Less Animal-Based Food, Better Weight Status: Associations of the Restriction of Animal-Based Product Intake with Body-Mass-Index, Depressive Symptoms and Personality in the General Population
Abstract
1. Introduction
2. Materials and Methods
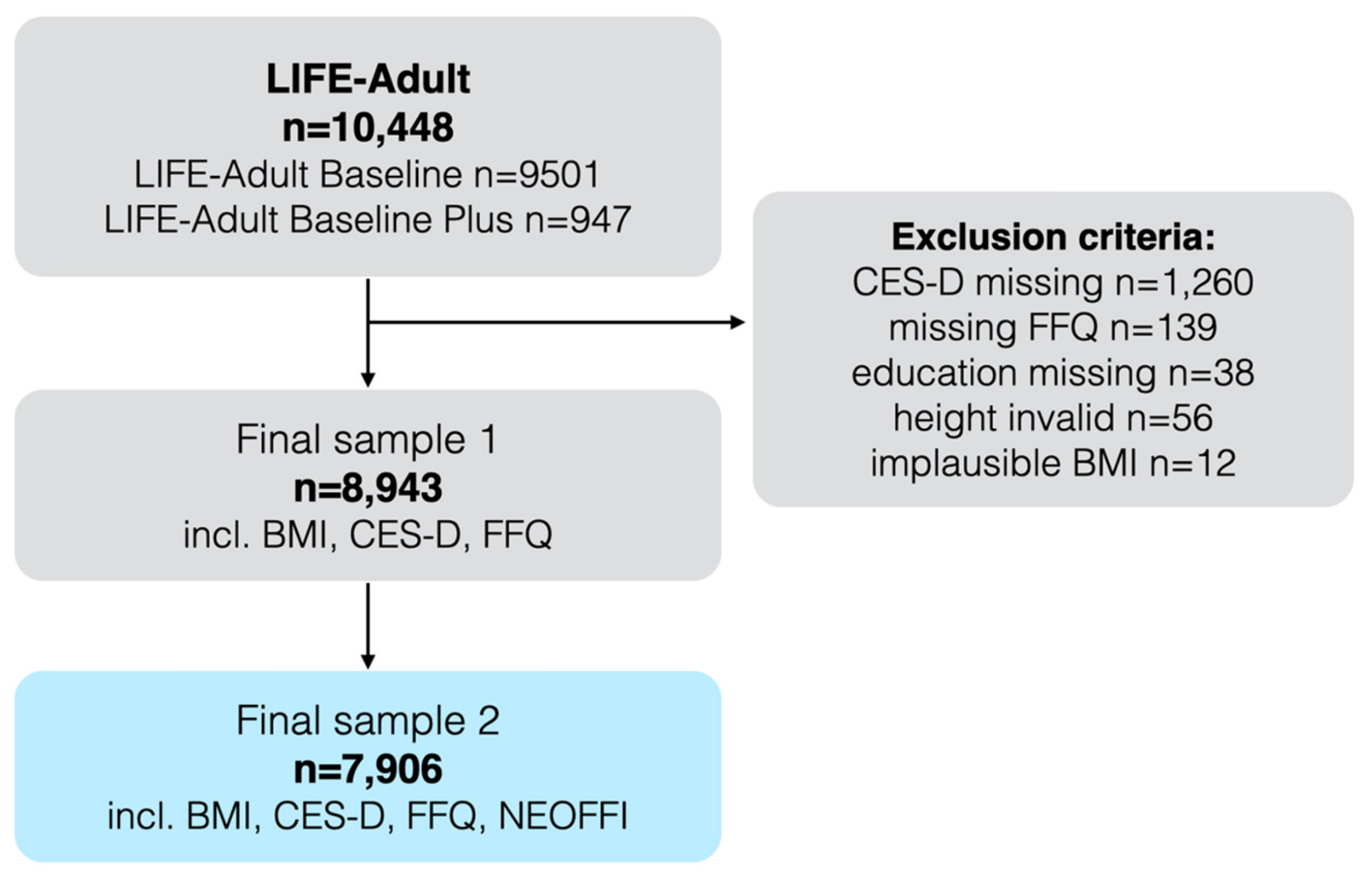
2.1. Inclusion Criteria
2.2. Ethics
2.3. Demographics
2.4. Anthropometry
2.5. Personality
2.6. Depressive Scores
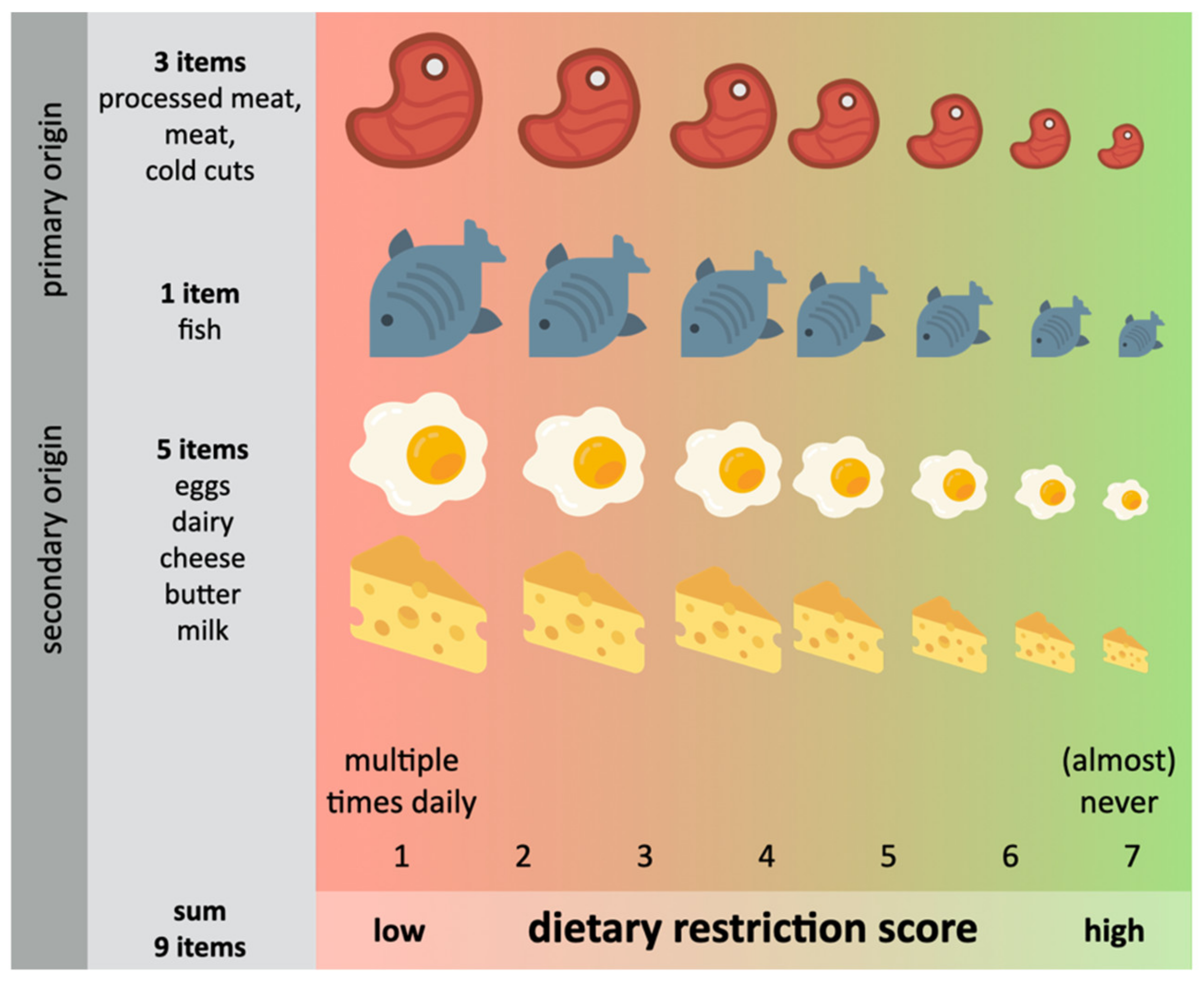
2.7. Dietary Restriction Scores (DRS)
2.8. Statistical Models
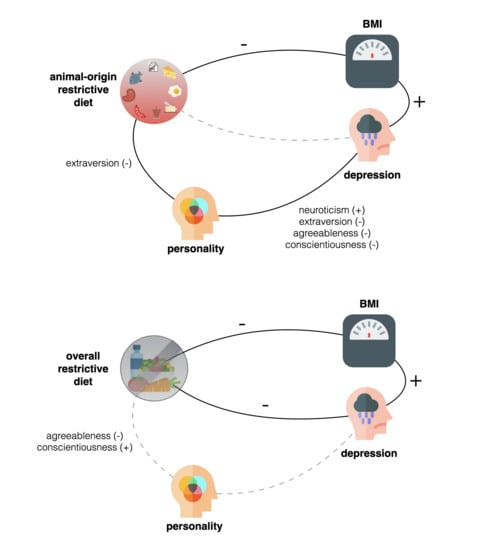
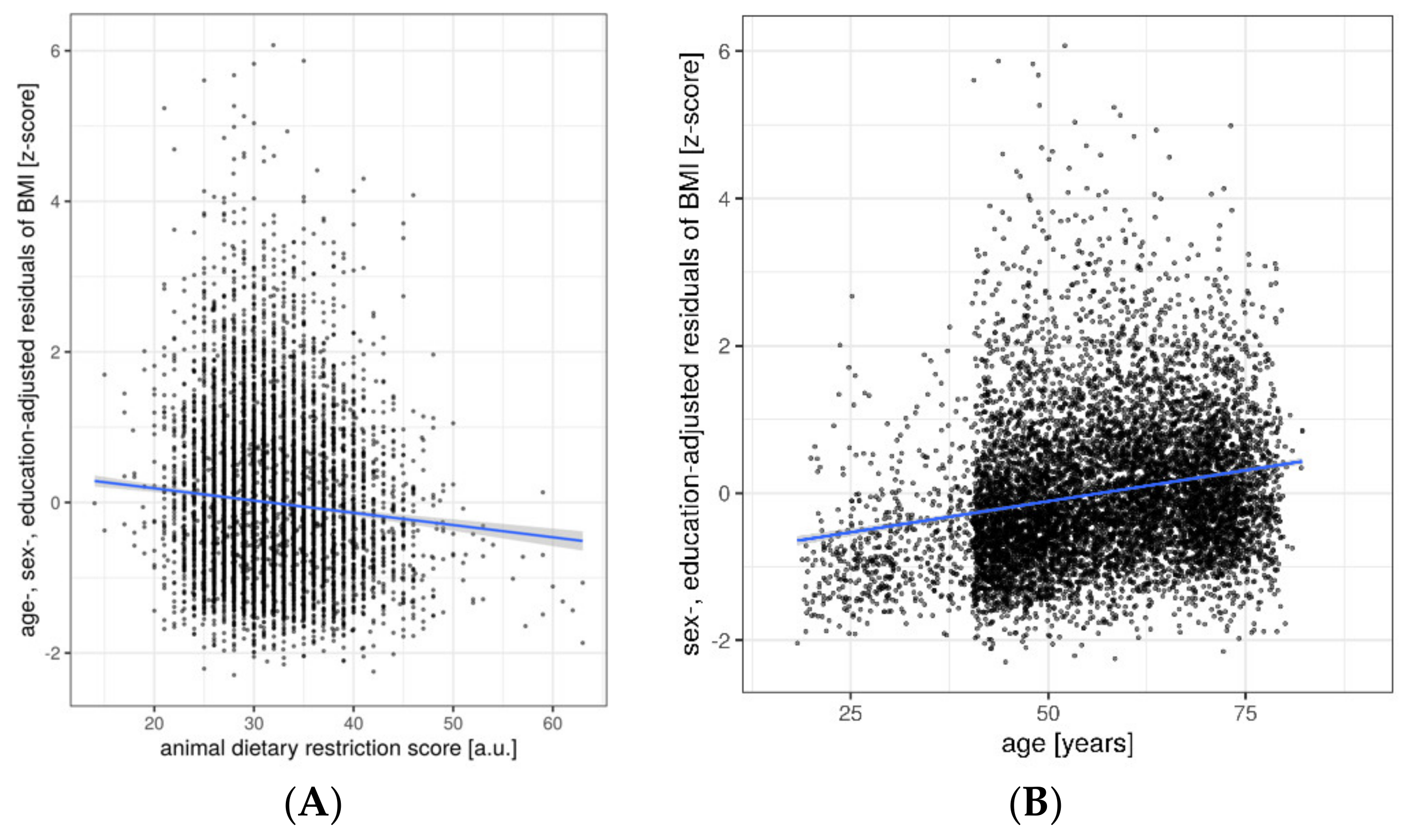
3. Results
Ancillary Analyses
4. Discussion
4.1. Weight Status
4.2. Depressive Symptoms & Personality Traits
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar]
- Le, L.T.; Sabaté, J. Beyond meatless, the health effects of vegan diets: Findings from the Adventist cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar]
- Key, T.J.; Appleby, P.N.; Spencer, E.A.; Travis, R.C.; Roddam, A.W.; Allen, N.E. Mortality in British vegetarians: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 2009, 89, 1613S–1619S. [Google Scholar]
- Mihrshahi, S.; Ding, D.; Gale, J.; Allman-Farinelli, M.; Banks, E.; Bauman, A.E. Vegetarian diet and all-cause mortality: Evidence from a large population-based Australian cohort-the 45 and up study. Prev. Med. 2017, 97, 1–7. [Google Scholar]
- Tong, T.Y.N.; Appleby, P.N.; Bradbury, K.E.; Perez-Cornago, A.; Travis, R.C.; Clarke, R.; Key, T.J. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: Results from the prospective EPIC-Oxford study. BMJ 2019, 366, l4897. [Google Scholar]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.A.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar]
- Lee, Y.-M.; Kim, S.-A.; Lee, I.-K.; Kim, J.-G.; Park, K.-G.; Jeong, J.-Y.; Jeon, J.-H.; Shin, J.-Y.; Lee, D.-H. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: A 12-week randomized clinical trial. PLoS ONE 2016, 11, e0155918. [Google Scholar]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N. A plant-based high-carbohydrate, low-fat diet in overweight individuals in a 16-week randomized clinical trial: The role of carbohydrates. Nutrients 2018, 10, 1302. [Google Scholar]
- Kahleova, H.; Fleeman, R.; Hlozkova, A.; Holubkov, R.; Barnard, N.D. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: Metabolic benefits of plant protein. Nutr. Diabetes 2018, 8, 1–1058. [Google Scholar]
- Kim, M.; Hwang, S.; Park, E.; Bae, J. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar]
- Glick-Bauer, M.; Yeh, M.-C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838. [Google Scholar]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar]
- Forestell, C.A.; Nezlek, J.B. Vegetarianism, depression, and the five factor model of personality. Ecol. Food Nutr. 2018, 57, 246–259. [Google Scholar]
- Hibbeln, J.R.; Northstone, K.; Evans, J.; Golding, J. Vegetarian diets and depressive symptoms among men. J. Affect. Disord. 2018, 225, 13–17. [Google Scholar]
