The Effects of Two Intervention Strategies to Reduce the Intake of Salt and the Sodium-To-Potassium Ratio on Cardiovascular Risk Factors. A 4-Month Randomised Controlled Study among Healthy Families
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
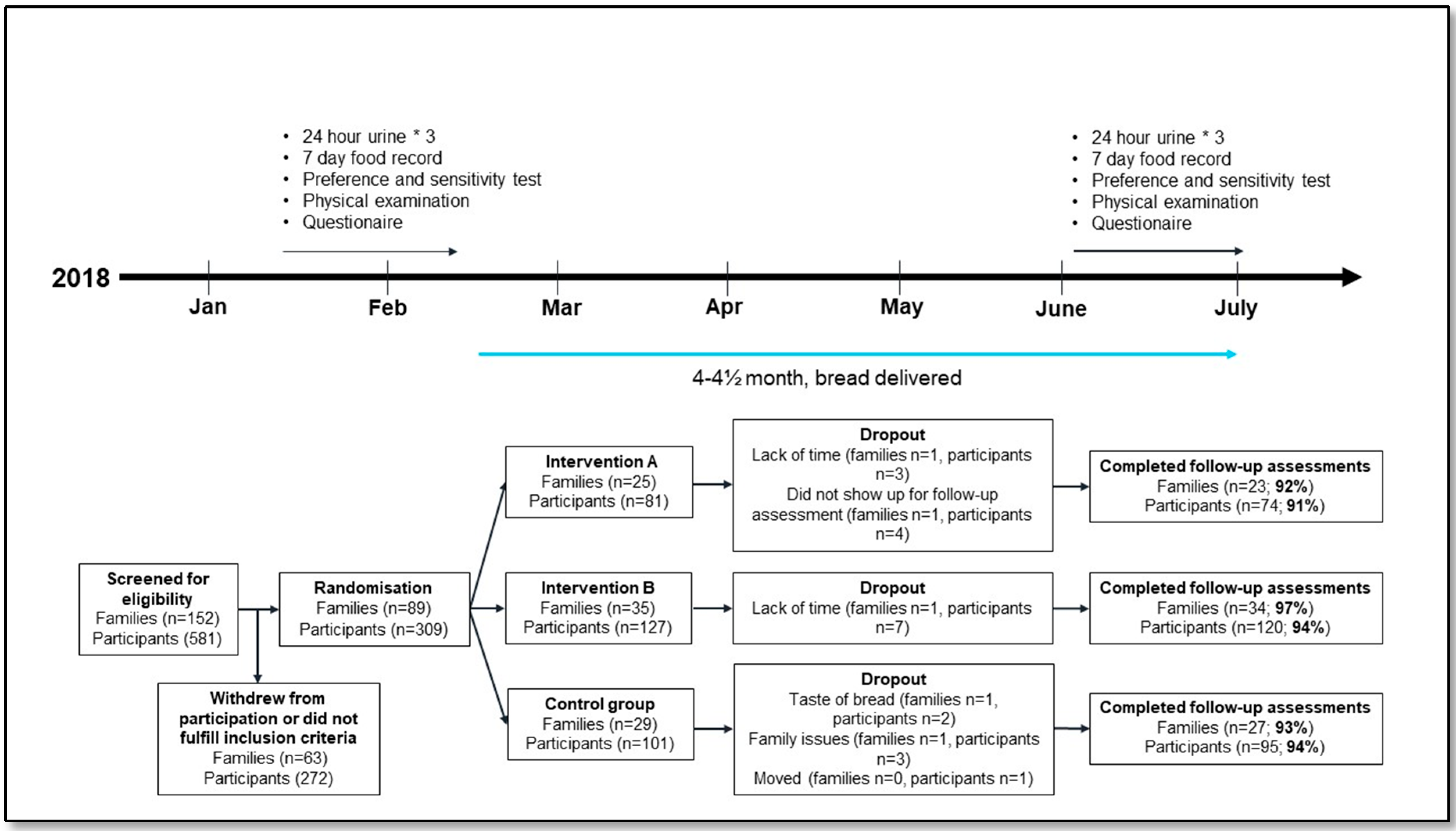
2.2. Study Design and Intervention
2.3. Outcome Measurements
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cappuccio, F.P.; Beer, M.; Strazzullo, P. European Salt Action Network. Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the European Salt Action Network. Metab. Cardiovasc. Dis. 2018, 29, 107–114. [Google Scholar] [CrossRef]
- Transforming European Food and Drink Policies for Cardiovascular Health; European Heart Network: Brussels, Belgium, 2017.
