Vitamin D’s Effect on Immune Function
Abstract
1. Introduction
2. Vitamin D’s Role in Immune Function: In Vitro Data
2.1. Innate
2.1.1. Monocyte/Macrophage
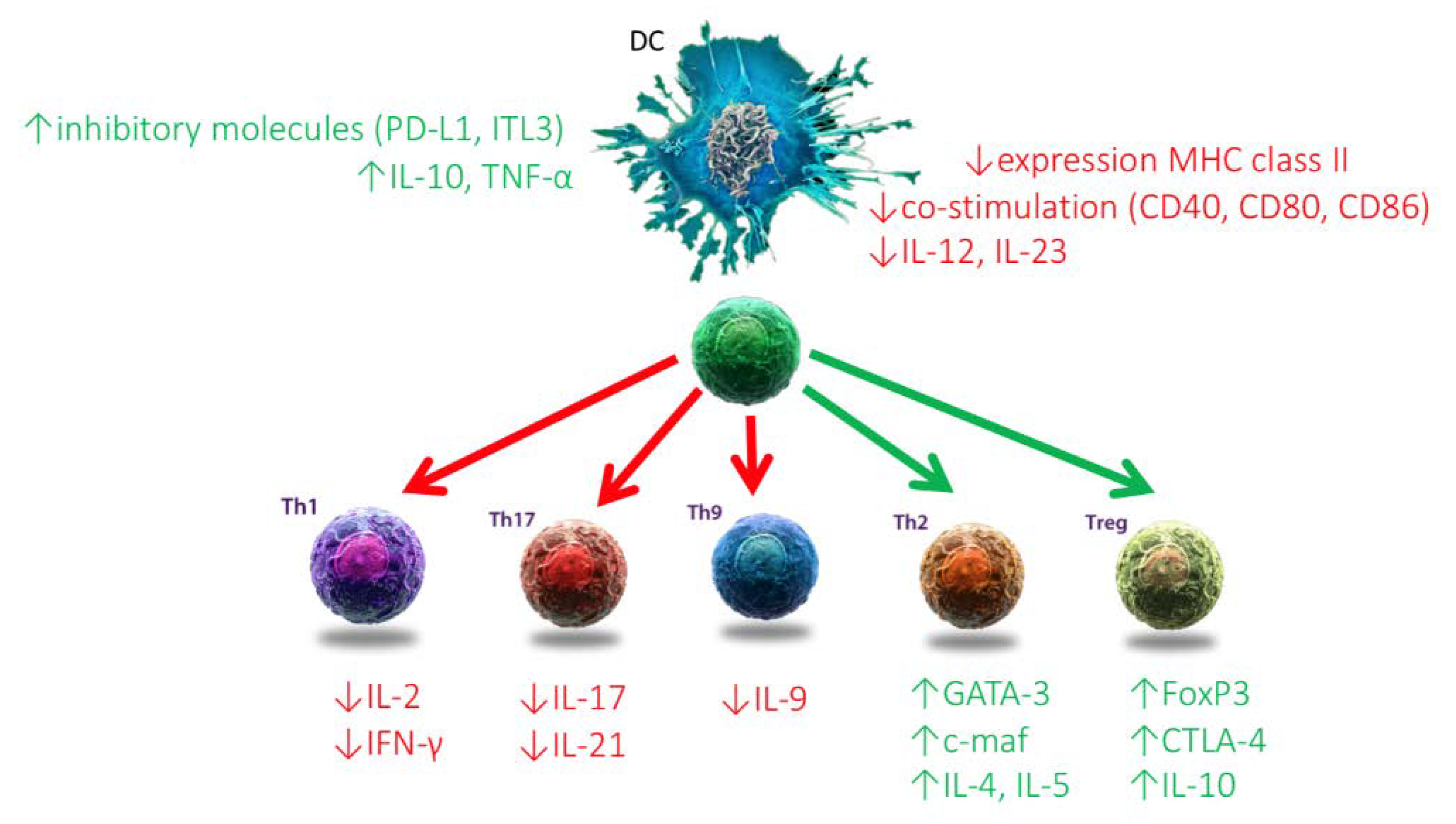
2.1.2. Dendritic Cells
2.1.3. Others
2.2. Adaptive
2.2.1. T-lymphocytes
2.2.2. B-lymphocytes
3. The Impact of Vitamin D Deficiency on the Immune System: In Vitro and In Vivo Data
4. Vitamin D Metabolites as Immune Modulators in Autoimmune Diseases: Animal Models and Human Data
5. Discrepancy between Promising in Vitro Data, Animal Models and Human Intervention Trials
5.1. Dose of Vitamin D Products
5.2. Timing of Intervention and Duration of Exposure
5.3. Relevance of Animal Models for Human Disease
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Nagata, A.; Akagi, Y.; Asano, L.; Kotake, K.; Kawagoe, F.; Mendoza, A.; Masoud, S.S.; Usuda, K.; Yasui, K.; Takemoto, Y.; et al. Synthetic Chemical Probes That Dissect Vitamin D Activities. ACS Chem. Biol. 2019, 14, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Carter, G.D. Toward Clarity in Clinical Vitamin D Status Assessment: 25(OH)D Assay Standardization. Endocrinol. Metab. Clin. N. Am. 2017, 46, 885–899. [Google Scholar] [CrossRef]
- Hollis, B.W. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 271–286. [Google Scholar] [CrossRef]
- Jimenez-Sousa, M.A.; Martinez, I.; Medrano, L.M.; Fernandez-Rodriguez, A.; Resino, S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front. Immunol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Serdar, M.A.; Batu Can, B.; Kilercik, M.; Durer, Z.A.; Aksungar, F.B.; Serteser, M.; Coskun, A.; Ozpinar, A.; Unsal, I. Analysis of Changes in Parathyroid Hormone and 25 (OH) Vitamin D Levels with Respect to Age, Gender and Season: A Data Mining Study. J. Med. Biochem. 2017, 36, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 2009, 124, e362–e370. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Parva, N.R.; Tadepalli, S.; Singh, P.; Qian, A.; Joshi, R.; Kandala, H.; Nookala, V.K.; Cheriyath, P. Prevalence of Vitamin D Deficiency and Associated Risk Factors in the US Population (2011–2012). Cureus 2018, 10, e2741. [Google Scholar] [CrossRef]
- Ganji, V.; Zhang, X.; Tangpricha, V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J. Nutr. 2012, 142, 498–507. [Google Scholar] [CrossRef]
- LeFevre, M.L.; Force, U.S.P.S.T. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2015, 162, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, M.; Kraak, V.; Hulver, M.; Epling, J. Clinical Management of Low Vitamin D: A Scoping Review of Physicians’ Practices. Nutrients 2018, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Ritz, C.; Kiely, M.; Odin, C. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef]
- Takacs, I.; Toth, B.E.; Szekeres, L.; Szabo, B.; Bakos, B.; Lakatos, P. Randomized clinical trial to comparing efficacy of daily, weekly and monthly administration of vitamin D3. Endocrine 2017, 55, 60–65. [Google Scholar] [CrossRef]
- De Niet, S.; Coffiner, M.; Da Silva, S.; Jandrain, B.; Souberbielle, J.C.; Cavalier, E. A Randomized Study to Compare a Monthly to a Daily Administration of Vitamin D (3) Supplementation. Nutrients 2018, 10, 659. [Google Scholar] [CrossRef]
- Giusti, A.; Barone, A.; Pioli, G.; Girasole, G.; Razzano, M.; Pizzonia, M.; Pedrazzoni, M.; Palummeri, E.; Bianchi, G. Heterogeneity in serum 25-hydroxy-vitamin D response to cholecalciferol in elderly women with secondary hyperparathyroidism and vitamin D deficiency. J. Am. Geriatr. Soc. 2010, 58, 1489–1495. [Google Scholar] [CrossRef]
