Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis—Possible Involvement of Clock System
Abstract
1. Introduction
2. Breakfast Skipping and Menstrual Disorders
3. Breakfast Skipping and Other Disorders
4. Past History of Dieting and Dysmenorrhea
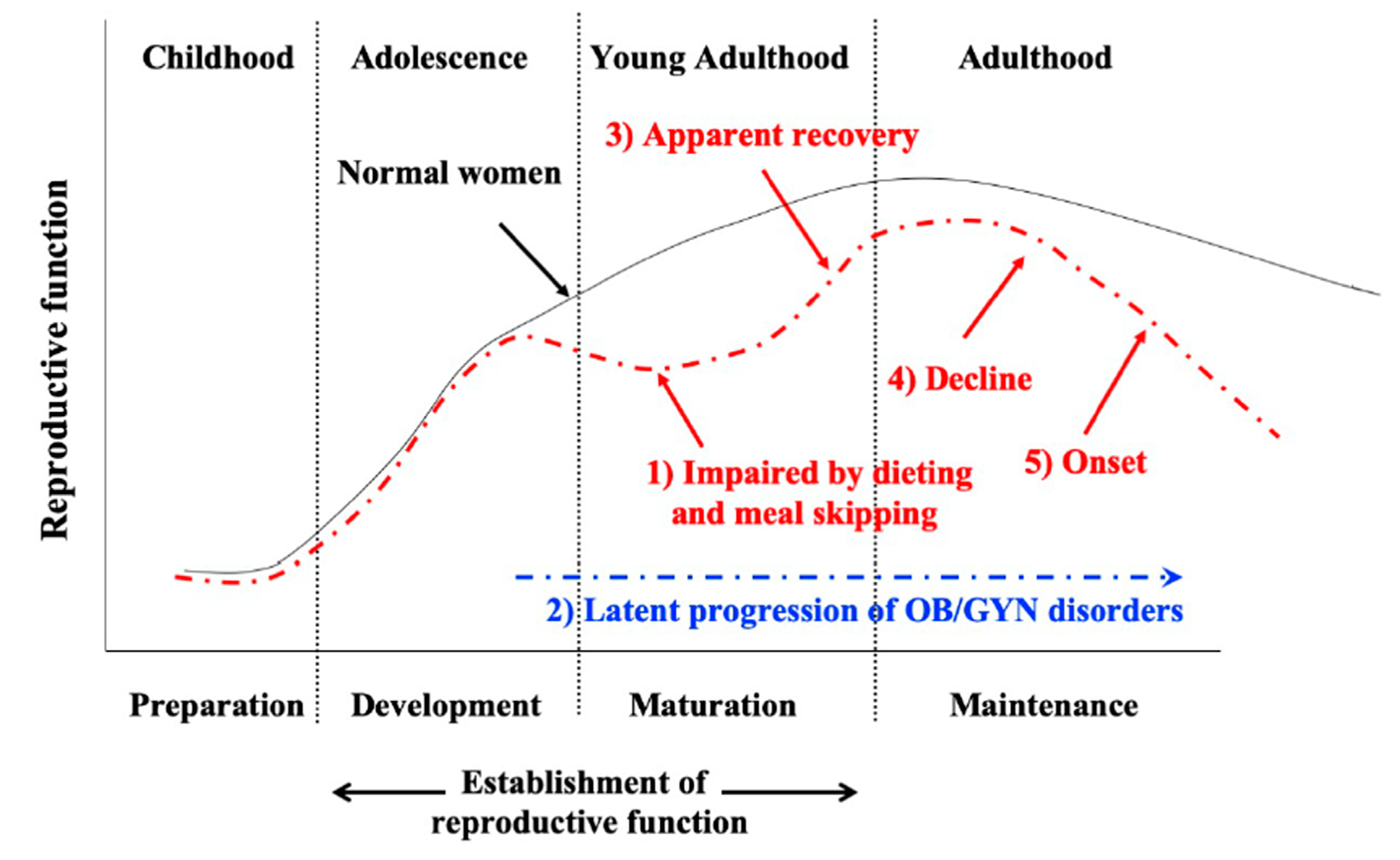
5. Adolescent Dietary Habit-Induced Obstetrics and Gynecologic Disease (ADHOGD)
6. Mechanism of Breakfast Skipping-Induced Dysmenorrhea
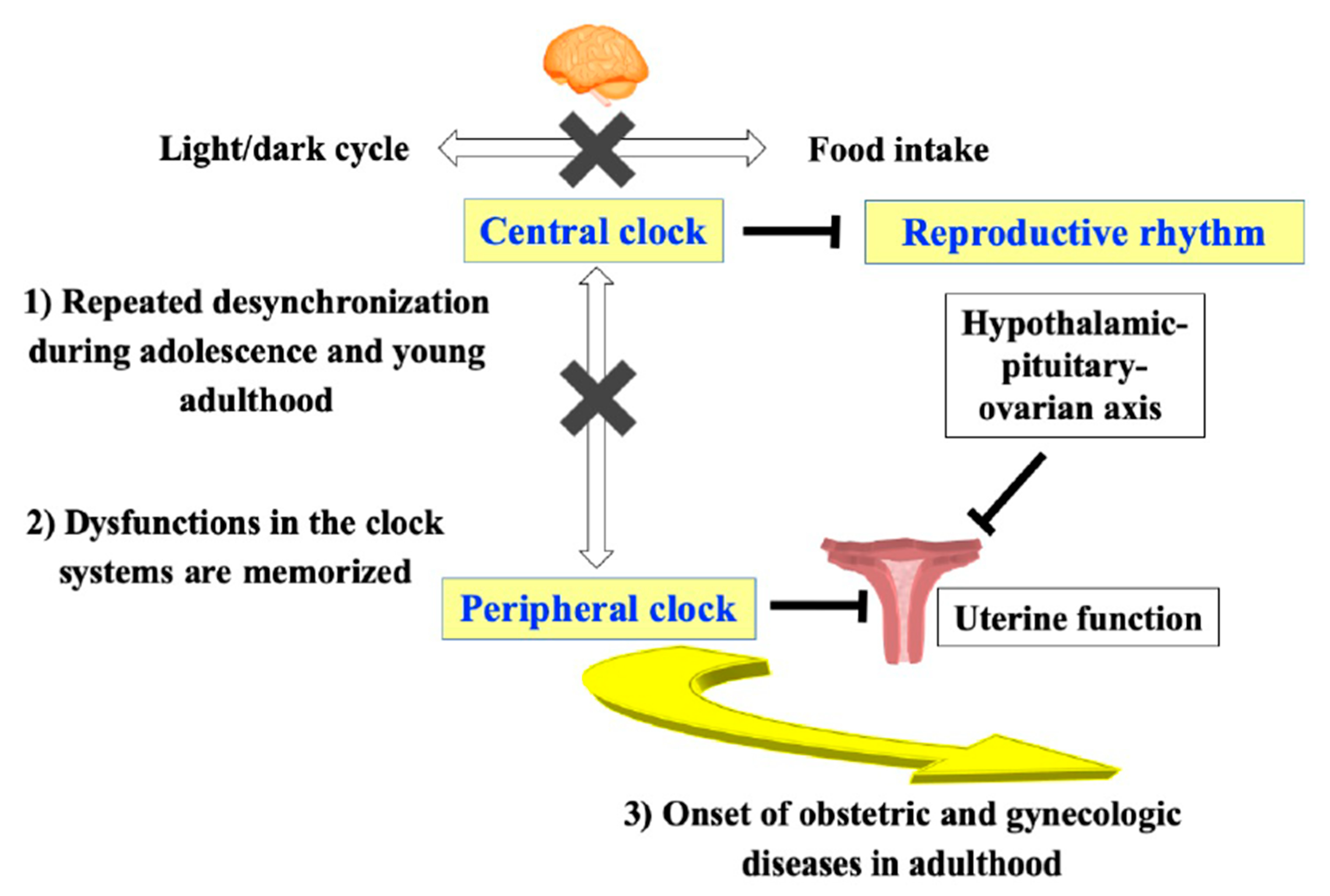
7. Possible Involvement of the Central Clock System in Reproductive Dysfunction
8. Possible Involvement of the Peripheral Clock System in Uterine Dysfunction
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Adolescent dietary habit-induced obstetric and gynecologic disease | ADHOGD |
| Body mass index | BMI |
| Brain and muscle aryl hydrocarbon receptor nuclear translocator like protein 1 | BMAL1 |
| Cryptochrome | CRY2 |
| Developmental origins of health and disease | DOHaD |
| Hypertensive disorders of pregnancy | HDP |
| Magnetic resonance imaging | MRI |
| Periods | Per |
References
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, F.J.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Correlates of meal skipping in young adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Ersig, A.L.; McCarthy, A.M. The influence of peers on diet and exercise among adolescents: A systematic review. J. Pediatr. Nurs. 2017, 36, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T. Diet during adolescence is a trigger for subsequent development of dysmenorrhea in young women. Int. J. Food. Sci. Nutr. 2007, 58, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Speakman, J. Update on human calorie restriction research. Adv. Nutr. 2013, 4, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Dorling, J.L.; Martin, C.K.; Redman, L.M. Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res. Rev. 2020, 101038. [Google Scholar] [CrossRef]
- Nazni, P. Association of western diet & lifestyle with decreased fertility. Indian J. Med. Res. 2014, 140, S78–S81. [Google Scholar]
- Bajalan, Z.; Alimoradi, Z.; Moafi, F. Nutrition as a potential factor of primary dysmenorrhea: A systematic review of observational studies. Gynecol. Obstet. Investig. 2019, 84, 209–224. [Google Scholar] [CrossRef]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 51. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, K.; Luo, L.; Liu, Y.; Liu, X.; Xu, L. Effects of exercise and dietary habits on the occurrence of polycystic ovary syndrome over 5 years of follow-up. Int. J. Gynaecol. Obstet. 2018, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, M.; Caetano, L.; Anastasiadou, N.; Karasu, T.; Lashen, H. Functional hypothalamic amenorrhoea: Leptin treatment, dietary intervention and counselling as alternatives to traditional practice-systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P.; Voussoughian, F.; Geer, E.B.; Hyle, E.P.; Adberg, C.L.; Ramos, R.H. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J. Clin. Endocrinol. Metab. 1999, 84, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Della Torre, S. The deep correlation between energy metabolism and reproduction: A view on the effects of nutrition for women fertility. Nutrients 2016, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Eke, A.C.; Chavarro, J.E.; Missmer, S.A. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T. Skipping breakfast is associated with dysmenorrhea in young women in Japan. Int. J. Food Sci. Nutr. 2003, 54, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nakata, R. Skipping breakfast is associated with reproductive dysfunction in post-adolescent female college students. Appetite 2010, 55, 714–717. [Google Scholar] [CrossRef]
- Wadolowska, L.; Hamulka, J.; Kowalkowska, J.; Ulewicz, N.; Gornicka, M.; Jeruszka-Bielak, M.; Kostecka, M.; Wawrzyniak, A. Skipping breakfast and a meal at school: Its correlates in adiposity context. Report from the ABC of healthy eating study of polish teenagers. Nutrients 2019, 11, 1563. [Google Scholar] [CrossRef]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Holmes, C.J.; Jernigan, T.L.; Toga, A.W. In Vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999, 2, 859–861. [Google Scholar] [CrossRef]
- Zhong, S.; He, Y.; Shu, H.; Gong, G. Developmental changes in topological asymmetry between hemispheric brain white matter networks from adolescence to young adulthood. Cereb. Cortex. 2017, 27, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Angelin, P.; Dileep, D.; Manju, T.; Veena, M.; Pradeep, D.; Amreen, K.; Soumitra, S. Effect of skipping breakfast on young girls’ menstruation. Ind. J. Youth Adol. Health 2017, 4, 17–20. [Google Scholar] [CrossRef]
- Abu Helwa, H.A.; Mitaeb, A.A.; Al-Hamshri, S.; Sweileh, W.M. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Womens Health 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tang, L.; Chen, L.; Kaminga, A.C.; Xu, H. Prevalence and risk factors associated with primary dysmenorrhea among chinese female university students: A cross-sectional study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.; Salmalian, H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran. Red Crescent Med. J. 2014, 16, e16307. [Google Scholar] [CrossRef]
- Gagua, T.; Tkeshelashvili, B.; Gagua, D. Primary dysmenorrhea: Prevalence in adolescent population of Tbilisi, Georgia and risk factors. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 162–168. [Google Scholar] [CrossRef]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A systematic review of the association of skipping breakfast with weight and cardiometabolic risk factors in children and adolescents. What should we better investigate in the future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef]