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliot, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, 1–20. [Google Scholar] [CrossRef]
- Campbell, N.; Correa-Rotter, R.; Neal, B.; Cappuccio, F. New evidence to the health impact of reducing salt intake. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Lawrence, J.; Appel, M.D.; Wehlton, M.B. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Djafarian, K.; Shab-Bidar, S. Dose–response association of dietary sodium intake with all-cause and cardiovascular mortality: A systematic review and metaanalysis of prospective studies. Public Health Nutr. 2019, 22, 295–306. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Integrating Nutrition and Physical Activity; Nordic Nutrition Recommendations: Copenhagen, Denmark, 2012. [CrossRef]
- World Health Organization. Report of the Formal Meeting of Member States to Conclude the Work on the Comprehensive Global Monitoring Framework, Including Indicators, and a Set of Voluntary Global Targets for the Prevention and Control of Non-Communicable Diseases; Worlds Health Organization Press: Geneva, Switzerland, 2012. [Google Scholar]
- Scientific Report of the 2015 Dietary Guidelines Advisory Committee. In Advisory Report to the Secretary of Health and Human Services and Secretary of Agriculture; U.S. Department of Agriculture, Agricultural Research Servicer: Washington, DC, USA, 2015.
- Cobiac, L.; Vos, T.; Veerman, J. Cost-effectiveness of interventions to reduce dietary salt intake. Heart 2010, 96, 1920–1925. [Google Scholar] [CrossRef]
- Hyseni, L.; Elliot-Green, A.; Lloyd-Williams, F.; Kypridemos, C.; O’Flaherty, M.; McGill, R.; Orton, L.; Bromley, H.; Cappuccio, F.P.; Capewell, S. Systematic review of dietary salt reduction policies: Evidence for an effectiveness hierarchy. PLoS ONE 2017, 12, e0177535. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am. J. Hypertens. 2012, 25, 1–15. [Google Scholar] [CrossRef]
- He, F.; Macgregor, G. Reducing population salt intake worldwide: From evidence to implementation. Prog. Cardiovasc. Dis. 2010, 52, 363–382. [Google Scholar] [CrossRef]
- Kasey, J.C.; Abigail, S.; Huffman, M.D.; Jenner, K.; Xavier, D.; Dunford, E.K. Differences in the sodium content of bread products in the USA-and UK: Implications for policy. Public Health Nutr. 2018, 21, 632–636. [Google Scholar] [CrossRef]
- Taylor, C.; Doyle, M.; Webb, D. The safety of sodium reduction in the food supply: A cross-discipline balancing act—Workshop proceedings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.B.; Wang, X.Y.; Liu, X.S.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. 2014, 371, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, D.; Hong, Y.; Gillespie, C.; Chang, M.-H.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and potassium intake and mortality among US adults. Arch. Int. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Hu, Y.-W.; Yue, C.-S.J.; Wen, Y.-W.; Yeh, W.-T.; Hsu, L.-S.; Tsai, S.-Y.; Pan, W.-H. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am. J. Lin. Nutr. 2006, 83, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Chang, E.T. Sodium-to-Potassium Ratio and Blood Pressure, Hypertension, and Related Factors. American Society for Nutrition. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef] [PubMed]
- Riis, N.L.; Bjoernsbo, K.S.; Toft, U.; Trolle, E.; Hyldig, G.; Hartley, I.E.; Keast, R.; Lassen, A.D. Impact of a sodium reduction intervention on salt taste sensitivity and preference, a cluster randomised controlled trial. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Bjoernsbo, K.S.; Riis, N.L.; Andreasen, A.H.; Petersen, J.; Lassen, A.D.; Trolle Frederiksen, A.K.S.; Munk, J.K.; Toft, U. Salt Reduction Intervention in Families Investigating Metabolic, Behavioral and Health Effects of Targeted Intake Reductions: Study Protocol for a Four Months Three-Armed, Randomized, Controlled ‘Real-Life’ Trial. Int. J. Environ. Res. Public Health 2019, 16, 3532. [Google Scholar] [CrossRef]
- Keyhole Label. Available online: https://altomkost.dk/english/#c41068 (accessed on 31 March 2020).