- Mittal, M.; Yadav, V.; Khadgawat, R.; Kumar, M.; Sherwani, P. Efficacy and Safety of 90,000 IU versus 300,000 IU Single Dose Oral Vitamin D in Nutritional Rickets: A Randomized Controlled Trial. Indian J. Endocrinol. Metab. 2018, 22, 760–765. [Google Scholar] [CrossRef]
- Ish-Shalom, S.; Segal, E.; Salganik, T.; Raz, B.; Bromberg, I.L.; Vieth, R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J. Clin. Endocrinol. Metab. 2008, 93, 3430–3435. [Google Scholar] [CrossRef]
- Chel, V.; Wijnhoven, H.A.; Smit, J.H.; Ooms, M.; Lips, P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar] [CrossRef]
- Mittal, H.; Rai, S.; Shah, D.; Madhu, S.V.; Mehrotra, G.; Malhotra, R.K.; Gupta, P. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: A randomized controlled trial. Indian Pediatr. 2014, 51, 265–272. [Google Scholar] [CrossRef]
- Bacon, C.J.; Gamble, G.D.; Horne, A.M.; Scott, M.A.; Reid, I.R. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos. Int. 2009, 20, 1407–1415. [Google Scholar] [CrossRef]
- Schleck, M.L.; Souberbielle, J.C.; Jandrain, B.; Da Silva, S.; De Niet, S.; Vanderbist, F.; Scheen, A.; Cavalier, E. A Randomized, Double-Blind, Parallel Study to Evaluate the Dose-Response of Three Different Vitamin D Treatment Schemes on the 25-Hydroxyvitamin D Serum Concentration in Patients with Vitamin D Deficiency. Nutrients 2015, 7, 5413–5422. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Safety of high dose vitamin D supplementation. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, M.; Li, Y.C. Vitamin D/Vitamin D Receptor Signaling Is Required for Normal Development and Function of Group 3 Innate Lymphoid Cells in the Gut. iScience 2019, 17, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; De Hertogh, G.; Kato, S.; Gysemans, C.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Impact on Experimental Colitis of Vitamin D Receptor Deletion in Intestinal Epithelial or Myeloid Cells. Endocrinology 2017, 158, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T. lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef]
- Chen, K.S.; DeLuca, H.F. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta 1995, 1263, 1–9. [Google Scholar] [CrossRef]
- Vidal, M.; Ramana, C.V.; Dusso, A.S. Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription. Mol. Cell. Biol. 2002, 22, 2777–2787. [Google Scholar] [CrossRef]
- Baeke, F.; Etten, E.V.; Overbergh, L.; Mathieu, C. Vitamin D3 and the immune system: Maintaining the balance in health and disease. Nutr. Res. Rev. 2007, 20, 106–118. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Bouillon, R.; Mathieu, C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: Unravelling the IFNgamma pathway. J. Steroid Biochem. Mol. Biol. 2007, 103, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Liu, P.T.; Schenk, M.; Walker, V.P.; Dempsey, P.W.; Kanchanapoomi, M.; Wheelwright, M.; Vazirnia, A.; Zhang, X.; Steinmeyer, A.; Zugel, U.; et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE 2009, 4, e5810. [Google Scholar]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. 1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J. Steroid Biochem. Mol. Biol. 2016, 159, 94–101. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef]