- Fujiwara, T.; Sato, N.; Awaji, H.; Sakamoto, H.; Nakata, R. Skipping breakfast adversely affects menstrual disorders in young college students. Int. J. Food Sci. Nutr. 2009, 60, 23–31. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.H. Household food insecurity and breakfast skipping: Their association with depressive symptoms. Psychiatry Res. 2019, 271, 83–88. [Google Scholar] [CrossRef]
- Fujiwara, T. Skipping breakfast is associated with constipation in post-adolescent female college students in Japan. In Constipation-Causes, Diagnosis and Treatment; Anthony, G.C.-S., Ed.; InTech Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Kunimoto, M.; Nishi, M.; Sasaki, K. The relation between irregular bowel movement and the lifestyle of working women. Hepatogastroenterology 1998, 45, 956–960. [Google Scholar]
- Sujatha, B.; Velayutham, D.R.; Deivamani, N.; Bavanandam, S. Normal bowel pattern in children and dietary and other precipitating factors in functional constipation. J. Clin. Diagn. Res. 2015, 9, SC12–SC15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Sekine, M.; Tatsuse, T. Lifestyle and bowel movements in school children: Results from the toyama birth cohort study. Pediatr. Int. 2017, 59, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and current therapeutic approaches. Handb. Exp. Pharmacol. 2017, 239, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Gibbins, I.; Patton, V.; Arkwright, J.W.; Lubowski, D.Z.; O’Grady, G.; Bampton, P.A.; Brookes, S.J.; et al. Quantification of In Vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. 2014, 26, 1443–1457. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef]
- Chisuwa-Hayami, N.; Haruki, T. Associations of body-related teasing with weight status, body image, and dieting behavior among Japanese adolescents. Health Promot. Perspect. 2017, 7, 80–87. [Google Scholar] [CrossRef]
- Dei, M.; Seravalli, V.; Bruni, V.; Balzi, D.; Pasqua, A. Predictors of recovery of ovarian function after weight gain in subjects with amenorrhea related to restrictive eating disorders. Gynecol. Endocrinol. 2008, 24, 459–464. [Google Scholar] [CrossRef]
- Montero, P.; Bernis, C.; Fernandez, V.; Castro, S. Influence of body mass index and slimming habits on menstrual pain and cycle irregularity. J. Biosoc. Sci. 1996, 28, 315–323. [Google Scholar] [CrossRef]
- Rodrigues, P.R.M.; Luiz, R.R.; Monteiro, L.S.; Ferreira, M.G.; Goncalves-Silva, R.M.V.; Pereira, R.A. Adolescents’ unhealthy eating habits are associated with meal skipping. Nutrition 2017, 42, 114–120. [Google Scholar] [CrossRef]
- Mandy, M.; Nyirenda, M. Developmental origins of health and disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Spencer, H.G. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.Y. Dysmenorrhoea and prostaglandins: pharmacological and therapeutic considerations. Drugs 1981, 22, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Research 2017, 6, 1645. [Google Scholar] [CrossRef]
- Deligeoroglou, E. Dysmenorrhea. Ann. N. Y. Acad. Sci. 2000, 900, 237–244. [Google Scholar] [CrossRef]