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomized trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef]
- Takada, T.; Imamoto, M.; Fukuma, S.; Yamamoto, Y.; Sasaki, S.; Uchida, M.; Miura, Y.; Shimizu, S.; Nihata, K.; Fukuhara, S. Effect of cooking classes for housewives on salt reduction in family members: A cluster randomized controlled trial. Public Health 2016, 140, 144–150. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kenny, S.; Kerry, J.P.; Leenhardt, F.; Arendt, E.K. ‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure. Nutrients 2019, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Wu, Y.; Feng, X.X.; Ma, J.; Ma, Y.; Wang, H.; Zhang, J.; Yuan, J.; Lin, C.P.; Nowson, C.; et al. School based education programme to reduce salt intake in children and their families (School-EduSalt): Cluster randomised controlled trial. BMJ 2015, 350, h770. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Violi, F.; D’Amico, R.; Vinceti, M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017, 4, CD004022. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst. Rev. 2013, 4, CD004937. [Google Scholar] [CrossRef]
- Alderman, M.H.; Cohen, H.W. Dietary sodium intake and cardiovascular mortality: Controversy resolved? Curr. Hypertens. Rep. 2012, 14, 193–201. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J. MacGregor: High Salt Intake. Independent Risk Factor for Obesity? Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Stambolliu, E.; Ntineri, A.; Anagnostopoulos, I.; Stergiou, G.S. Seasonal blood pressure variation assessed by different measurement methods: Systematic review and meta-analysis. J. Hypertens. 2020, 38, 791–798. [Google Scholar] [CrossRef]
- Hughes, R.A.; Heron, J.; Sterne, J.A.C.; Tilling, K. Accounting for missing data in statistical analyses: Multiple imputation is not always the answer. Int. J. Epidemiol. 2019, 48, 1294–1304. [Google Scholar] [CrossRef]
- Mukaka, M.; White, S.A.; Terlouw, D.J.; Mwapasa, V.; Kalilani-Phiri, L.; Faragher, E.B. Is using multiple imputation better than complete case analysis for estimating a prevalence (risk) difference in randomized controlled trials when binary outcome observations are missing? Trials 2016, 17, 341. [Google Scholar] [CrossRef]
| Intervention A | Intervention B | Control | |
|---|---|---|---|
| Cluster level | |||
| Families (n) | 25 (28.1) | 35 (39.3) | 29 (32.6) |
| Participants (n) | 81 (26.2) | 127 (41.1) | 101 (32.7) |
| Family size | 3.2 ± 0.8 | 3.6 ± 1.1 | 3.5 ± 1.1 |
| Parental education | |||
| Short higher education < 3y or shorter b (n) | 9 (37.5) | 8 (24.2) | 8 (28.6) |
| Medium higher education 3–4y (n) | 6 (25.0) | 12 (36.4) | 7 (25.0) |
| Long higher education > 4y (n) | 9 (37.5) | 13 (39.4) | 13 (46.4) |
| Individual level | |||
