Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies
Abstract
1. Introduction
2. Search of the Literature
3. Carnitine
4. Flavonoids, Isoflavonoids, Soy
5. Melatonin
6. Omega-3 Polyunsaturated Fatty Acids (Or Fish Oil)
7. Resveratrol
8. Selenium
9. Vitamins
9.1. Vitamin A
9.2. Vitamin D
9.3. Vitamin E
10. Zinc
11. Inositol
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benvenga, S.; Feldt-Rasmussen, U.; Bonofiglio, D.; Asamoah, E. Nutraceutical Supplements in the Thyroid Setting: Health Benefits beyond Basic Nutrition. Nutrients 2019, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, G.B. The quality of commercially available nutraceutical supplements and food sources. J. Pharm. Pharmacol. 2011, 63, 3–10. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Regulation EU 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32015R2283 (accessed on 13 March 2020).
- Galetta, F.; Franzoni, F.; Bernini, G.; Poupak, F.; Carpi, A.; Cini, G.; Tocchini, L.; Antonelli, A.; Santoro, G. Cardiovascular complications in patients with pheochromocytoma: A mini-review. Biomed. Pharmacother. 2010, 64, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Galetta, F.; Franzoni, F.; Fallahi, P.; Tocchini, L.; Braccini, L.; Santoro, G.; Antonelli, A. Changes in heart rate variability and QT dispersion in patients with overt hypothyroidism. Eur. J. Endocrinol. 2008, 158, 85–90. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Frascerra, S.; Di Domenicantonio, A.; Nicolini, A.; Ferrari, P.; Ferrannini, E.; Fallahi, P. Increase of circulating CXCL9 and CXCL11 associated with euthyroid or subclinically hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 2011, 96, 1859–1863. [Google Scholar] [CrossRef][Green Version]
- Benvenga, S.; Lakshmanan, M.; Trimarchi, F. Carnitine is a naturally occurring inhibitor of thyroid hormone nuclear uptake. Thyroid 2000, 12, 1043–1050. [Google Scholar] [CrossRef]
- Rotzsch, W.; Strack, E. Umstaz Und Wirkung des Carnitins im Tierkorper. Int. Abstr. Biol. Sci. 1958, 11, 80. [Google Scholar]
- Hellthaler, G.; Wenzel, K.W.; Rotzsch, W. Aminotransferasen unter thyroxin und Karnitin. Acta Biol. German 1967, 19, 641–652. [Google Scholar]
- Yildirim, S.; Yildirim, A.; Dane, S.; Aliyev, E.; Yigitoglu, R. Dose-dependent protective effect of L-carnitine on oxidative stress in the livers of hyperthyroid rats. Eurasian J. Med. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Huang, H.; Liu, N.; Guo, H.; Liao, S.; Li, X.; Yang, C.; Liu, S.; Song, W.; Liu, C.; Guan, L.; et al. L-Carnitine Is an Endogenous HDAC Inhibitor Selectively Inhibiting Cancer Cell Growth In Vivo and In Vitro. PLoS ONE 2012, 7, e49062. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, Y.; Su, D.; Gong, B.; He, X.; Zhou, X.; Pang, Z.; Cheng, L.; Chen, Y.; Yao, Z. Cpt1c regulated by AMPK promotes papillary thyroid carcinomas cells survival under metabolic stress conditions. J. Cancer 2017, 8, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Payab, M.; Sarvari, M.; Gilany, K.; Larijani, B.; Arjmand, B.; Tavangar, S.M. Oncometabolites as biomarkers in thyroid cancer: A systematic review. Cancer Manag. Res. 2019, 11, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Torun, N.; Muratli, A.; Serim, B.D.; Ergulen, A.; Altun, G.D. Radioprotective Effects of Amifostine, L-Carnitine and Vitamin E in Preventing Early Salivary Gland Injury due to Radioactive Iodine Treatment. Curr. Med. Imag. Rev. 2019, 15, 395–404. [Google Scholar] [CrossRef]
- Spencer, J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 99, ES60–ES77. [Google Scholar] [CrossRef]