- Matta, J.; Czernichow, S.; Kesse-Guyot, E.; Hoertel, N.; Limosin, F.; Goldberg, M.; Zins, M.; Lemogne, C. Depressive symptoms and vegetarian diets: Results from the Constances cohort. Nutrients 2018, 10, 1695. [Google Scholar]
- Luck-Sikorski, C.; Jung, F.; Schlosser, K.; Riedel-Heller, S.G. Is orthorexic behavior common in the general public? A large representative study in Germany. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2019, 24, 267–273. [Google Scholar]
- Beezhold, B.L.; Johnston, C.S. Restriction of meat, fish, and poultry in omnivores improves mood: A pilot randomized controlled trial. Nutr. J. 2012, 11, 9. [Google Scholar]
- Kahleova, H.; Hrachovinova, T.; Hill, M.; Pelikanova, T. Vegetarian diet in type 2 diabetes—Improvement in quality of life, mood and eating behaviour. Diabet. Med. 2013, 30, 127–129. [Google Scholar] [PubMed]
- Agarwal, U.; Mishra, S.; Xu, J.; Levin, S.; Gonzales, J.; Barnard, N.D. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: The GEICO study. Am. J. Health Promot. 2015, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Beezhold, B.; Radnitz, C.; Rinne, A.; DiMatteo, J. Vegans report less stress and anxiety than omnivores. Nutr. Neurosci. 2015, 18, 289–296. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.L.; Kotwal, R.; Malhotra, S.; Nelson, E.B.; Keck, P.E., Jr.; Nemeroff, C.B. Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry 2004, 65, 634–651. [Google Scholar] [CrossRef]
- Vainik, U.; Dagher, A.; Realo, A.; Colodro-Conde, L.; Mortensen, E.L.; Jang, K.; Juko, A.; Kandler, C.; Sørensen, T.I.A.; Mõttus, R. Personality-obesity associations are driven by narrow traits: A meta-analysis. Obes. Rev. 2019, 20, 1121–1131. [Google Scholar] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W.J.H. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [PubMed]
- Ouakinin, S.R.S.; Barreira, D.P.; Gois, C.J. Depression and obesity: Integrating the role of stress, neuroendocrine dysfunction and inflammatory pathways. Front. Endocrinol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the Nutrinet-Sante study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef]
- Loeffler, M.; Engel, C.; Ahnert, P.; Alfermann, D.; Arelin, K.; Baber, R.; Beutner, F.; Binder, H.; Brähler, E.; Burkhardt, R. The LIFE-Adult-Study: Objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015, 15, 691. [Google Scholar] [CrossRef]
- Priyanka, T.K.; Kapoor, S. Personality variations of vegetarian and non-vegetarian adolescentss. Indian J. Res. 2016, 5, 68–70. [Google Scholar]
- König, W.; Lüttinger, P.; Müller, W. A Comparative Analysis of the Development and Structure of Educational Systems: Methodological Foundations and the Construction of a Comparative Educational Scale; Universität Mannheim, Institut für Sozialwissenschaften: Mannheim, Germany, 1988. [Google Scholar]
- Herzberg, P.Y.; Brähler, E. Assessing the Big-Five personality domains via short forms. Eur. J. Psychol. Assess. 2006, 22, 139–148. [Google Scholar] [CrossRef]