| Children < 18y | |||
| N | 40 | 64 | 52 |
| Sex (boys, %) | 21 (52.5) | 33 (51.6) | 27 (51.9) |
| Age (y) | 9.5 ± 4.2 | 9.1 ± 4.2 | 8.4 ± 3.5 |
| Weight (kg) | 40.3 ± 18.3 | 37.3 ± 19.6 | 32.0 ± 16.1 |
| Height (cm) | 144.1 ± 25.9 | 140.4 ± 27.4 | 133.1 ± 23.2 |
| BMI (kg/m2) | 18.0 ± 2.9 | 17.4 ± 2.8 | 16.9 ± 2.8 |
| Physical activity | |||
| Sedentary (n) | 4 (10.0) | 6 (10.0) | 6 (12.0) |
| Moderately active (n) | 7 (17.5) | 10 (16.7) | 6 (12.0) |
| Vigorously active (n) | 29 (72.5) | 44 (73.3) | 38 (76.0) |
| Alcohol drinkers (n) | 6 (15.0) | 3 (5.0) | 1 (2.0) |
| Smokers (n) | 2 (5.0) | 1 (1.7) | 0 (0.0) |
| Salt intake (g/day) c | 6.2 ± 2.9 | 6.0 ± 3.4 | 5.5 ± 2.2 |
| Sodium/potassium excretion ratio c,d | 2.2 [1.7,2.7] | 2.2 [1.6,2.9] | 2.4 [1.9,2.9] |
| Adults ≥ 18y | |||
| N | 41 (50.6) | 63 (49.6) | 49 (48.5) |
| Sex (men, %) | 18 (43.9) | 29 (46.0) | 23 (46.9) |
| Age (y) | 41.5 ± 9.5 | 40.5 ± 9.0 | 40.9 ± 8.0 |
| Weight (kg) | 78.6 ± 14.3 | 77.4 ± 16.3 | 75.5 ± 14.6 |
| Height (cm) | 174.2 ± 9.7 | 174.1 ± 9.3 | 174.0 ± 8.7 |
| BMI (kg/m2) | 25.8 ± 3.8 | 25.6 ± 5.6 | 24.8 ± 4.1 |
| Physical activity | |||
| Sedentary (n) | 8 (20.0) | 8 (13.3) | 7 (14.6) |
| Moderately active (n) | 23 (57.5) | 30 (50.0) | 31 (64.6) |
| Vigorously active (n) | 9 (22.5) | 22 (36.7) | 10 (20.8) |
| Alcohol drinkers (n) | 31 (77.5) | 49 (81.7) | 38 (79.2) |
| Smokers (n) | 4 (10.0) | 5 (8.3) | 5 (10.4) |
| Salt intake (g/day) c | 9.1 ± 2.9 | 8.9 ± 2.4 | 9.1 ± 2.9 |
| Sodium/potassium excretion ratio c,d | 2.2 [1.8,2.6] | 2.3 [1.9,2.7] | 2.2 [1.8,2.8] |
| Control | Intervention Group A | Intervention Group B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | |
| N = 101 | N = 81 | N = 127 | |||||||
| Systolic BP (mmHg) | 106 (12) | 101 (9) | 0.0103 | 107 (9) | 103 (8) | 0.0277 | 106 (14) | 102(12) | 0.0142 |
| Diastolic BP (mmHg) | 66 (6) | 62 (6) | <0.0001 | 66 (5) | 64 (6) | 0.0699 | 67 (9) | 64 (6) | 0.0216 |
| BMI (kg/m2) | 16.42 (14.73–18.13) | 16.13 (14.57–18.65) | 0.9529 | 17.64 (15.54–19.29) | 16.82 (15.04–19.26) | 0.2875 | 16.34 (15.51–19.21) | 16.29 (15.17–18.98) | 0.7830 |
| Adults (>18 years) | |||||||||
| Diastolic BP (mmHg) | 77.40 (10.83) | 75.40 (9.97) | 0.0183 | 79.71 (9.50) | 77.86 (7.73) | 0.0488 | 78.38 (9.85) | 75.41 (9.47) | 0.0006 |
| Systolic BP (mmHg) | 120.58 (16.31) | 116.69 (13.97) | 0.0041 | 124.41 (14.29) | 121.45 (10.81) | 0.0407 | 123.16 (13.22) | 116.93 (15.95) | <0.0001 |
| Plasma total cholesterol (mmol/L) | 4.49 (0.77) | 4.46 (0.85) | 0.7596 | 4.31 (0.90) | 4.17 (0.77) | 0.0007 | 4.49 (0.99) | 4.32 (0.96) | 0.0211 |
| Plasma triglycerides ** (mmol/L) | 0.86 (0.66–1.30) | 0.92 (0.61–1.28) | 0.2995 | 0.89 (0.63–1.26) | 1.03 (0.66–1.32) | 0.7308 | 1.04 (0.67–1.56) | 0.98 (0.67–1.60) | 0.3346 |