- de Souza Dos Santos, M.C.; Gonçalves, C.F.; Vaisman, M.; Ferreira, A.C.; de Carvalho, D.P. Impact of flavonoids on thyroid function. Food Chem. Toxicol. 2011, 49, 2495–2502. [Google Scholar] [CrossRef]
- Lehmann, L.; Soukup, S.T.; Gerhäuser, C.; Vollmer, G.; Kulling, S.E. [Isoflavone-containing dietary supplements]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2017, 60, 305–313. [Google Scholar] [CrossRef]
- D’Adamo, C.R.; Sahin, A. Soy foods and supplementation: A review of commonly perceived health benefits and risks. Altern. Ther. Health Med. 2014, 20, 39–51. [Google Scholar]
- Silverstein, M.G.; Kaplan, J.R.; Appt, S.E.; Register, T.C.; Shively, C.A. Effect of soy isoflavones on thyroid hormones in intact and ovariectomized cynomolgus monkeys (Macaca fascicularis). Menopause 2014, 21, 1136–1142. [Google Scholar] [CrossRef]
- Doerge, D.R.; Chang, H.C. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J. Chromatogr. B 2002, 777, 269–279. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishikawa, A.; Imazawa, T.; Kimura, S.; Hirose, M. Dramatic synergism between excess soybean intake and iodine deficiency on the development of rat thyroid hyperplasia. Carcinogenesis 2000, 21, 707–713. [Google Scholar] [CrossRef]
- Son, H.Y.; Nishikawa, A.; Ikeda, T.; Imazawa, T.; Kimura, S.; Hirose, M. Lack of effect of soy isoflavone on thyroid hyperplasia in rats receiving an iodine-deficient diet. Jpn. J. Cancer Res. 2001, 92, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Polito, F.; Adamo, E.B.; Bitto, A.; Squadrito, F.; Benvenga, S. Update on genistein and thyroid: An overall message of safety. Front. Endocrinol. 2012, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Antonelli, A.; Guidi, P.; Bernardeschi, M.; Scarcelli, V.; Fallahi, P.; Frenzilli, G. Genotoxicity Evaluation of the Soybean Isoflavone Genistein in Human Papillary Thyroid Cancer Cells. Study of Its Potential Use in Thyroid Cancer Therapy. Nutr. Cancer 2019, 71, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Doerge, D.R. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 2000, 168, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Prince, P.S.; Sathya, B. Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. Eur. J. Pharmacol. 2010, 635, 142–148. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Antiinflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef]
- Giuliani, C.; Noguchi, Y.; Harii, N.; Napolitano, G.; Tatone, D.; Bucci, I.; Piantelli, M.; Monaco, F.; Kohn, L.D. The flavonoid quercetin regulates growth and gene expression in rat FRTL-5 thyroid cells. Endocrinology 2008, 149, 84–92. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Di Santo, S.; Rossi, C.; Grassadonia, A.; Piantelli, M.; Monaco, F.; Napolitano, G. The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Food Chem. Toxicol. 2014, 66, 23–29. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Doseff, A.I.; Ringel, M.D.; Saji, M.; Rousset, B.; Zhang, X.; Jhiang, S.M. Apigenin in combination with Akt inhibition significantly enhances thyrotropin-stimulated radioiodide accumulation in thyroid cells. Thyroid 2014, 24, 878–887. [Google Scholar] [CrossRef]
- Tran, L.; Hammuda, M.; Wood, C.; Xiao, C.W. Soy extracts suppressed iodine uptake and stimulated the production of autoimmunogen in rat thyrocytes. Exp. Biol. Med. 2013, 238, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.K.; De, N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem. Toxicol. 2010, 48, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Perri, A.; Vizza, D.; Russo, A.; Panno, M.L.; Bonofiglio, D.; Giordano, C.; Mauro, L.; Aquila, S.; Tramontano, D.; et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J. Cell Physiol. 2013, 228, 2054–2062. [Google Scholar] [CrossRef]