- Costa, P.T., Jr.; McCrae, R.R. The revised NEO personality inventory (NEO-PI-R); SAGE Publications Inc: London, UK, 2008. [Google Scholar]
- Körner, A.; Geyer, M.; Roth, M.; Drapeau, M.; Schmutzer, G.; Albani, C.; Schumann, S.; Brähler, E. Persönlichkeitsdiagnostik mit dem neo-fünf-faktoren-inventar: Die 30-item-kurzversion (neo-ffi-30). PPmP-Psychother. Psychosom. Med. Psychol. 2008, 58, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Kurth, T.; Moore, S.C.; Gaziano, J.M.; Kase, C.S.; Stampfer, M.J.; Berger, K.; Buring, J.E. Healthy lifestyle and the risk of stroke in women. Arch. Intern. Med. 2006, 166, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Flöel, A.; Witte, A.V.; Lohmann, H.; Wersching, H.; Ringelstein, E.B.; Berger, K.; Knecht, S. Lifestyle and memory in the elderly. Neuroepidemiology 2008, 31, 39–47. [Google Scholar] [CrossRef]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Heal. Sci. Educ. 2010, 15, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Yan, R.; Butler, T.L.; Jaceldo-Siegl, K.; Beeson, W.L.; Chan, J. Missing data in a long food frequency questionnaire: Are imputed zeroes correct? Epidemiology 2009, 20, 289. [Google Scholar] [CrossRef]
- Enzenbach, C.; Wicklein, B.; Wirkner, K.; Loeffler, M. Evaluating selection bias in a population-based cohort study with low baseline participation: The LIFE-Adult-Study. BMC medical research methodology. BMC Med. Res. Methodol. 2019, 19, 135. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. 2003, 27, 728–734. [Google Scholar] [CrossRef]
- Wardle, J.; Waller, J.; Jarvis, M.J. Sex differences in the association of socioeconomic status with obesity. Am. J. Public Health 2002, 92, 1299–1304. [Google Scholar] [CrossRef]
- Groth, M.; Fagt, S.; Brøndsted, L. Social determinants of dietary habits in Denmark. Eur. J. Clin. Nutr. 2001, 55, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping neural endophenotypes in addiction and obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef]
- Curtain, F.; Grafenauer, S. Plant-based meat substitutes in the flexitarian age: An audit of products on supermarket shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef]
- Biswal, B.B.; Mennes, M.; Zuo, X.-N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef]
- Muga, M.A.; Owili, P.O.; Hsu, C.-Y.; Rau, H.-H.; Chao, J.C.J. Dietary patterns, gender, and weight status among middle-aged and older adults in Taiwan: A cross-sectional study. BMC Geriatr. 2017, 17, 268. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V. Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019, 30, 67–77. [Google Scholar] [CrossRef]