| Plasma HDL cholesterol ** (mmol/L) | 1.22 (1.04–1.40) | 1.20 (1.02–1.47) | 0.7278 | 1.15 (1.04–1.33) | 1.12 (1.03–1.32) | 0.2209 | 1.15 (0.96–1.40) | 1.20 (0.99–1.32) | 0.5863 |
| Plasma LDL cholesterol ** (mmol/L) | 2.70 (2.40–3.20) | 2.80 (2.10–3.10) | 0.1375 | 2.70 (2.10–3.10) | 2.30 (2.00–3.00) | 0.0042 | 2.60 (2.20–2.90) | 2.30 (2.00–2.90) | 0.0252 |
| Plasma VLDL cholesterol ** (mmol/L) | 0.40 (0.30–0.60) | 0.40 (0.30–0.60) | 0.4201 | 0.40 (0.30–0.60) | 0.50 (0.30–0.60) | 0.8809 | 0.50 (0.30–0.70) | 0.40 (0.30–0.70) | 0.3418 |
| Plasma Aldosterone ** pmol/L | 190.00 (103–261) | 150.00 (103–270) | 0.9338 | 124.00 (103–201) | 138.00 (103–202) | 0.8742 | 122.00 (103–217) | 147.00 (103–222) | 0.4465 |
| Plasma Renin ** IU/L | 16.50 (9.85–22) | 17.00 (11–25) | 0.0312 | 13.00 (8.00–18) | 12.00 (7.75–21) | 0.7128 | 12.00 (7.90–19) | 15.00 (12–25) | 0.0012 |
| Plasma Metanephrine (adrenaline) nmol/L | 0.11 (0.08) | 0.12 (0.08) | 0.4116 | 0.12 (0.05) | 0.10 (0.06) | 0.0225 | 0.12 (0.07) | 0.12 (0.09) | 0.3920 |
| Plasma Normetanephrine (noradrenaline) nmol/L | 0.25 (0.14) | 0.21 (0.08) | 0.0435 | 0.31 (0.11) | 0.21 (0.09) | <0.0001 | 0.32 (0.13) | 0.22 (0.11) | <0.0001 |
| Plasma-glucose ** (mmol/L) | 5.10 (4.90–5.40) | 5.10 (4.90–5.40) | 0.7877 | 5.10 (4.90–5.50) | 5.20 (4.90–5.50) | 0.2934 | 5.20 (4.80–5.40) | 5.15 (5.00–5.50) | 0.2586 |
| HbA1c (mmol/mol) | 34.35 (2.25) | 34.81 (2.42) | 0.0069 | 33.90 (3.00) | 34.94 (3.10) | 0.0021 | 34.23 (3.92) | 34.39 (4.38) | 0.4874 |
| BMI (kg/m2) ** | 24.77 (21.76–26.99) | 24.34 (21.57–27.28) | 0.1599 | 26.01 (23.07–27.99) | 25.98 (23.19–28.49) | 0.6562 | 24.38 (22.16–27.44) | 23.87 (22.06–27.04) | 0.7070 |
| Fat percent (%) | 26.61 (9.59) | 26.35 (8.58) | 0.6193 | 28.39 (9.91) | 27.13 (9.88) | 0.0004 | 27.37 (10.55) | 26.74 (10.79) | 0.0366 |
| Intervention A Versus Control | Intervention B Versus Control | Intervention B Versus A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Difference (95% CI) | p-Value | Difference (95% CI) | p-Value | Difference (95% CI) | p-Value | ||||
| Children < 18 years | ||||||||||
| Systolic BP (mmHg) | 124 | 0.115 | (−4.469–4.700) | 0.9607 | −0.178 | (−4.253–3.897) | 0.9317 | −0.293 | (−4.875–4.288) | 0.9001 |
| Diastolic BP (mmHg) | 124 | 1.282 | (−1.598–4.162) | 0.3829 | 1.643 | (−0.865–4.151) | 0.1991 | 0.361 | (−2.504–3.227) | 0.8047 |
| BMI ** (kg/m2) | 137 | −0.026 | (−0.067–0.015) | 0.2124 | 0.001 | (−0.035–0.038) | 0.9359 | 0.027 | (−0.015–0.069) | 0.2001 |
| 18+ years | ||||||||||
| Diastolic BP (mmHg) | 141 | −0.116 | (−2.842–2.611) | 0.9338 | −0.807 | (−3.254–1.640) | 0.5179 | −0.692 | (−3.312–1.929) | 0.6049 |
| Systolic BP (mmHg) | 141 | 0.729 | (−3.918–5.377) | 0.7584 | −1.497 | (−5.690–2.695) | 0.4839 | −2.227 | (−6.657–2.204) | 0.3246 |