- Garcia-Marin, R.; Fernandez-Santos, J.M.; Morillo-Bernal, J.; Gordillo-Martinez, F.; Vazquez-Roman, V.; Utrilla, J.C.; Carrillo-Vico, A.; Guerrero, J.M.; Martin-Lacave, I. Melatonin in the thyroid gland: Regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J. Physiol. Pharmacol. 2015, 66, 643–652. [Google Scholar] [PubMed]
- Pierpaoli, G.; Dall’Ara, A.; Pedrinis, E.; Regelson, W. The pineal control of aging: The effects of melatonin and pineal grafting on the survival of older mice. Ann. N. Y. Acad. Sci. 1991, 621, 291–313. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Bediz, C.S.; Kul, A.; Ugur, A. Pinealectomy and zinc deficiency have opposite effects on thyroid hormones in rats. Endocr. Res. 2003, 29, 473–481. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Kul, A.; Bediz, C.S.; Ugur, A. Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology 2004, 195, 65–75. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Leptin, N.P.Y. Melatonin and Zinc Levels in Experimental Hypothyroidism and Hyperthyroidism: The Relation to Zinc. Biochem. Genet. 2017, 55, 223–233. [Google Scholar] [CrossRef]
- Zou, Z.W.; Liu, T.; Li, Y.; Chen, P.; Peng, X.; Ma, C.; Zhang, W.J.; Li, P.D. Melatonin suppresses thyroid cancer growth and overcomes radioresistance via inhibition of p65 phosphorylation and induction of ROS. Redox Biol. 2018, 16, 226–236. [Google Scholar] [CrossRef]
- Soukup, T. Effects of long-term thyroid hormone level alterations, n-3 polyunsaturated fatty acid supplementation and statin administration in rats. Physiol. Res. 2014, 63, S119–S131. [Google Scholar]
- Sinha, R.A.; Khare, P.; Rai, A.; Maurya, S.K.; Pathak, A.; Mohan, V.; Nagar, G.K.; Mudiam, M.K.; Godbole, M.M.; Bandyopadhyay, S. Anti-apoptotic role of omega-3-fatty acids in developing brain: Perinatal hypothyroid rat cerebellum as apoptotic model. Int. J. Dev. Neurosci. 2009, 27, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, E.S.; Gomaa, A.M.; Sayed, M.M. The effect of omega-3 on cognition in hypothyroid adult male rats. Acta Physiol. Hung. 2014, 101, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Rauchová, H.; Vokurková, M.; Pavelka, S.; Behuliak, M.; Tribulová, N.; Soukup, T. N-3 polyunsaturated fatty acids supplementation does not affect changes of lipid metabolism induced in rats by altered thyroid status. Horm. Metab. Res. 2013, 45, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gani, O.A. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, S.; Momenzadeh, S.; Seyed Forootan, F.; Ghaedi, K.; Nasr Esfahani, M.H. The influence of peroxisome proliferator-activated receptor γ (PPARγ) ligands on cancer cell tumorigenicity. Gene 2018, 649, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Endo, T.; Haraguchi, K.; Hershman, J.M.; Onaya, T. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2001, 86, 2170–2177. [Google Scholar] [CrossRef][Green Version]
- Hayashi, N.; Nakamori, S.; Hiraoka, N.; Tsujie, M.; Xundi, X.; Takano, T.; Amino, N.; Sakon, M.; Monden, M. Antitumor effects of peroxisome proliferator activate receptor gamma ligands on anaplastic thyroid carcinoma. Int. J. Oncol. 2004, 24, 89–95. [Google Scholar]
- Bonofiglio, D.; Qi, H.; Gabriele, S.; Catalano, S.; Aquila, S.; Belmonte, M.; Andò, S. Peroxisome proliferator-activated receptor gamma inhibits follicular and anaplastic thyroid carcinoma cells growth by upregulating p21Cip1/WAF1 gene in a Sp1-dependent manner. Endocr. Relat. Cancer 2008, 15, 545–557. [Google Scholar] [CrossRef]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Santini, F.; Minuto, M.; Galleri, D.; Miccoli, P. New targeted therapies for thyroid cancer. Curr. Genom. 2011, 12, 626–631. [Google Scholar] [CrossRef]