- Forestell, C.A. Flexitarian diet and weight control: Healthy or risky eating behavior? Front. Nutr. 2018, 5, 59. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Kuna, S.T.; Reboussin, D.M.; Borradaile, K.E.; Sanders, M.H.; Millman, R.P.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Jakicic, J.M.; Wing, R.R. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013, 36, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Borradaile, K.E.; Sanders, M.H.; Millman, R.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Pi-Sunyer, F.X. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The Sleep AHEAD study. Arch. Intern. Med. 2009, 169, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, L.M.; Das, D.; Majumdar, S.R.; Johnson, J.A.; Padwal, R.S. The effect of weight loss on health-related quality of life: Systematic review and meta-analysis of randomized trials. Obes. Rev. 2014, 15, 169–182. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef]
- Pfeiler, T.M.; Egloff, B. Examining the “Veggie” personality: Results from a representative German sample. Appetite 2018, 120, 246–255. [Google Scholar] [CrossRef]
- Cole, M.; Morgan, K. Vegaphobia: Derogatory discourses of veganism and the reproduction of speciesism in UK national newspapers 1. Br. J. Sociol. 2011, 62, 134–153. [Google Scholar] [CrossRef]
- MacInnis, C.C.; Hodson, G. It ain’t easy eating greens: Evidence of bias toward vegetarians and vegans from both source and target. Group Process. Intergroup Relat. 2017, 20, 721–744. [Google Scholar] [CrossRef]
- Thomas, M.A. Are vegans the same as vegetarians? The effect of diet on perceptions of masculinity. Appetite 2016, 97, 79–86. [Google Scholar] [CrossRef]
- Garnett, E.E.; Balmford, A.; Sandbrook, C.; Pilling, M.A.; Marteau, T.M. Impact of increasing vegetarian availability on meal selection and sales in cafeterias. Proc. Natl. Acad. Sci. USA 2019, 116, 20923–20929. [Google Scholar] [CrossRef]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wójcik, G. Unprocessed red meat and processed meat consumption: Dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef]
- Slanger, T.; Mutschelknauss, E.; Kropp, S.; Braendle, W.; Flesch-Janys, D.; Chang-Claude, J. Test–retest reliability of self-reported reproductive and lifestyle data in the context of a German case–control study on breast cancer and postmenopausal hormone therapy. Ann. Epidemiol. 2007, 17, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Finger, J.D.; Tylleskär, T.; Lampert, T.; Mensink, G.B. Dietary behaviour and socioeconomic position: The role of physical activity patterns. PLoS ONE 2013, 8, e78390. [Google Scholar] [CrossRef] [PubMed]
- Temple, N. The possible importance of income and education as covariates in cohort studies that investigate the relationship between diet and disease. F1000Res. 2015, 4, 690. [Google Scholar] [CrossRef]
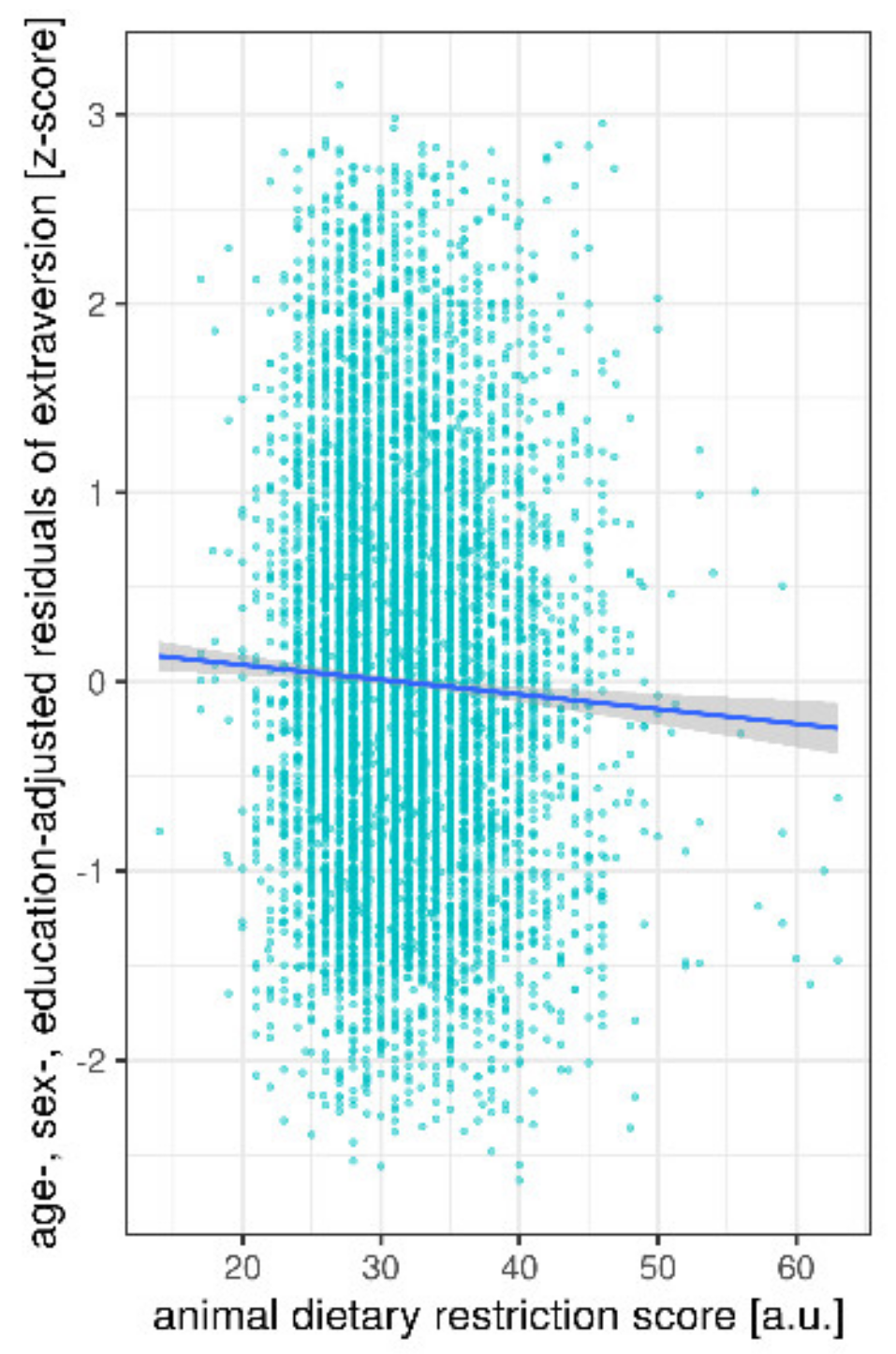
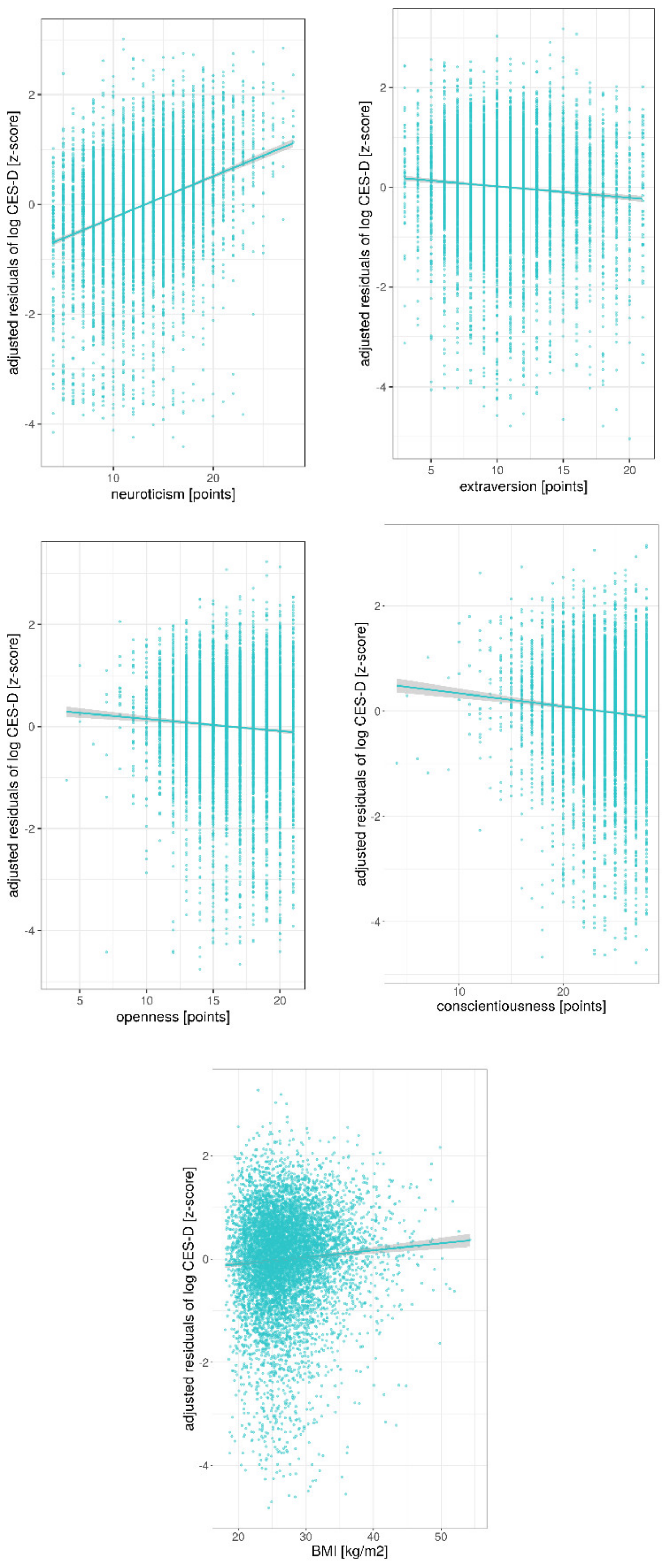
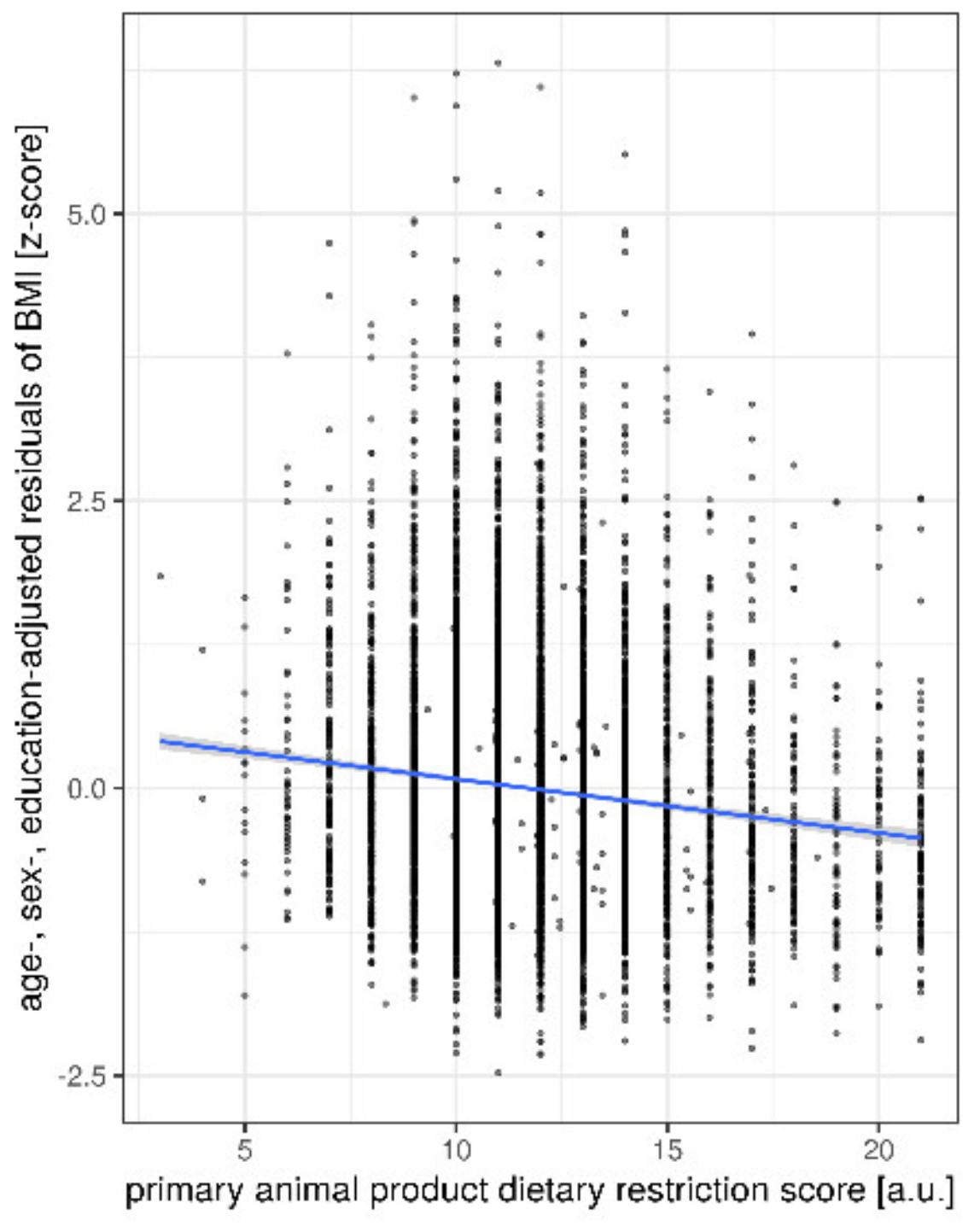
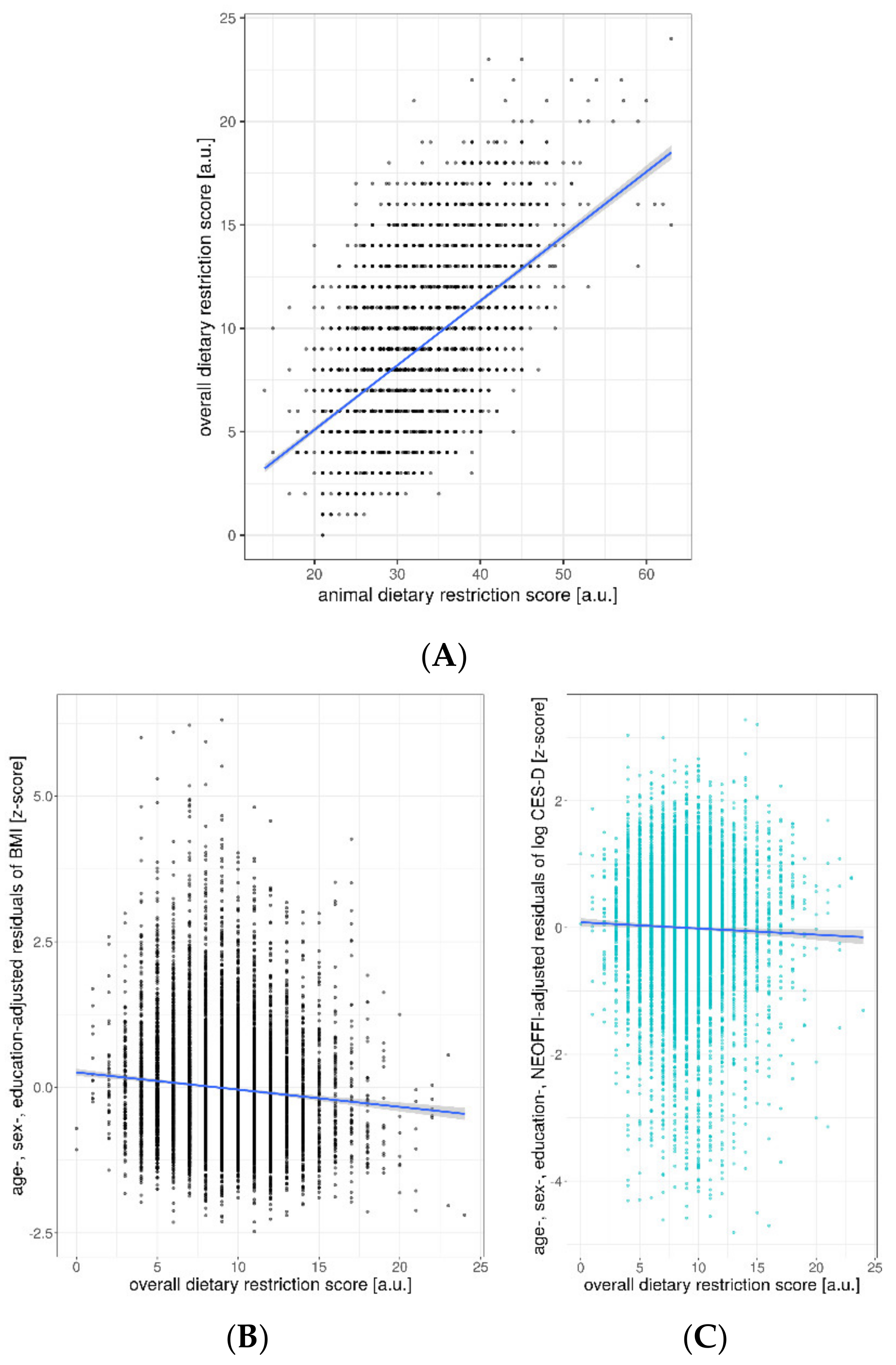
| Age (Years) | Sex | Education (CASMIN Levels) | Animal DRS (9–63) | BMI (kg/m2) | CES-D (0–60) | ||
|---|---|---|---|---|---|---|---|
| Sample 1 (n = 8943) | mean | 56.6 | 8943 | 2.28 | 31.53 | 27.25 | 10.69 |