| Total cholesterol (mmol/L) | 137 | −0.251 | (−0.509–0.008) | 0.0574 | −0.117 | (−0.334–0.100) | 0.2915 | 0.134 | (−0.114–0.382) | 0.2897 |
| Triglycerides ** (mmol/L) | 137 | −0.030 | (−0.255–0.195) | 0.7950 | −0.040 | (−0.227–0.148) | 0.6784 | −0.010 | (−0.233–0.213) | 0.9306 |
| HDL cholesterol ** (mmol/L) | 137 | −0.035 | (−0.172–0.102) | 0.6156 | 0.016 | (−0.090–0.122) | 0.7671 | 0.051 | (−0.081–0.183) | 0.4466 |
| LDL cholesterol ** (mmol/L) | 135 | −0.099 | (−0.264–0.065) | 0.2360 | −0.033 | (−0.159–0.093) | 0.6053 | 0.066 | (−0.091–0.223) | 0.4078 |
| VLDL cholesterol ** (mmol/L) | 135 | −0.037 | (−0.278–0.204) | 0.7651 | −0.047 | (−0.249–0.156) | 0.6521 | −0.010 | (−0.233–0.214) | 0.9312 |
| Aldosterone ** (pmol/L) | 137 | −0.054 | (−0.265–0.158) | 0.6196 | −0.020 | (−0.208–0.168) | 0.8362 | 0.034 | (−0.169–0.236) | 0.7441 |
| Renin ** (IU/L) | 137 | −0.193 | (−0.388–0.003) | 0.0535 | 0.040 | (−0.135–0.214) | 0.6568 | 0.232 | (0.045–0.420) | 0.0152 |
| Metanephrine (nmol/L) | 112 | −0.017 | (−0.059–0.025) | 0.4305 | −0.008 | (−0.049–0.033) | 0.7001 | 0.009 | (−0.037–0.055) | 0.6999 |
| Normetanephrine (nmol/L) | 112 | −0.023 | (−0.085–0.040) | 0.4753 | −0.005 | (−0.061–0.050) | 0.8538 | 0.018 | (−0.045–0.080) | 0.5814 |
| Plasma-glucose ** (mmol/L) | 135 | 0.023 | (−0.023–0.068) | 0.3260 | 0.007 | (−0.032–0.046) | 0.7390 | −0.016 | (−0.061–0.029) | 0.4840 |
| HbA1c (mmol/mol) | 137 | 0.654 | (−0.076–1.384) | 0.0793 | −0.318 | (−0.971–0.335) | 0.3400 | −0.972 | (−1.689–0.255) | 0.0079 |
| BMI ** (kg/m2) | 142 | 0.004 | (−0.010–0.018) | 0.5572 | 0.008 | (−0.004–0.019) | 0.2155 | 0.003 | (−0.009–0.016) | 0.5975 |
| Fat percent (%) | 142 | −1.314 | (−2.399–0.229) | 0.0177 | −0.706 | (−1.666–0.255) | 0.1498 | 0.608 | (−0.431–1.647) | 0.2515 |
| Intervention A Versus Control | Intervention B Versus Control | Intervention B Versus A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Difference (95% CI) | p-Value | Difference (95% CI) | p-Value | Difference (95% CI) | p-Value | ||||
| Children < 18 years | ||||||||||
| Diastolic BP (mmHg) | 124 | 1.243 | (−1.182–3.669) | 0.3082 | 1.540 | (−0.679–3.759) | 0.1693 | 0.296 | (−2.135–2.727) | 0.8078 |
| Systolic BP (mmHg) | 124 | −0.615 | (−4.116–2.886) | 0.7273 | 0.214 | (−2.991–3.418) | 0.8946 | 0.829 | (−2.662–4.321) | 0.6376 |
| BMI ** (kg/m2) | 137 | −0.014 | (−0.043–0.015) | 0.3409 | 0.005 | (−0.021–0.031) | 0.7160 | 0.019 | (−0.010–0.047) | 0.1924 |
| 18+ years | ||||||||||
| Diastolic BP (mmHg) | 141 | −0.138 | (−2.640–2.363) | 0.9127 | −1.129 | (−3.412–1.154) | 0.3276 | −0.991 | (−3.385–1.404) | 0.4122 |
| Systolic BP (mmHg) | 141 | 0.923 | (−3.278–5.124) | 0.6629 | −1.770 | (−5.616–2.077) | 0.3622 | −2.693 | (−6.720–1.335) | 0.1868 |
| Total cholesterol (mmol/L) | 137 | −0.286 | (−0.495–0.077) | 0.0079 | −0.151 | (−0.339–0.036) | 0.1122 | 0.135 | (−0.065–0.335) | 0.1835 |
