Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer
Abstract
1. Introduction
2. Materials and Methods
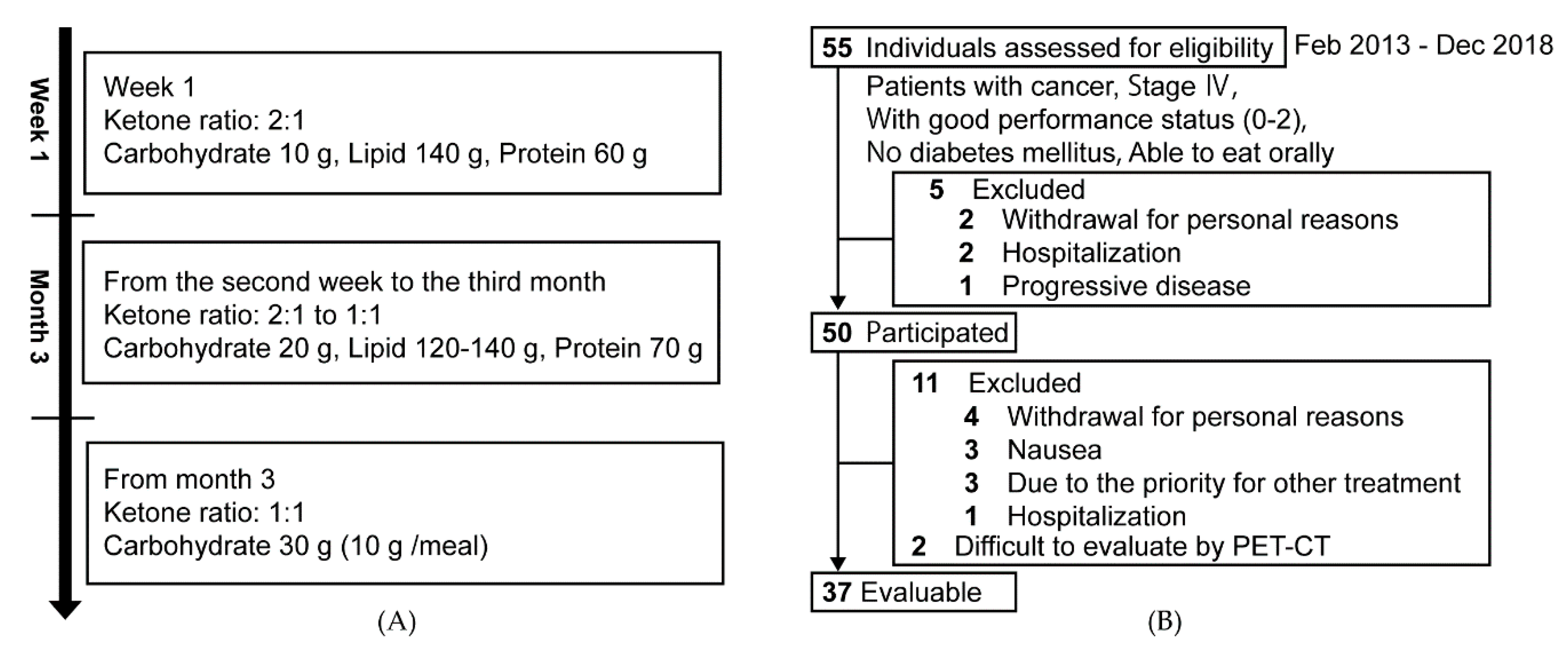
2.1. Design and Participants
2.2. Ketogenic Diet for Cancer Patients
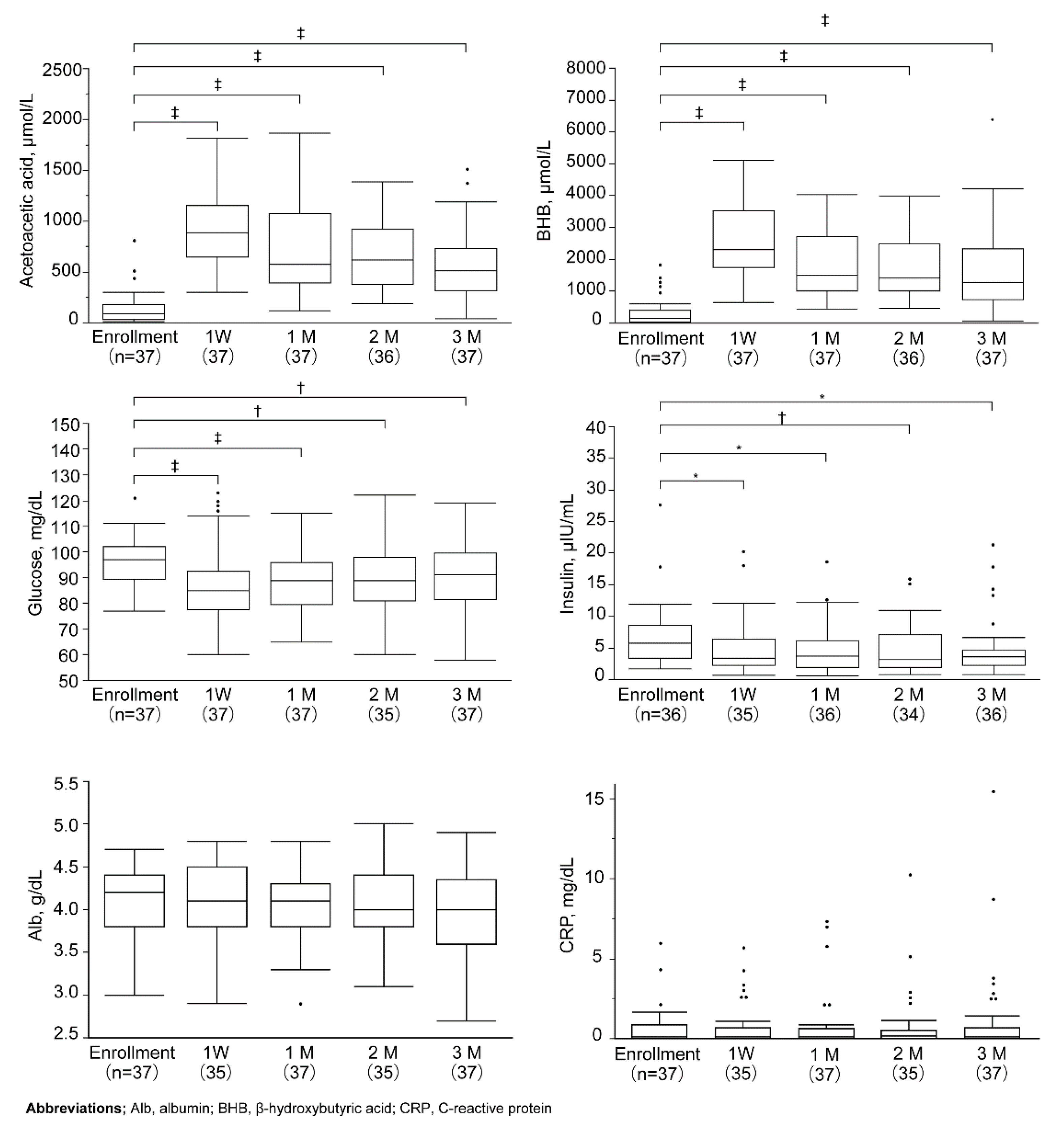
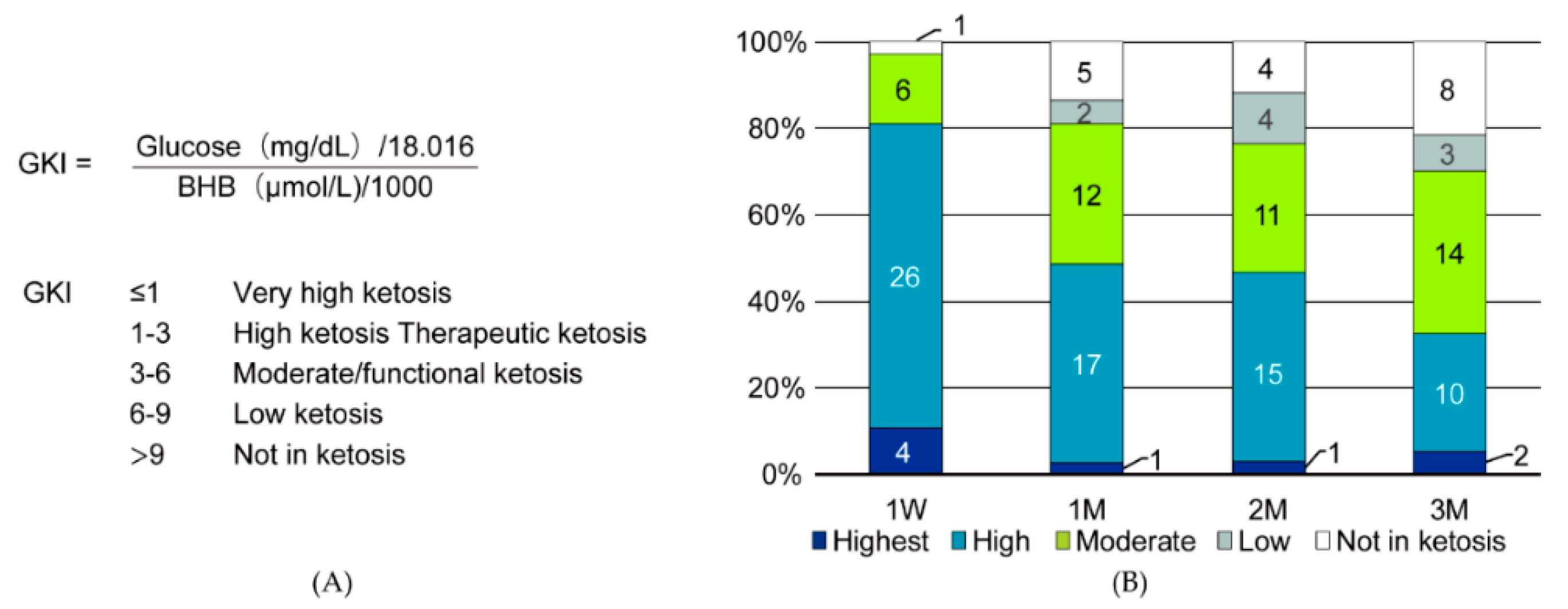
2.3. Evaluation and Statistical Analysis
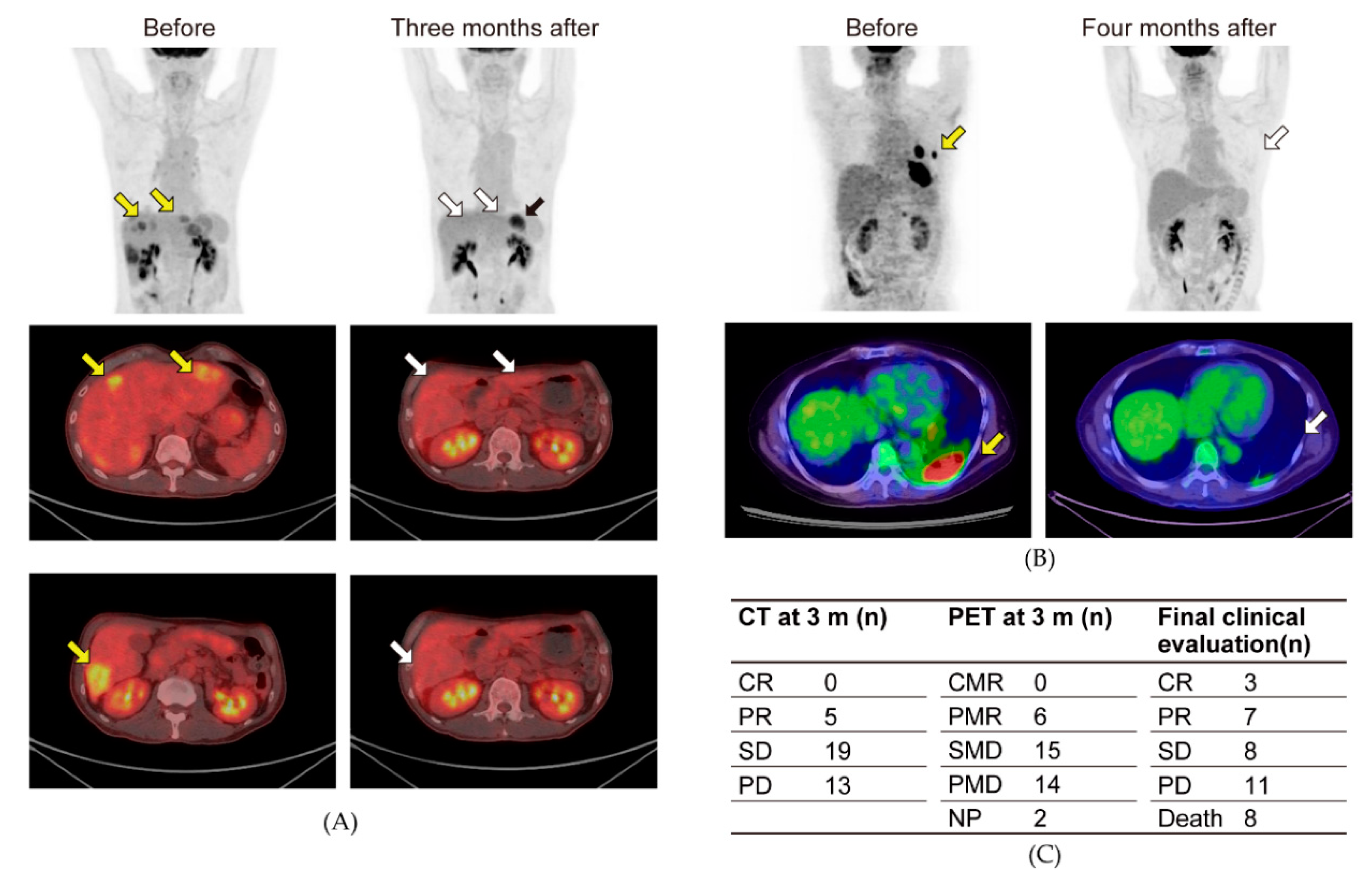
2.4. PET-CT
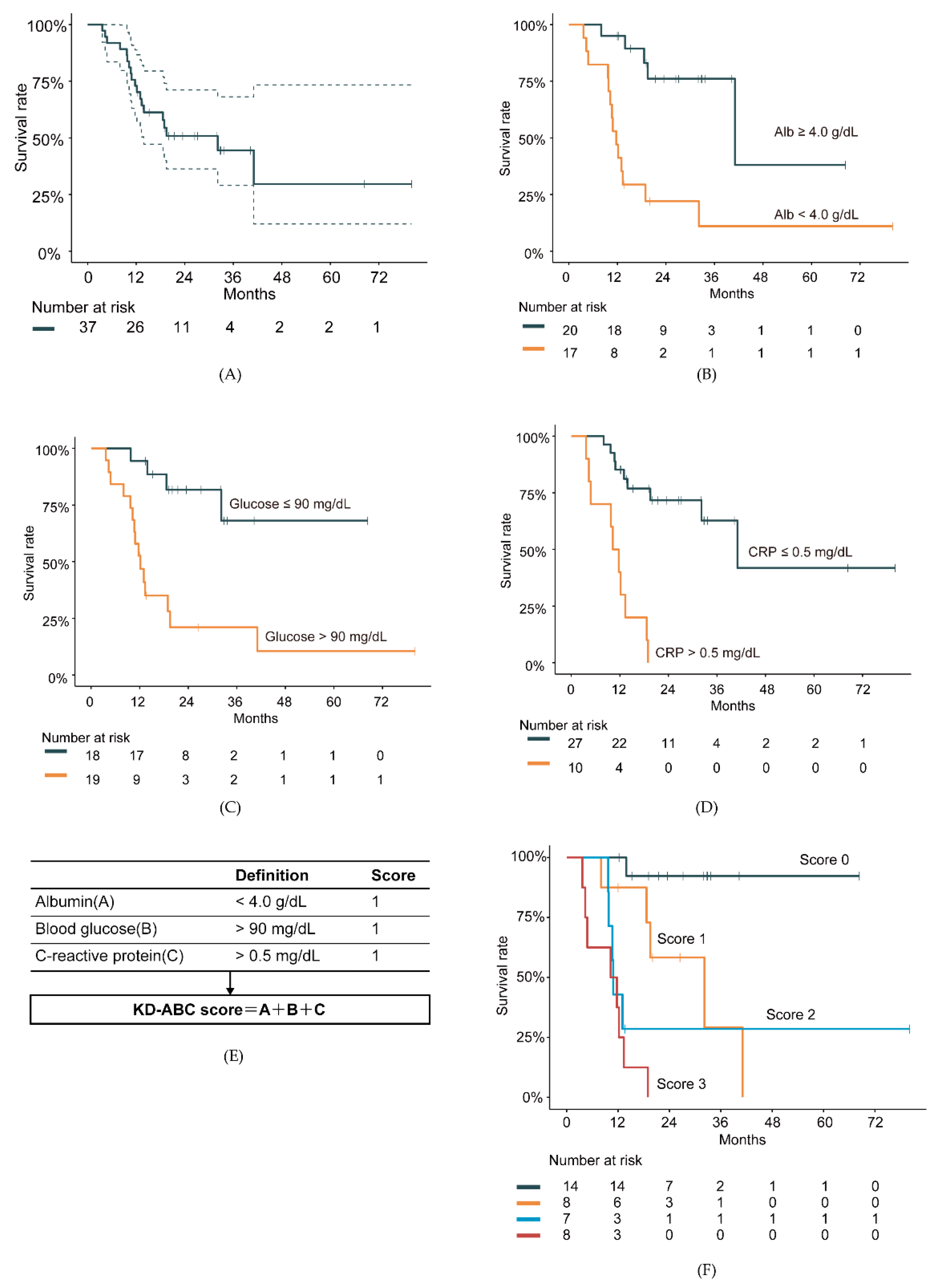
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Ericksong, N.; Lavianoh, A.; Lisantii, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.; Norman, A.R.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Flood, A.; Mai, V.; Pfeiffer, R.; Kahle, L.; Remaley, A.T.; Lanza, E.; Schatzkin, A. Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology 2007, 133, 1423–1429. [Google Scholar] [CrossRef]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Hopkins, B.D.; Cantley, L.C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 2018, 379, 2052–2062. [Google Scholar] [CrossRef]
- Warburg, O. Origin of cancer cells. Oncologia 1956, 9, 75–83. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2019, 33, 102–121. [Google Scholar] [CrossRef]