| (18–82) | (4609F) | (1–3) | (14–63) | (16.2–57.3) | (0–53) | ||
| SD | 12.5 | - | 0.6 | 5.1 | 4.9 | 6.9 | |
| Sample 2 (n = 7906) | mean | 55.7 | 7906 | 2.31 | 31.55 | 27.16 | 10.57 |
| (18–82) | (4010F) | (1–3) | (14–63) | (16.2–57.3) | (0–53) | ||
| SD | 12.4 | - | 0.6 | 5.1 | 4.7 | 6.9 |
| Neuroticism | Extraversion | Openness | Agreeable-Ness | Conscientious-Ness | ||
|---|---|---|---|---|---|---|
| Sample 2 (n = 7906) | mean | 13.2 | 10.9 | 16.3 | 11.7 | 23.6 |
| (4–28) | (3–21) | (4–21) | (2–14) | (4–28) | ||
| SD | 4.4 | 3.7 | 2.7 | 2.0 | 3.2 |
| Adj. R2 | B | C.I. | Beta | p | |
|---|---|---|---|---|---|
| BMI (model 1) | |||||
| Model | 0.06 | <0.001 | |||
| sex | −0.59 | [−0.79 −0.40] | −0.06 | <0.001 | |
| education | −0.67 | [−0.83 −0.50] | −0.08 | <0.001 | |
| age | 0.08 | [0.07 0.09] | 0.21 | <0.001 | |
| animal DRS | −0.07 | [−0.09 −0.05] | −0.06 | <0.001 | |
| BMI (model 1)—sample 2 (df = 7896), corrected for personality | |||||
| Model | 0.08 | <0.001 | |||
| sex | −0.55 | [−0.78 −0.33] | −0.06 | <0.001 | |
| education | −0.65 | [−0.83 −0.47] | −0.08 | <0.001 | |
| age | 0.09 | [0.09 0.10] | 0.24 | <0.001 | |
| animal DRS | −0.07 | [−0.09 −0.05] | −0.07 | <0.001 | |
| neuroticism | −0.05 | [−0.08 −0.03] | −0.05 | 0.001 | |
| extraversion | 0.01 | [−0.02 0.04] | 0.01 | 0.42 | |
| openness | −0.05 | [−0.10 −0.01] | −0.03 | 0.01 | |
| agreeableness | 0.13 | [0.07 0.19] | 0.05 | <0.001 | |
| conscientiousness | −0.20 | [−0.23 −0.16] | −0.13 | <0.001 | |
| Pillai’s Trace | F | df | num df | den df | p | |
|---|---|---|---|---|---|---|
| NEOFFI (model 2) (all factors, corrected for age, sex, education) | ||||||
| sex | 0.17 | 322.2 | 1 | 5 | 7897 | <0.001 |
| education | 0.04 | 66.9 | 1 | 5 | 7897 | <0.001 |
| age | 0.04 | 69.3 | 1 | 5 | 7897 | <0.001 |
| animal DRS | 0.002 | 2.8 | 1 | 5 | 7897 | 0.016 |
| NEOFFI Neuroticism | ||||||
| sex | 327.6 | 1 | 5 | 7897 | <0.001 | |
| education | 113.5 | 1 | 5 | 7897 | <0.001 | |
| age | 28.5 | 1 | 5 | 7897 | <0.001 | |
| animal DRS | 3.5 | 1 | 5 | 7897 | 0.06 | |
| NEOFFI Extraversion | ||||||
| sex | 15.9 | 1 | 5 | 7897 | <0.001 | |
| education | 71.1 | 1 | 5 | 7897 | <0.001 | |
| age | 152.7 | 1 | 5 | 7897 | <0.001 | |
| animal DRS | 9.8 | 1 | 5 | 7897 | 0.002 | |
| NEOFFI Openness | ||||||
| sex | 7.3 | 1 | 5 | 7897 | 0.007 | |
| education | 208.4 | 1 | 5 | 7897 | <0.001 | |
| age | 4.6 | 1 | 5 | 7897 | 0.03 | |
| animal DRS | 3.4 | 1 | 5 | 7897 | 0.07 | |
| NEOFFI Agreeableness | ||||||
| sex | 953.5 | 1 | 5 | 7897 | <0.001 | |
| education | 1.0 | 1 | 5 | 7897 | 0.33 | |
| age | 0.7 | 1 | 5 | 7897 | 0.39 | |
| animal DRS | 0.03 | 1 | 5 | 7897 | 0.87 | |
| NEOFFI Conscientiousness | ||||||
| sex | 137.4 | 1 | 5 | 7897 | <0.001 | |
| education | 10.7 | 1 | 5 | 7897 | 0.001 | |
| age | 148.4 | 1 | 5 | 7897 | <0.001 | |
| animal DRS | 0.0006 | 1 | 5 | 7897 | 0.98 | |
| Adj. R2 | B | C.I. | Beta | p | |
|---|---|---|---|---|---|