| Triglycerides ** (mmol/L) | 137 | −0.065 | (−0.228–0.097) | 0.4271 | −0.065 | (−0.213–0.082) | 0.3800 | −0.000 | (−0.157–0.157) | 0.9984 |
| HDL cholesterol ** (mmol/L) | 137 | −0.029 | (−0.088–0.030) | 0.3371 | 0.006 | (−0.047–0.059) | 0.8191 | 0.035 | (−0.022–0.092) | 0.2253 |
| LDL cholesterol ** (mmol/L) | 135 | −0.076 | (−0.148–0.003) | 0.0413 | −0.034 | (−0.098–0.031) | 0.3065 | 0.042 | (−0.027–0.111) | 0.2296 |
| VLDL cholesterol ** (mmol/L) | 135 | −0.097 | (−0.258–0.065) | 0.2366 | −0.079 | (−0.223–0.066) | 0.2833 | 0.018 | (−0.138–0.174) | 0.8162 |
| Aldosterone ** (pmol/L) | 137 | −0.084 | (−0.281–0.113) | 0.3982 | −0.071 | (−0.248–0.106) | 0.4277 | 0.013 | (−0.174–0.200) | 0.8894 |
| Renin ** (IU/L) | 137 | −0.231 | (−0.414–0.049) | 0.0134 | 0.007 | (−0.157–0.171) | 0.9319 | 0.238 | (0.064–0.412) | 0.0076 |
| Metanephrine (nmol/L) | 112 | −0.031 | (−0.056–0.007) | 0.0131 | −0.011 | (−0.032–0.010) | 0.2891 | 0.020 | (−0.004–0.044) | 0.0943 |
| Normetanephrine (nmol/L) | 112 | −0.033 | (−0.074–0.009) | 0.1252 | −0.010 | (−0.046–0.026) | 0.5784 | 0.022 | (−0.018–0.062) | 0.2660 |
| Plasma-glucose ** (mmol/L) | 135 | 0.013 | (−0.021–0.047) | 0.4386 | 0.007 | (−0.023–0.038) | 0.6280 | −0.006 | (−0.038–0.027) | 0.7193 |
| HbA1c (mmol/mol) | 137 | 0.641 | (−0.056–1.338) | 0.0708 | −0.382 | (−1.003–0.240) | 0.2254 | −1.023 | (−1.690–0.356) | 0.0031 |
| BMI ** (kg/m2) | 142 | 0.005 | (−0.008–0.017) | 0.4698 | 0.008 | (−0.004–0.019) | 0.1908 | 0.003 | (−0.009–0.015) | 0.6280 |
| Fat percent (%) | 142 | −1.527 | (−2.512–0.543) | 0.0027 | −0.765 | (−1.655–0.125) | 0.0908 | 0.762 | (−0.185–1.709) | 0.1132 |
| A Reduction in Salt Intake from Baseline to Follow-Up of at Least 3 g per day (N = 31) | A Reduction in the Sodium to Potassium Ratio of at Least 20 Percent from Baseline to Follow-Up (N = 33) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Baseline | Follow-up | p-Value | N | Baseline | Follow-up | p-Value | |
| Systolic BP (mmHg) | 30 | 123 (11) | 115 (11) | <0.0001 | 32 | 123 (11) | 117 (12) | <0.0001 |
| Diastolic BP (mmHg) | 30 | 78 (8) | 76 (8) | 0.0336 | 32 | 80 (8) | 77 (9) | 0.0212 |
| Plasma total cholesterol (mmol/L) | 30 | 4.48 (1.04) | 4.28 (0.89) | 0.0296 | 32 | 4.48 (1.04) | 4.22 (0.97) | 0.0005 |
| Plasma triglycerides ** (mmol/L) | 30 | 1.04 (0.66–1.32) | 0.89 (0.62–1.12) | 0.0794 | 32 | 1.07 (0.65–1.42) | 0.83 (0.62–1.14) | 0.0993 |
| Plasma HDL cholesterol ** (mmol/L) | 30 | 1.23 (0.97–1.40) | 1.20 (1.06–1.37) | 0.5019 | 32 | 1.22 (1.00–1.39) | 1.14 (1.04–1.32) | 0.9353 |
| Plasma LDL cholesterol ** (mmol/L) | 30 | 2.50 (2.10–3.20) | 2.50 (2.00–3.20) | 0.1503 | 31 | 2.40 (2.00–3.20) | 2.30 (2.00–3.20) | 0.0283 |