- Noorlag, L.; De Vos, F.Y.; Kok, A.; Broekman, M.L.D.; Seute, T.; Robe, P.A.; Snijders, T.J. Treatment of malignant gliomas with ketogenic or caloric restricted diets: A systematic review of preclinical and early clinical studies. Clin. Nutr. 2019, 38, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.; Marcello, N.; Pisanello, A.; Servadei, F.; Vaccaro, S.; Mukherjee, P.; Seyfried, T.N. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr. Metab. 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, A.M.A.; Bary, M.A.; Abdelzaher, E.; Elnaggar, M.; Kalamian, M.; Mukherjee, P.; Seyfried, T.N. Management of glioblastoma multiforme in a patient treated with ketogenic metabolic therapy and modified standard of care: A 24-month follow-up. Front. Nutr. 2018, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pfetzer, N.; Schwab, M.; Strauss, I.; Kämmerer, U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ito, Y.; Takahashi, S.; Shimono, K.; Natsume, J.; Yanagihara, K.; Oguni, H. Outcome of ketogenic diets in GLUT1 deficiency syndrome in Japan: A nationwide survey. Brain Dev. 2016, 38, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Danceyg, J.; Arbuckh, S.; Gwytheri, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 261–267. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P.; on behalf of the European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Meidenbauer, J.J.; Mukherjee, P.; Seyfried, T.N. The glucose ketone index calculator: A simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr. Metab. 2015, 12, 12. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; MiguelSanchez, J.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Okusaka, T.; Omuro, Y.; Isayama, H.; Fukutomi, A.; Ikeda, M.; Mizuno, N.; Fukuzawa, K.; Furukawa, M.; Iguchi, H.; et al. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann. Oncol. 2016, 27, 502–508. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. CA Cancer J. Clin. 2010, 60, 207–221. [Google Scholar] [CrossRef]
- Yang, I.P.; Miao, Z.F.; Huang, C.W.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Chang, T.K.; Chang, S.F.; Wang, J.Y. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866964. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef] [PubMed]
- Stafford, P.; Abdelwahab, M.G.; Kim, D.Y.; Preul, M.C.; Rho, J.M.; Scheck, A.C. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. 2010, 7, 74. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, M.H.; Ha, S.; Bang, E.J.; Lee, Y.; Lee, A.; Lee, J.; Yu, B.P.; Young, H. Anti-inflammatory action of β-hydroxybutyrate via modulation of PGC-1α and FoxO1, mimicking calorie restriction. Aging 2019, 11, 1283–1304. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A signaling metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Winter, S.F.; Loebel, F.; Dietrich, J. Role of ketogenic metabolic therapy in malignant glioma: A systematic review. Crit. Rev. Oncol. Hematol. 2017, 112, 41–58. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P.; Iyikesici, M.S.; Slocum, A.; Kalamian, M.; Spinosa, J.P.; Chinopoulos, C. Consideration of Ketogenic Metabolic Therapy as a Complementary or Alternative Approach for Managing Breast Cancer. Front. Nutr. 2020, 7, 21. [Google Scholar] [CrossRef]
- Klement, R.J.; Sweeney, R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: II. Protocol of a randomised phase I study (KETOCOMP). Clin. Nutr. ESPEN 2016, 12, e1–e6. [Google Scholar] [CrossRef] [PubMed]
| Age, y | 54.8 ± 12.6 |
| Sex (male/female), n | 15/22 |
| Body height, cm | 162.5 ± 9.5 |
| Body weight, kg | 55.5 ± 13.2 |
| Body mass index, kg/m2 | 20.9 ± 3.7 |
| Primary cancer | |
| Colorectal cancer, n | 8 |
| Non-small cell lung cancer, n | 6 |
| Breast cancer, n | 5 |