| CES-D (model 3)—sample 1 (df = 8938) | |||||
| Model | 0.04 | <0.001 | |||
| sex | 0.04 | [0.029 0.051] | 0.071 | <0.001 | |
| education | −0.09 | [−0.10 −0.08] | −0.184 | <0.001 | |
| age | 0.001 | [0.0007 0.0016] | 0.050 | <0.001 | |
| animal DRS | 0.001 | [−0.0002 0.0020] | 0.016 | 0.12 | |
| CES-D (model 3)—sample 2 (df = 7901) | |||||
| Model | 0.04 | ||||
| sex | 0.04 | [0.0273 0.0523] | 0.069 | <0.001 | |
| education | −0.09 | [−0.1001 −0.0786] | −0.180 | <0.001 | |
| age | 0.001 | [0.0006 0.0016] | 0.049 | <0.001 | |
| animal DRS | 0.001 | [−0.0002 0.0022] | 0.018 | 0.10 | |
| CES-D (model 3)—sample 2 (df = 7896), corrected for personality | |||||
| Model | 0.21 | ||||
| sex | 0.011 | [−0.001 0.024] | 0.02 | 0.08 | |
| education | −0.06 | [−0.07 −0.05] | −0.12 | <0.001 | |
| age | 0.0006 | [0.0001 0.0011] | 0.03 | 0.015 | |
| animal DRS | 0.0005 | [−0.0006 0.0015] | 0.009 | 0.40 | |
| neuroticism | 0.024 | [0.022 0.025] | 0.36 | <0.001 | |
| extraversion | −0.006 | [−0.008 −0.005] | −0.08 | <0.001 | |
| openness | −0.007 | [−0.010 −0.005] | −0.07 | <0.001 | |
| agreeableness | −0.0004 | [−0.004 0.003] | −0.003 | 0.80 | |
| conscientiousness | −0.008 | [−0.009 −0.006] | −0.08 | <0.001 | |
| CES-D (model 3)—sample 2 (df = 7895), corrected for personality and BMI | |||||
| Model | 0.21 | <0.001 | |||
| sex | 0.013 | [0.0008 0.026] | 0.02 | 0.04 | |
| education | −0.06 | [−0.082 −0.039] | −0.11 | <0.001 | |
| age | 0.0002 | [−0.066 −0.046] | 0.01 | 0.32 | |
| animal DRS | 0.001 | [−0.004 0.002] | 0.013 | 0.20 | |
| neuroticism | 0.024 | [0.022 0.025] | 0.36 | <0.001 | |
| extraversion | −0.006 | [−0.008 −0.005] | −0.08 | <0.001 | |
| openness | −0.007 | [−0.010 −0.005] | −0.07 | 0.14 | |
| agreeableness | −0.0009 | [−0.004 0.003] | −0.006 | 0.60 | |
| conscientiousness | −0.007 | [−0.009 −0.005] | −0.08 | <0.001 | |
| BMI | 0.004 | [0.002 0.005] | 0.06 | <0.001 | |
| Adj. R2 | B | C.I. | Beta | p | |
|---|---|---|---|---|---|
| BMI (model 1)—primary animal DRS | |||||
| Model | 0.07 | <0.001 | |||
| sex | −0.18 | [−0.38 0.03] | −0.018 | 0.10 | |
| education | −0.61 | [−0.76 −0.44] | −0.074 | <0.001 | |
| age | 0.09 | [0.08 0.10] | 0.225 | <0.001 | |
| primary animal DRS | −0.25 | [−0.29 −0.21] | −0.132 | <0.001 | |
| BMI (model 1)—secondary animal DRS | |||||
| Model | 0.06 | <0.001 | |||
| sex | −0.63 | [−0.84 −0.43] | −0.065 | <0.001 | |
| education | −0.65 | [−0.82 −0.49] | −0.079 | <0.001 | |
| age | 0.08 | [0.07 0.09] | 0.209 | <0.001 | |
| secondary animal DRS | −0.02 | [−0.04 −0.01] | −0.015 | 0.16 | |
| BMI (model 1)—overall DRS | |||||
| Model | 0.07 | <0.001 | |||
| sex | −0.50 | [−0.69 −0.30] | −0.051 | <0.001 | |
| education | −0.70 | [−0.83 −0.49] | −0.080 | <0.001 | |
| age | 0.09 | [0.08 0.10] | 0.221 | <0.001 | |
| overall DRS | −0.15 | [−0.18 −0.11] | −0.091 | <0.001 | |
| Adj. R2 | B | C.I. | Beta | p | |
|---|---|---|---|---|---|
| CES-D—sample 1 (df = 8938) | |||||