- Lundstrom, V.; Green, K. Endogenous levels of prostaglandin F2alpha and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am. J. Obstet. Gynecol. 1978, 130, 640–646. [Google Scholar] [CrossRef]
- Dawood, M.Y. Dysmenorrhea. Clin. Obstet. Gynecol. 1990, 33, 168–178. [Google Scholar] [CrossRef]
- Morotti, M.; Vincent, K.; Becker, C.M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 8–13. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, E. Endometriosis and female pelvic pain. Semin. Reprod. Med. 2018, 36, 143–151. [Google Scholar] [CrossRef]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Marchetti, C.; Perniola, G.; Pecorini, F.; Benedetti Panici, P. Management of endometriosis from diagnosis to treatment: Roadmap for the future. Minerva Ginecol. 2019, 71, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Flyckt, R. Clinical management of endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Ono, D.; Hasegawa, E.; Okamoto, H.; Honma, K.; Honma, S.; Sakurai, T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 2015, 85, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Sakurai, T. Bmal1 in the nervous system is essential for normal adaptation of circadian locomotor activity and food intake to periodic feeding. J. Neurosci. 2011, 31, 15391–15396. [Google Scholar] [CrossRef]
- Mieda, M. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci. Res. 2019. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakata, R.; Ono, M.; Mieda, M.; Ando, H.; Daikoku, T.; Fujiwara, H. Time restriction of food intake during the circadian cycle is a possible regulator of reproductive function in postadolescent female rats. Curr. Dev. Nutr. 2019, 3, nzy093. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Sen, A.; Sellix, M.T. The circadian timing system and environmental circadian disruption: From follicles to fertility. Endocrinology 2016, 157, 3366–3373. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Quignon, C.; Kriegsfeld, L.J.; Simonneaux, V. Functional implications of RFRP-3 in the central control of daily and seasonal rhythms in reproduction. Front. Endocrinol. 2019, 10, 183. [Google Scholar] [CrossRef]
- Simonneaux, V.; Bahougne, T. A multi-oscillatory circadian system times female reproduction. Front. Endocrinol. 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef] [PubMed]
- Nakao, N.; Yasuo, S.; Nishimura, A.; Yamamura, T.; Watanabe, T.; Anraku, T.; Okano, T.; Fukada, Y.; Sharp, P.J.; Ebihara, S.; et al. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology 2007, 148, 3031–3038. [Google Scholar] [CrossRef]