- Antonelli, A.; Miccoli, P.; Derzhitski, V.E.; Panasiuk, G.; Solovieva, N.; Baschieri, L. Epidemiologic and clinical evaluation of thyroid cancer in children from the Gomel region (Belarus). World J. Surg. 1996, 20, 867–871. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Limmongkon, A.; Janhom, P.; Amthong, A.; Kawpanuk, M.; Nopprang, P.; Poohadsuan, J.; Somboon, T.; Saijeen, S.; Surangkul, D.; Srikummool, M.; et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pac. J. Trop. Biomed. 2017, 7, 332–338. [Google Scholar] [CrossRef]
- Ge, J.F.; Xu, Y.Y.; Li, N.; Zhang, Y.; Qiu, G.L.; Chu, C.H.; Wang, C.Y.; Qin, G.; Chen, F.H. Resveratrol improved the spatial learning and memory in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. Endocr. J. 2015, 62, 927–938. [Google Scholar] [CrossRef]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Cheng, J.Q.; Chen, F.H. Resveratrol Ameliorates the Anxiety- and Depression-Like Behavior of Subclinical Hypothyroidism Rat: Possible Involvement of the HPT Axis, HPA Axis, and Wnt/β-Catenin Pathway. Front. Endocrinol. 2016, 7, 44. [Google Scholar] [CrossRef]
- Sarkar, C.; Pal, S. Ameliorative effect of resveratrol against fluoride-induced alteration of thyroid function in male wistar rats. Biol. Trace Elem. Res. 2014, 162, 278–287. [Google Scholar] [CrossRef]
- Ho, Y.; Lin, Y.S.; Liu, H.L.; Shih, Y.J.; Lin, S.Y.; Shih, A.; Chin, Y.T.; Chen, Y.R.; Lin, H.Y.; Davis, P.J. Biological Mechanisms by Which Antiproliferative Actions of Resveratrol Are Minimized. Nutrients 2017, 9, 1046. [Google Scholar] [CrossRef]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Di Santo, S.; Rossi, C.; Grassadonia, A.; Mariotti, M.; Piantelli, M.; Monaco, F.; Napolitano, G. Resveratrol inhibits sodium/iodide symporter gene expression and function in rat thyroid cells. PLoS ONE 2014, 9, e107936. [Google Scholar] [CrossRef]
- Giuliani, C.; Iezzi, M.; Ciolli, L.; Hysi, A.; Bucci, I.; Di Santo, S.; Rossi, C.; Zucchelli, M.; Napolitano, G. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem. Toxicol. 2017, 107, 237–247. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.T.; Tian, X.T.; Liu, Y.S.; Wu, M.L.; Li, P.N.; Liu, J. STAT3 signaling statuses determine the fate of resveratrol-treated anaplastic thyroid cancer cells. Cancer Biomark. 2020, 27, 461–469. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wu, M.L.; Wu, J.; Sun, Y.; Zhang, K.L.; Liu, J. Resveratrol Reverses Retinoic Acid Resistance of Anaplastic Thyroid Cancer Cells via Demethylating CRABP2 Gene. Front. Endocrinol. 2019, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Hossein, S.A.H. Resveratrol Sensitizes Selectively Thyroid Cancer Cell to 131-Iodine Toxicity. J. Toxicol. 2014, 2014, 839597. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, I.; Fetoui, H.; Guermazi, F.; Zeghal, N. Dietary selenium addition improves cerebrum and cerebellum impairments induced by methimazole in suckling rats. Int. J. Dev. Neurosci. 2009, 27, 719–726. [Google Scholar] [CrossRef]
- Laureano-Melo, R.; Império, G.E.; da Silva-Almeida, C.; Kluck, G.E.; Cruz Seara Fde, A.; da Rocha, F.F.; da Silveira, A.L.; Reis, L.C.; Ortiga-Carvalho, T.M.; da Silva Côrtes, W. Sodium selenite supplementation during pregnancy and lactation promotes anxiolysis and improves mnemonic performance in wistar rats’ offspring. Pharmacol. Biochem. Behav. 2015, 138, 123–132. [Google Scholar] [CrossRef]