| Model | 0.04 | <0.001 | |||
| sex | 0.05 | [0.031 0.058] | 0.08 | <0.001 | |
| education | −0.09 | [−0.100 −0.078] | −0.18 | <0.001 | |
| age | 0.001 | [0.0007 0.0017] | 0.05 | <0.001 | |
| primary DRS | −0.003 | [−0.005 −0.00008] | −0.02 | 0.04 | |
| CES-D—sample 2 (df = 7896), corrected for personality | |||||
| Model | 0.21 | <0.001 | |||
| sex | 0.014 | [0.0008 0.0270] | 0.02 | 0.04 | |
| education | −0.06 | [−0.068 −0.048] | −0.12 | <0.001 | |
| age | 0.0006 | [0.0001 0.0011] | 0.03 | 0.01 | |
| primary DRS | −0.002 | [−0.004 −0.001] | −0.01 | 0.21 | |
| neuroticism | 0.024 | [0.022 0.025] | 0.36 | <0.001 | |
| extraversion | −0.006 | [−0.008 −0.005] | −0.08 | <0.001 | |
| openness | −0.007 | [−0.010 −0.005] | −0.07 | <0.001 | |
| agreeableness | −0.0003 | [−0.004 0.003] | −0.002 | 0.84 | |
| conscientiousness | −0.007 | [−0.009 −0.006] | −0.08 | <0.001 | |
| CES-D—sample 1 (df = 8938) | |||||
| Model | 0.04 | <0.001 | |||
| sex | 0.04 | [0.032 0.055] | 0.08 | <0.001 | |
| education | −0.09 | [−0.10 −0.08] | −0.20 | <0.001 | |
| age | 0.001 | [0.0007 0.0016] | 0.05 | <0.001 | |
| secondary DRS | 0.002 | [0.0003 0.003] | −0.03 | 0.02 | |
| CES-D—sample 2 (df = 7896), corrected for personality | |||||
| Model | 0.21 | <0.001 | |||
| sex | 0.013 | [0.0010 0.0261] | 0.02 | 0.05 | |
| education | −0.06 | [−0.068 −0.048] | −0.12 | <0.001 | |
| age | 0.0006 | [0.0001 0.0011] | 0.03 | 0.01 | |
| secondary DRS | 0.001 | [−0.005 0.002] | 0.01 | 0.20 | |
| neuroticism | 0.024 | [0.022 0.025] | 0.36 | <0.001 | |
| extraversion | −0.006 | [−0.008 −0.005] | −0.08 | <0.001 | |
| openness | −0.007 | [−0.010 −0.005] | −0.07 | <0.001 | |
| agreeableness | −0.0003 | [−0.004 0.003] | −0.002 | 0.84 | |
| conscientiousness | −0.008 | [−0.009 −0.006] | −0.08 | <0.001 | |
| Pillai’s Trace | F | df | num df | den df | p | |
|---|---|---|---|---|---|---|
| NEOFFI (all factors)—sample 2, corrected for age, sex, education | ||||||
| sex | 0.169 | 320.0 | 1 | 5 | 7897 | <0.001 |
| education | 0.041 | 67.4 | 1 | 5 | 7897 | <0.001 |
| age | 0.040 | 65.2 | 1 | 5 | 7897 | <0.001 |
| overall DRS | 0.007 | 11.8 | 1 | 5 | 7897 | <0.001 |
| NEOFFI Neuroticism | ||||||
| sex | 342.0 | 1 | 5 | 7897 | <0.001 | |
| education | 114.5 | 1 | 5 | 7897 | <0.001 | |
| age | 28.9 | 1 | 5 | 7897 | <0.001 | |
| overall DRS | 0.6 | 1 | 5 | 7897 | 0.44 | |
| NEOFFI Extraversion | ||||||
| sex | 14.5 | 1 | 5 | 7897 | <0.001 | |
| education | 72.6 | 1 | 5 | 7897 | <0.001 | |
| age | 149.3 | 1 | 5 | 7897 | <0.001 | |
| overall DRS | 0.3 | 1 | 5 | 7897 | 0.6 | |
| NEOFFI Openness | ||||||
| sex | 6.1 | 1 | 5 | 7897 | 0.01 | |
| education | 209.8 | 1 | 5 | 7897 | <0.001 | |
| age | 4.9 | 1 | 5 | 7897 | 0.03 | |
| overall DRS | 1.6 | 1 | 5 | 7897 | 0.21 | |
| NEOFFI Agreeableness | ||||||
| sex | 937.3 | 1 | 5 | 7897 | <0.001 | |
| education | 0.9 | 1 | 5 | 7897 | 0.34 | |
| age | 0.2 | 1 | 5 | 7897 | 0.7 | |
| overall DRS | 15.7 | 1 | 5 | 7897 | <0.001 | |