- Miller, B.H.; Olson, S.L.; Turek, F.W.; Levine, J.E.; Horton, T.H.; Takahashi, J.S. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 2004, 14, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, C.K.; Boehle, K.L.; Muglia, L.J. Impaired steroidogenesis and implantation failure in Bmal1-/- mice. Endocrinology 2009, 150, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Johnson, B.P.; Shen, A.L.; Wallisser, J.A.; Krentz, K.J.; Moran, S.M.; Sullivan, R.; Glover, E.; Parlow, A.F.; Drinkwater, N.R.; et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci. USA 2014, 111, 14295–14300. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Matsumoto, H.; Sato, Y.; Horie, A.; Ono, M.; Nakamura, M.; Mizumoto, Y.; Kagami, K.; Fujiwara, T.; Hattori, A.; et al. Factors regulating human extravillous trophoblast invasion: Chemokine-peptidase and CD9-integrin Systems. Curr. Pharm. Biotechnol. 2018, 19, 764–770. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian clocks: Setting time by food. J. Neuroendocrinol. 2007, 19, 127–137. [Google Scholar] [CrossRef]
- Johnson, M.H.; Lim, A.; Fernando, D.; Day, M.L. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod. Biomed. Online 2002, 4, 140–145. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Moriya, T.; Inoue, S.; Shimazoe, T.; Watanabe, S.; Ebihara, S.; Shinohara, K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J. Neurosci. Res. 2005, 82, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, N.; Ma, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Impaired decidualization caused by downregulation of circadian clock gene BMAL1 contributes to human recurrent miscarriagedagger. Biol. Reprod. 2019, 101, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef]
- Brosens, I.; Benagiano, G. The endometrium from the neonate to the adolescent. J. Matern. Fetal Neonatal Med. 2016, 29, 1195–1199. [Google Scholar] [CrossRef]
- Cramer, S.F.; Oshri, A.; Heller, D.S. A study of myometrial growth and development. J. Pediatr. Adolesc. Gynecol. 2015, 28, 387–394. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, T.; Ono, M.; Mieda, M.; Yoshikawa, H.; Nakata, R.; Daikoku, T.; Sekizuka-Kagami, N.; Maida, Y.; Ando, H.; Fujiwara, H. Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis—Possible Involvement of Clock System. Nutrients 2020, 12, 1294. https://doi.org/10.3390/nu12051294
Fujiwara T, Ono M, Mieda M, Yoshikawa H, Nakata R, Daikoku T, Sekizuka-Kagami N, Maida Y, Ando H, Fujiwara H. Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis—Possible Involvement of Clock System. Nutrients. 2020; 12(5):1294. https://doi.org/10.3390/nu12051294
Chicago/Turabian StyleFujiwara, Tomoko, Masanori Ono, Michihiro Mieda, Hiroaki Yoshikawa, Rieko Nakata, Takiko Daikoku, Naomi Sekizuka-Kagami, Yoshiko Maida, Hitoshi Ando, and Hiroshi Fujiwara. 2020. "Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis—Possible Involvement of Clock System" Nutrients 12, no. 5: 1294. https://doi.org/10.3390/nu12051294
APA StyleFujiwara, T., Ono, M., Mieda, M., Yoshikawa, H., Nakata, R., Daikoku, T., Sekizuka-Kagami, N., Maida, Y., Ando, H., & Fujiwara, H. (2020). Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis—Possible Involvement of Clock System. Nutrients, 12(5), 1294. https://doi.org/10.3390/nu12051294