| Pancreatic cancer, n | 4 |
| Head and neck cancer, n | 3 |
| Bone and soft tissue sarcoma, n | 3 |
| Ovarian cancer and peritoneal cancer, n | 2 |
| Endometrial cancer, n | 1 |
| Bladder cancer, n | 1 |
| Brain tumor, n | 1 |
| Biliary tract cancer, n | 1 |
| Gastric cancer, n | 1 |
| Prostate cancer, n | 1 |
| Treatment history | |
| Chemohormonal therapy, n (%) | 33 (89.2) |
| Radiation therapy, n (%) | 13 (35.1) |
| Surgical therapy, n (%) | 26 (70.3) |
| CTCAE v5.0 Term | Grade | Total n = 55 (%) | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | ||
| Hyperuricemia | 8 | 24 | 0 | 0 | 32 (58.2) |
| Hypoglycemia | 4 | 6 | 0 | 0 | 10 (18.2) |
| Cholesterol high | 12 | 13 | 1 | 0 | 26 (47.3) |
| Hypertriglyceridemia | 11 | 2 | 0 | 0 | 13 (23.6) |
| Hyperlipidemia | 14 | 15 | 0 | 0 | 29 (52.7) |
| Hypokalemia | 1 | 4 | 0 | 0 | 5 (9.1) |
| Hypocalcemia | 0 | 1 | 0 | 0 | 1 (1.8) |
| Abdominal pain | 0 | 2 | 0 | 0 | 2 (3.6) |
| Constipation | 0 | 17 | 0 | 0 | 17 (30.9) |
| Vomiting | 0 | 1 | 0 | 0 | 1 (1.8) |
| Nausea | 0 | 1 | 0 | 0 | 1 (1.8) |
| Stomach pain | 0 | 8 | 0 | 0 | 8 (14.5) |
| Diarrhea | 0 | 7 | 0 | 0 | 7 (12.7) |
| Dyspepsia | 0 | 1 | 0 | 0 | 1 (1.8) |
| Anorexia | 0 | 1 | 0 | 0 | 1 (1.8) |