| NEOFFI Conscientiousness | ||||||
| sex | 122.4 | 1 | 5 | 7897 | <0.001 | |
| education | 10.7 | 1 | 5 | 7897 | 0.001 | |
| age | 130.7 | 1 | 5 | 7897 | <0.001 | |
| overall DRS | 53.9 | 1 | 5 | 7897 | <0.001 | |
| Adj. R2 | B | C.I. | Beta | p | |
|---|---|---|---|---|---|
| CES-D—sample 1 (df = 8938) | |||||
| Model | 0.05 | <0.001 | |||
| sex | 0.04 | [0.032 0.055] | 0.076 | <0.001 | |
| education | −0.09 | [−0.100 −0.080] | −0.185 | <0.001 | |
| age | 0.001 | [0.0008 0.0017] | 0.054 | <0.001 | |
| overall DRS | −0.004 | [−0.006 −0.002] | −0.043 | <0.001 | |
| CES-D—sample 2 (df = 7901) | |||||
| Model | 0.04 | <0.001 | |||
| sex | 0.04 | [0.031 0.056] | 0.075 | <0.001 | |
| education | −0.09 | [−0.100 −0.080] | −0.180 | <0.001 | |
| age | 0.001 | [0.0008 0.0017] | 0.054 | <0.001 | |
| overall DRS | −0.005 | [−0.007 −0.002] | −0.048 | <0.001 | |
| CES-D—sample 2 (df = 7896), corrected for personality | |||||
| Model | 0.21 | <0.001 | |||
| sex | 0.014 | [0.0010 0.0261] | 0.02 | 0.04 | |
| education | −0.06 | [−0.068 −0.048] | −0.12 | <0.001 | |
| age | 0.0007 | [0.0002 0.0011] | 0.03 | 0.007 | |
| overall DRS | −0.003 | [−0.004 −0.001] | −0.03 | 0.007 | |
| neuroticism | 0.024 | [0.022 0.025] | 0.36 | <0.001 | |
| extraversion | −0.006 | [−0.008 −0.005] | −0.08 | <0.001 | |
| openness | −0.007 | [−0.010 −0.005] | −0.07 | <0.001 | |
| agreeableness | −0.0005 | [−0.004 0.003] | −0.004 | 0.76 | |
| conscientiousness | −0.007 | [−0.009 −0.006] | −0.08 | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medawar, E.; Enzenbach, C.; Roehr, S.; Villringer, A.; Riedel-Heller, S.G.; Witte, A.V. Less Animal-Based Food, Better Weight Status: Associations of the Restriction of Animal-Based Product Intake with Body-Mass-Index, Depressive Symptoms and Personality in the General Population. Nutrients 2020, 12, 1492. https://doi.org/10.3390/nu12051492
Medawar E, Enzenbach C, Roehr S, Villringer A, Riedel-Heller SG, Witte AV. Less Animal-Based Food, Better Weight Status: Associations of the Restriction of Animal-Based Product Intake with Body-Mass-Index, Depressive Symptoms and Personality in the General Population. Nutrients. 2020; 12(5):1492. https://doi.org/10.3390/nu12051492
Chicago/Turabian StyleMedawar, Evelyn, Cornelia Enzenbach, Susanne Roehr, Arno Villringer, Steffi G. Riedel-Heller, and A. Veronica Witte. 2020. "Less Animal-Based Food, Better Weight Status: Associations of the Restriction of Animal-Based Product Intake with Body-Mass-Index, Depressive Symptoms and Personality in the General Population" Nutrients 12, no. 5: 1492. https://doi.org/10.3390/nu12051492
APA StyleMedawar, E., Enzenbach, C., Roehr, S., Villringer, A., Riedel-Heller, S. G., & Witte, A. V. (2020). Less Animal-Based Food, Better Weight Status: Associations of the Restriction of Animal-Based Product Intake with Body-Mass-Index, Depressive Symptoms and Personality in the General Population. Nutrients, 12(5), 1492. https://doi.org/10.3390/nu12051492