| Plasma VLDL cholesterol ** (mmol/L) | 30 | 0.50 (0.30–0.60) | 0.40 (0.30–0.50) | 0.1071 | 31 | 0.50 (0.30–0.60) | 0.40 (0.30–0.50) | 0.0375 |
| Plasma Aldosterone ** (pmol/L) | 30 | 119.50 (103–231) | 127.00 (103–242) | 0.6131 | 32 | 123.00 (103–286) | 158.00 (103–262) | 0.4074 |
| Plasma Renin ** (IU/L) | 30 | 10.50 (6.80–18) | 13.00 (7.80–18) | 0.3039 | 32 | 10.50 (6.60–18) | 13.50 (7.80–18) | 0.0710 |
| Plasma Adrenalin (nmol/L) | 23 | 0.12 (0.07) | 0.10 (0.08) | 0.0412 | 24 | 0.12 (0.08) | 0.11 (0.08) | 0.2433 |
| Plasma Noradrenalin (nmol/L) | 23 | 0.31 (0.13) | 0.22 (0.09) | 0.0006 | 24 | 0.30 (0.13) | 0.21 (0.10) | 0.0001 |
| Plasma-glucose ** (mmol/L) | 28 | 5.25 (4.80–5.50) | 5.25 (4.95–5.65) | 0.3402 | 30 | 5.20 (4.90–5.50) | 5.25 (4.80–5.50) | 0.7358 |
| HbA1c (mmol/mol) | 30 | 34.77 (2.86) | 35.27 (2.64) | 0.0961 | 32 | 34.38 (2.81) | 34.84 (2.83) | 0.0917 |
| BMI ** (kg/m2) | 31 | 25.35 (21–27) | 25.24 (22–27) | 0.9294 | 33 | 25.77 (21–27) | 25.40 (21–27) | 0.3039 |
| Fat percent (%) | 31 | 27.71 (10.33) | 27.39 (10.13) | 0.2355 | 33 | 27.81 (9.73) | 27.04 (9.81) | 0.0047 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toft, U.; Riis, N.L.; Lassen, A.D.; Trolle, E.; Andreasen, A.H.; Frederiksen, A.K.S.; Joergensen, N.R.; Munk, J.K.; Bjoernsbo, K.S. The Effects of Two Intervention Strategies to Reduce the Intake of Salt and the Sodium-To-Potassium Ratio on Cardiovascular Risk Factors. A 4-Month Randomised Controlled Study among Healthy Families. Nutrients 2020, 12, 1467. https://doi.org/10.3390/nu12051467
Toft U, Riis NL, Lassen AD, Trolle E, Andreasen AH, Frederiksen AKS, Joergensen NR, Munk JK, Bjoernsbo KS. The Effects of Two Intervention Strategies to Reduce the Intake of Salt and the Sodium-To-Potassium Ratio on Cardiovascular Risk Factors. A 4-Month Randomised Controlled Study among Healthy Families. Nutrients. 2020; 12(5):1467. https://doi.org/10.3390/nu12051467
Chicago/Turabian StyleToft, Ulla, Nanna Louise Riis, Anne Dahl Lassen, Ellen Trolle, Anne Helms Andreasen, Amalie Kruse Sigersted Frederiksen, Niklas Rye Joergensen, Jens Kristian Munk, and Kirsten Schroll Bjoernsbo. 2020. "The Effects of Two Intervention Strategies to Reduce the Intake of Salt and the Sodium-To-Potassium Ratio on Cardiovascular Risk Factors. A 4-Month Randomised Controlled Study among Healthy Families" Nutrients 12, no. 5: 1467. https://doi.org/10.3390/nu12051467
APA StyleToft, U., Riis, N. L., Lassen, A. D., Trolle, E., Andreasen, A. H., Frederiksen, A. K. S., Joergensen, N. R., Munk, J. K., & Bjoernsbo, K. S. (2020). The Effects of Two Intervention Strategies to Reduce the Intake of Salt and the Sodium-To-Potassium Ratio on Cardiovascular Risk Factors. A 4-Month Randomised Controlled Study among Healthy Families. Nutrients, 12(5), 1467. https://doi.org/10.3390/nu12051467