- Xue, H.; Wang, W.; Li, Y.; Shan, Z.; Li, Y.; Teng, X.; Gao, Y.; Fan, C.; Teng, W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr. J. 2010, 57, 595–601. [Google Scholar] [CrossRef]
- Kato, M.A.; Finley, D.J.; Lubitz, C.C.; Zhu, B.; Moo, T.A.; Loeven, M.R.; Ricci, J.A.; Zarnegar, R.; Katdare, M.; Fahey, T.J. 3rd. Selenium decreases thyroid cancer cell growth by increasing expression of GADD153 and GADD34. Nutr. Cancer 2010, 62, 66–73. [Google Scholar] [CrossRef]
- de Oliveira Maia, M.; Batista, B.A.M.; Sousa, M.P.; de Souza, L.M.; Maia, C.S.C. Selenium and thyroid cancer: A systematic review. Nutr. Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Biebinger, R.; Arnold, M.; Koss, M.; Kloeckener-Gruissem, B.; Langhans, W.; Hurrell, R.F.; Zimmermann, M.B. Effect of concurrent vitamin A and iodine deficiencies on the thyroid-pituitary axis in rats. Thyroid 2006, 16, 961–965. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Interactions of vitamin A and iodine deficiencies: Effects on the pituitary-thyroid axis. Int. J. Vitam. Nutr. Res. 2007, 77, 236–240. [Google Scholar] [CrossRef]
- Li, H.; Bai, B.; Zhang, Q.; Bao, Y.; Guo, J.; Chen, S.; Miao, C.; Liu, X.; Zhang, T. Ectopic cross-talk between thyroid and retinoic acid signaling: A possible etiology for spinal neural tube defects. Gene 2015, 573, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J. Effects of all-trans retinoic acid on sodium/iodide symporter and CCAAT/enhancer-binding protein-homologous protein under condition of endoplasmic reticulum stress in FRTL5 thyroid cells. Horm. Metab. Res. 2011, 43, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Basourakos, S.; Cui, D.; Zuo, X.; Deng, W.; Huo, L.; Chen, H.; Zhang, G.; Deng, L.; Shi, B.; et al. ATRA increases iodine uptake and inhibits the proliferation and invasiveness of human anaplastic thyroid carcinoma SW1736 cells: Involvement of β-catenin phosphorylation inhibition. Oncol. Lett. 2017, 14, 7733–7738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, R.; Xu, H.; Zhang, M.; Li, B. Retinoic acid and tributyrin induce in-vitro radioiodine uptake and inhibition of cell proliferation in a poorly differentiated follicular thyroid carcinoma. Nucl. Med. Commun. 2011, 32, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Albano, L.; Ungaro, P.; Colao, A.; Macchia, P.E. Sunshine vitamin and thyroid. Rev. Endocr. Metab. Disord. 2017, 18, 347–354. [Google Scholar] [CrossRef]
- Fournier, C.; Gepner, P.; Sadouk, M.; Charreire, J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin. Immunol. Immunopathol. 1990, 54, 53–63. [Google Scholar] [CrossRef]
- Chen, W.; Lin, H.; Wang, M. Immune intervention effects on the induction of experimental autoimmune thyroiditis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2002, 22, 343–345. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. [Effects of 1,25- dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis]. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 1573–1576. [Google Scholar]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapaport, B.; McLachlan, S.M. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009, 150, 1051–1060. [Google Scholar] [CrossRef]
- Williams, T.L.; Elliott, J.; Berry, J.; Syme, H.M. Investigation of the pathophysiological mechanism for altered calcium homeostasis in hyperthyroid cats. J. Small Anim. Pract. 2013, 54, 367–373. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef] [PubMed]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; Van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, L.; Sun, W.; Qin, Y.; Zhang, P.; Zhang, H. 1,25-Dihydroxyvitamin D3 enhances the susceptibility of anaplastic thyroid cancer cells to adriamycin-induced apoptosis by increasing the generation of reactive oxygen species. Mol. Med. Rep. 2019, 20, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, K.; Zheng, R.; Derwahl, M. 1,25 dihydroxyvitamin D3 inhibits the proliferation of thyroid cancer stem-like cells via cell cycle arrest. Endocr. Res. 2016, 41, 71–80. [Google Scholar] [CrossRef]
