–225. [Google Scholar] [CrossRef]
- GarcíA-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary gland—Contribution to presence of nutrients and drugs into milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Alhadeff, R.; Warshel, A.; Assaraf, Y.G. ZnT2 is an electroneutral proton-coupled vesicular antiporter displaying an apparent stoichiometry of two protons per zinc ion. PLoS Comput. Biol. 2019, 15, e1006882. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Protein Name | Effect of Mutations on Human Milk | The Effect on the Infant or Related Disease | Treatment |
|---|---|---|---|---|
| SLC30A2 | ZnT2 | LoF homozygous mutation in ZnT2 were found in mothers producing zinc-deficient human milk [25,26,27,28,29,30,31,32,33]. | Transient neonatal zinc deficiency (TNZD), a disorder that leads to severe zinc deficiency in exclusively breastfed infants | Zinc supplementation (5 mg/day) and continuing breastfeeding [32]. No supplementation is needed after weaning. |
| ABCG2 | BCRP | Mothers harboring the c.421C > A polymorphism in ABCG2, secreted threefold more nifedipine to human milk [41]. | Unknown | |
| SLC5A5 | Sodium iodide symporter (NIS) | Mother carrying homozygous T354P mutation in the NIS transporter was reported to produce iodine-deficient milk. Other known LoF mutations in SLC5A5: V59E [54], G93R [51], R124H [55], Δ143-323 [56], Q267E [57], V270E [58], C272X [59], Δ287-288 [60], T354P [61], G395R [62], Δ439-443 [63], G543E [64], fs515X [65], and Y531X [65]. | The mother was diagnosed with ITD, treated with levothyroxine from the age of five years old, therefore, iodine supplementation was given after birth to prevent deficiencies in the infant. | Mother supplementation with 50 mg potassium iodide tablet daily starting on the fifth day postpartum to increase iodine concentration in human milk. |
| MTHFR | Methylene tetrahydrof-olate reductase | The MTHFR 677C > T SNP was associated with higher levels of human milk unmetabolized folic acid (UMFA) [41]. | Unknown | |
| MTHFD1 | Methylene tetrahydrof-olate dehydroge-nase 1 | rs1076991, rs2983733, rs2987981, rs8003379, and rs17824591 SNPs in the methylene tetrahydrofolate dehydrogenase 1 (MTHFD1) gene were found to be associated with very high human milk choline concentrations in three subjects [22]. | Unknown | |
| FADS1 and FADS2 | Fatty acid desaturase 1/2 | The minor allele homozygotes of rs174553 (GG), rs99780 (TT), and rs174583 (TT) were associated with significantly lower 14:0, arachidonic (ARA, 20:4 ) and eicosapentanoic acid (EPA, 20:5), but higher 20:2 (n − 6) fatty acid in human milk [66]. Mothers carrying the minor homozygous allele G/G of rs174575, showed lower ARA, EPA, and docosahexanoic acids (DHA, 22:6 (n − 3)) and 22:5 (n − 3) levels in human milk [66]. Mothers carrying FADS1 rs174561, FADS2 rs174575, and intergenic rs3834458 minor alleles were found to have lower proportions of DHA in human milk [23]. | Mothers carrying genetic variants associated with lower FADS1 activity (regulating AA and EPA synthesis), higher FADS2 activity (regulating DHA synthesis), and with higher EPA/AA and DHA/AA ratios in colostrum showed a significant advantage in cognition at 14 months. | |
| FUT2 | Fucosyltra-nsferase 2 | Nonsense mutation W143X that introduces a premature stop codon in the FUT2 gene (rs601338) abolished the ability to synthesize α (1-2)-fucosylated HMOs (non-secretor status). Non-secretors where found to express less HMOs compared to mothers with secretor status (active FUT2) [67,68,69,70]. In addition, maternal secretor status was shown to be associated with the human milk microbiota composition [71]. | Infants fed by non-secretor mothers, were delayed in the establishment of their gut microbiota, specifically bifidobacterial-laden [72,73]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golan, Y.; Assaraf, Y.G. Genetic and Physiological Factors Affecting Human Milk Production and Composition. Nutrients 2020, 12, 1500. https://doi.org/10.3390/nu12051500
Golan Y, Assaraf YG. Genetic and Physiological Factors Affecting Human Milk Production and Composition. Nutrients. 2020; 12(5):1500. https://doi.org/10.3390/nu12051500
Chicago/Turabian StyleGolan, Yarden, and Yehuda G. Assaraf. 2020. "Genetic and Physiological Factors Affecting Human Milk Production and Composition" Nutrients 12, no. 5: 1500. https://doi.org/10.3390/nu12051500
APA StyleGolan, Y., & Assaraf, Y. G. (2020). Genetic and Physiological Factors Affecting Human Milk Production and Composition. Nutrients, 12(5), 1500. https://doi.org/10.3390/nu12051500