| Muscle cramp | 0 | 1 | 0 | 0 | 1 (1.8) |
| Malaise | 0 | 1 | 0 | 0 | 1 (1.8) |
| Weight loss | 10 | 2 | 0 | 0 | 12 (21.8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagihara, K.; Kajimoto, K.; Osaga, S.; Nagai, N.; Shimosegawa, E.; Nakata, H.; Saito, H.; Nakano, M.; Takeuchi, M.; Kanki, H.; et al. Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients 2020, 12, 1473. https://doi.org/10.3390/nu12051473
Hagihara K, Kajimoto K, Osaga S, Nagai N, Shimosegawa E, Nakata H, Saito H, Nakano M, Takeuchi M, Kanki H, et al. Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients. 2020; 12(5):1473. https://doi.org/10.3390/nu12051473
Chicago/Turabian StyleHagihara, Keisuke, Katsufumi Kajimoto, Satoshi Osaga, Naoko Nagai, Eku Shimosegawa, Hideyuki Nakata, Hitomi Saito, Mai Nakano, Mariko Takeuchi, Hideaki Kanki, and et al. 2020. "Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer" Nutrients 12, no. 5: 1473. https://doi.org/10.3390/nu12051473
APA StyleHagihara, K., Kajimoto, K., Osaga, S., Nagai, N., Shimosegawa, E., Nakata, H., Saito, H., Nakano, M., Takeuchi, M., Kanki, H., Kagitani-Shimono, K., & Kijima, T. (2020). Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients, 12(5), 1473. https://doi.org/10.3390/nu12051473