- Yu, J.; Shan, Z.; Chong, W.; Mao, J.; Geng, Y.; Zhang, C.; Xing, Q.; Wang, W.; Li, N.; Fan, C.; et al. Vitamin E ameliorates iodine-induced cytotoxicity in thyroid. J. Endocrinol. 2011, 209, 299–306. [Google Scholar] [CrossRef][Green Version]
- Pan, T.; Zhong, M.; Zhong, X.; Zhang, Y.; Zhu, D. Levothyroxine replacement therapy with vitamin E supplementation prevents oxidative stress and cognitive deficit in experimental hypothyroidism. Endocrine 2013, 43, 434–439. [Google Scholar] [CrossRef]
- Guo, Y.; Wan, S.Y.; Zhong, X.; Zhong, M.K.; Pan, T.R. Levothyroxine replacement therapy with vitamin E supplementation prevents the oxidative stress and apoptosis in hippocampus of hypothyroid rats. Neuroendocrinol. Lett. 2014, 35, 684–690. [Google Scholar]
- Subudhi, U.; Das, K.; Paital, B.; Bhanja, S.; Chainy, G.B. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Biol. Interact. 2008, 173, 105–114. [Google Scholar] [CrossRef]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Nitrite-induced alterations in sex steroids and thyroid hormones of Labeo rohita juveniles: Effects of dietary vitamin E and L-tryptophan. Fish Physiol. Biochem. 2013, 39, 1297–1307. [Google Scholar] [CrossRef]
- Esposito, T.; Lucariello, A.; Hay, E.; Contieri, M.; Tammaro, P.; Varriale, B.; Guerra, G.; De Luca, A.; Perna, A. Effects of curcumin and its adjuvant on TPC1 thyroid cell line. Chem. Biol. Interact. 2019, 305, 112–118. [Google Scholar] [CrossRef]
- Pathak, R.; Dhawan, D.; Pathak, A. Effect of zinc supplementation on the status of thyroid hormones and Na, K, and Ca levels in blood following ethanol feeding. Biol. Trace Elem. Res. 2011, 140, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.D.; Lin, P.Y.; Lin, W.H. Zinc supplementation on serum levels and hepatic conversion of thyroid hormones in obese (ob/ob) mice. Biol. Trace Elem. Res. 1998, 61, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Keçeci, T.; Keskin, E. Zinc supplementation decreases total thyroid hormone concentration in small ruminants. Acta Vet. Hung. 2002, 50, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baydas, B.; Karagoz, S.; Meral, I. Effects of oral zinc and magnesium supplementation on serum thyroid hormone and lipid levels in experimentally induced diabetic rats. Biol. Trace Elem. Res. 2002, 88, 247–253. [Google Scholar] [CrossRef]
- Guantario, B.; Capolupo, A.; Monti, M.C.; Leoni, G.; Ranaldi, G.; Tosco, A.; Marzullo, L.; Murgia, C.; Perozzi, G. Proteomic Analysis of Zn Depletion/Repletion in the Hormone-Secreting Thyroid Follicular Cell Line FRTL-5. Nutrients 2018, 10, 1981. [Google Scholar] [CrossRef]
- Benvenga, S.; Antonelli, A. Inositol(s) in thyroid function, growth and autoimmunity. Rev. Endocr. Metab. Disord. 2016, 17, 471–484. [Google Scholar] [CrossRef]
- Lewin, L.M.; Yannai, Y.; Sulimovici, S.; Kraicer, P.F. Studies on the Metabolic Role of Myo-Inositol. Distribution of Radioactive Myo-Inositol in the Male Rat. Biochem. J. 1976, 156, 375–380. [Google Scholar] [CrossRef]
- Grafton, G.; Baxter, M.A.; Sheppard, M.C.; Eggo, M.C. Regulation of Myo-Inositol Transport During the Growth and Differentiation of Thyrocytes: A Link With Thyroid-Stimulating Hormone-Induced Phospholipase A2 Activity. Biochem. J. 1995, 309, 667–675. [Google Scholar] [CrossRef]
- Hasegawa, R.; Eisenberg, F., Jr. Selective Hormonal Control of Myo-Inositol Biosynthesis in Reproductive Organs and Liver of the Male Rat. Proc. Natl. Acad. Sci. USA 1981, 78, 4863–4866. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Caruso, C.; Guglielmi, G.; Antonelli, A. Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev. Endocr. Metab. Disord. 2018, 19, 349–354. [Google Scholar] [CrossRef]
- Gerloff, B.J.; Herdt, T.H.; Wells, W.W.; Nachreiner, R.F.; Emery, R.S. Inositol and Hepatic Lipidosis. II. Effect of Inositol Supplementation and Time from Parturition on Serum Insulin, Thyroxine and Triiodothyronine and Their Relationship to Serum and Liver Lipids in Dairy Cows. J. Anim. Sci. 1986, 62, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Pajalich, R. Combined treatment with Myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J. Thyroid. Res. 2013, 2013, 424163. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Di Bari, F.; Vita, R.; Benvenga, S.; Antonelli, A. Myo-inositol and selenium reduce the risk of developing overt hypothyroidism in patients with autoimmune thyroiditis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 36–42. [Google Scholar]
- Benvenga, S.; Vicchio, T.; Di Bari, F.; Vita, R.; Fallahi, P.; Ferrari, S.M.; Catania, S.; Costa, C.; Antonelli, A. Favorable effects of myo-inositol, selenomethionine or their combination on the hydrogen peroxide-induced oxidative stress of peripheral mononuclear cells from patients with Hashimoto’s thyroiditis: Preliminary in vitro studies. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 89–101. [Google Scholar] [PubMed]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Caruso, C.; Benvenga, S.; Fallahi, P.; Antonelli, A. The protective effect of myo-inositol on human thyrocytes. Rev. Endocr. Metab. Disord. 2018, 19, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Heyliger, C.E.; McNeill, J.H. Effect of Myo-Inositol and T3 on Myocardial Lipids and Cardiac Function in Streptozocin-Induced Diabetic Rats. Diabetes 1988, 37, 1542–1548. [Google Scholar] [CrossRef]
| n. of Items. | Entry | Humans | Other Animals |
|---|---|---|---|
| 1 | nutraceuticals | 55,737 | 31,391 |
| 2 | nutraceuticals AND thyroid | 522 (0.9%) | 224 (0.9%) |
| 3 | carnitine | 8134 | 8778 |
| 4 | carnitine AND thyroid | 71 (0.8%) | 95 (1.1%) |
| 5 | flavonoids | 44,187 | 49,719 |
| 6 | flavonoids AND thyroid | 222 (0.5%) | 248 (0.5%) |
| 7 | isoflavonoids | 404 | 281 |
| 8 | isoflavonoids AND thyroid | 4 (0.9%) | 4 (1.4%) |
| 9 | soy | 7965 | 6531 |
| 10 | soy AND thyroid | 93 (1.2%) | 75 (1.1%) |
| 11 | melatonin | 11,142 | 14,477 |
| 12 | melatonin AND thyroid | 200 (1.8%) | 364 (2.5%) |
| 13 | omega-3 polyunsaturated fatty acids | 17,168 | 12,783 |
| 14 | omega-3 polyunsaturated fatty acids AND thyroid | 37 (0.21%) | 38 (0.3%) |
| 15 | resveratrol | 5823 | 5961 |
| 16 | resveratrol AND thyroid | 54 (0.9%) | 42 (0.7%) |
| 17 | selenium | 13,794 | 13,888 |
| 18 | selenium AND thyroid | 600 (4.3%) | 372 (2.7%) |
| 19 | vitamin A | 32,637 | 22,296 |
| 20 | vitamin A AND thyroid | 495 (1.5%) | 593 (2.7%) |
| 21 | vitamin D | 61,418 | 20311 |
| 22 | vitamin D AND thyroid | 1280 (2.1%) | 554 (2.7%) |
| 23 | vitamin E | 22,004 | 18,811 |
| 24 | vitamin E AND thyroid | 96 (0.4%) | 123 (0.6%) |
| 25 | zinc | 58,247 | 50,628 |
| 26 | zinc AND thyroid | 503 (0.86%) | 401 (0.7%) |
| 27 | inositol | 17,144 | 27,226 |
| 28 | inositol AND thyroid | 147 (0.86%) | 205 (0.75%) |
| Groups | Age (Months) | Melatonin (Duration of Treatments, Months) | T3 (ng/mL) | T4 (μg/dL) |
|---|---|---|---|---|
| Untreated (n = 10) | 19 | ------------ | 0.854 ± 0.165 | 5.48 ± 1.09 |
| Treated (n = 10) | 19 | 3 | 0.873 ± 0.160 (+ 2.2%) P > 0.05 (NS) | 5.46 ± 1.51 (− 0.36%) P > 0.05 (NS) |
| Untreated (n = 4) | 23 | ------------ | 0.850 ± 0.028 | 4.94 ± 1.10 |
| Treated (n = 8) | 23 | 7 | 0.682 ± 0.049 (− 19.8%) * P < 0.001 | 3.79 ± 1.37 (− 23.3%) § P > 0.05 (NS) |
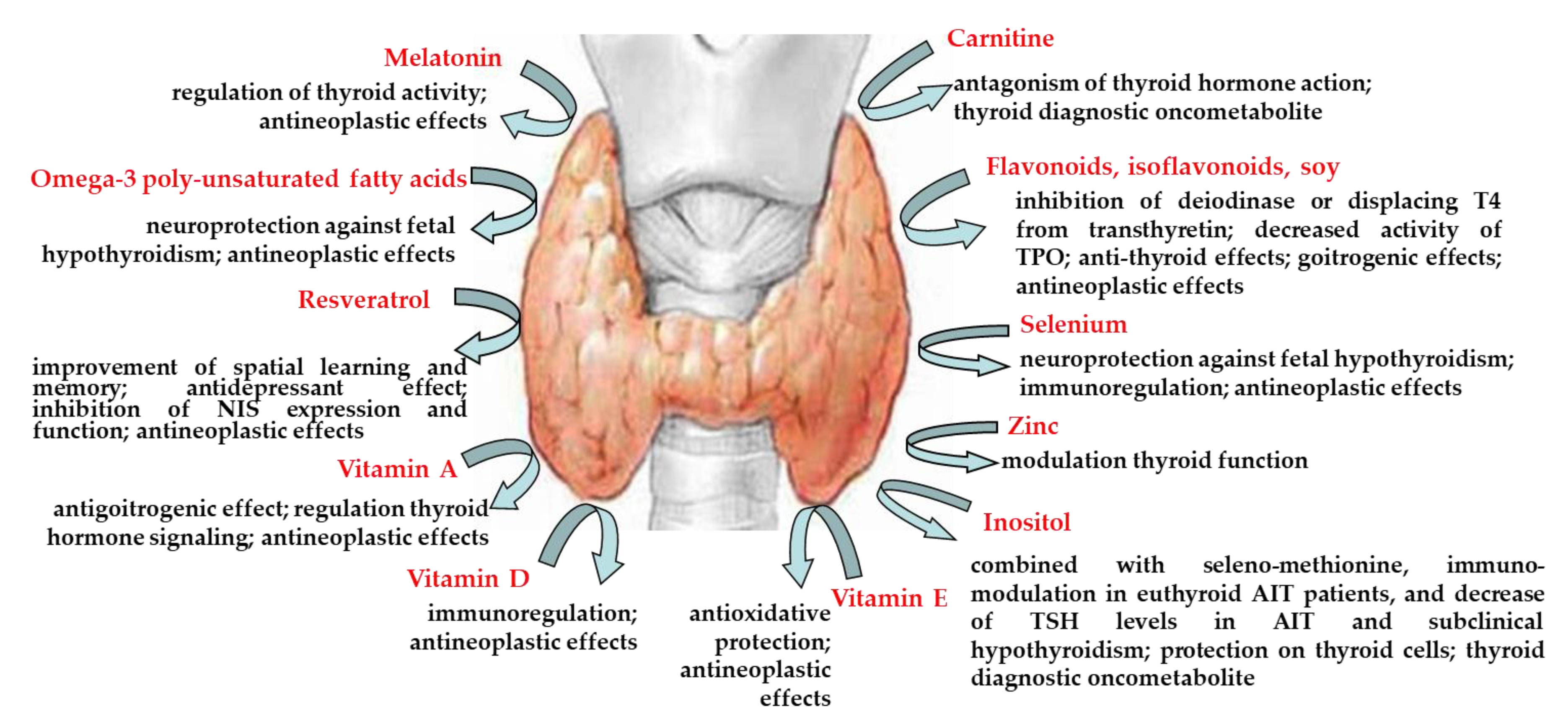
| Compounds | Main Findings | References |
|---|---|---|
| carnitine | antagonism of thyroid hormone action, thyroid diagnostic oncometabolite | [9] [13] |
| flavonoids, isoflavonoids, soy | inhibition of deiodinase or displacing T4 from transthyretin, decreased activity of thyroid peroxidase anti-thyroid effects goitrogenic effect antineoplastic effects | [16,33] [20,29,30,31] [22] [24,34] |
| melatonin | regulation of thyroid activity antineoplastic effects | [37,38,39] [40] |
| omega-3 poly-unsaturated fatty acids | neuroprotection against fetal hypothyroidism antineoplastic effects | [42,43] [45] |
| resveratrol | improvement of spatial learning and memory antidepressant effect inhibition of sodium/iodide symporter expression and function antineoplastic effects | [54] [55] [59] [57,58,61,62,63] |
| selenium | neuroprotection against fetal hypothyroidism immunoregulation antineoplastic effects | [65,66] [67] [68] |
| vitamin A | antigoitrogenic effect regulation thyroid hormone signaling antineoplastic effects | [71] [72] [73,74,75] |
| vitamin D | immunoregulation antineoplastic effects | [77,78,79,80] [82] |
| vitamin E | antioxidative protection antineoplastic effects | [86,87,88,89] [91] |
| zinc | modulation thyroid function | [36,37,38,92,93,94,95,96] |
| inositol | involvement in the intracellular TSH signaling, via PIP-3 inositol supplementation decreased circulating T3 and FT3 concentrations thyroid diagnostic oncometabolite the treatment, in combination with seleno-methionine, declined the elevated levels of TSH in patients with AIT and subclinical hypothyroidism immune-modulatory effect of myo-inositol in association with seleno-methionine in patients with euthyroid AIT beneficial effects of myo-inositol, seleno-methionine or their combination on PBMC exposed in vitro to H2O2-induced oxidative stress in both control and women with HT protective effect of myo-inositol on thyroid cells myo-inositol, either alone or in association with T3 improved cardiac lipid content and function of streptozocin-induced diabetic rats | [101] [102] [13] [103] [104] [105] [106] [107] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benvenga, S.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Camastra, S.; Bonofiglio, D.; Antonelli, A.; Fallahi, P. Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients 2020, 12, 1337. https://doi.org/10.3390/nu12051337
Benvenga S, Ferrari SM, Elia G, Ragusa F, Patrizio A, Paparo SR, Camastra S, Bonofiglio D, Antonelli A, Fallahi P. Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients. 2020; 12(5):1337. https://doi.org/10.3390/nu12051337
Chicago/Turabian StyleBenvenga, Salvatore, Silvia Martina Ferrari, Giusy Elia, Francesca Ragusa, Armando Patrizio, Sabrina Rosaria Paparo, Stefania Camastra, Daniela Bonofiglio, Alessandro Antonelli, and Poupak Fallahi. 2020. "Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies" Nutrients 12, no. 5: 1337. https://doi.org/10.3390/nu12051337
APA StyleBenvenga, S., Ferrari, S. M., Elia, G., Ragusa, F., Patrizio, A., Paparo, S. R., Camastra, S., Bonofiglio, D., Antonelli, A., & Fallahi, P. (2020). Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients, 12(5), 1337. https://doi.org/10.3390/nu12051337