- Rockett, K.A.; Brookes, R.; Udalova, I.; Vidal, V.; Hill, A.V.; Kwiatkowski, D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 1998, 66, 5314–5321. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Kuo, M.C.; Kuo, H.T.; Hwang, S.J.; Tsai, J.C.; Chen, H.C.; Lai, YH. 1-alpha,25-Dihydroxyvitamin D3 regulates inducible nitric oxide synthase messenger RNA expression and nitric oxide release in macrophage-like RAW 264.7 cells. J. Lab. Clin. Med. 2004, 143, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017, 453, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar] [CrossRef]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Gysemans, C.A.; Demengeot, J.; da Cunha, J.P.; Vanherwegen, A.S.; Overbergh, L.; Van Belle, T.L.; Pauwels, F.; Verstuyf, A.; Korf, H.; et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 2014, 192, 4210–4220. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Zaldumbide, A.; Nikolic, T.; Roep, B.O. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J. Immunol. 2010, 185, 1412–1418. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Hoe, E.; Nathanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection. Nutrients 2016, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bjorklund, G.; Sboarina, A.; Vella, A. The Role of Vitamin D in the Immune System as a Pro-survival Molecule. Clin. Ther. 2017, 39, 894–916. [Google Scholar] [CrossRef]
- Barnado, A.; Crofford, L.J.; Oates, J.C. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 2016, 99, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Agraz-Cibrian, J.M.; Giraldo, D.M.; Urcuqui-Inchima, S. 1,25-Dihydroxyvitamin D3 induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: A pilot study. Steroids 2019, 141, 14–22. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Lee, G.Y.; Park, C.Y.; Cha, K.S.; Lee, S.E.; Pae, M.; Han, S.N. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J. Nutr. Biochem. 2018, 55, 178–184. [Google Scholar] [CrossRef]
- Xu, H.; Soruri, A.; Gieseler, R.K.; Peters, J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- van Halteren, A.G.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Van Belle, T.; Gysemans, C.; Mathieu, C. Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: Relevance for type 1 diabetes. Pediatr. Diabetes 2013, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Ker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Baeke, F.; Gysemans, C.; Korf, H.; Mathieu, C. Vitamin D insufficiency: Implications for the immune system. Pediatr. Nephrol. 2010, 25, 1597–1606. [Google Scholar] [CrossRef]
- Staeva-Vieira, T.P.; Freedman, L.P. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 2002, 168, 1181–1189. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.; Kang, I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Vanherwegen, A.S.; Feyaerts, D.; De Clercq, P.; Verstuyf, A.; Korf, H.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PLoS ONE 2014, 9, e109194. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Geldmeyer-Hilt, K.; Heine, G.; Hartmann, B.; Baumgrass, R.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D3 impairs NF-kappaB activation in human naive B cells. Biochem. Biophys. Res. Commun. 2011, 407, 699–702. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Lang, P.O.; Aspinall, R. Vitamin D Status and the Host Resistance to Infections: What It Is Currently (Not) Understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef]
- Huang, S.J.; Wang, X.H.; Liu, Z.D.; Cao, W.L.; Han, Y.; Ma, A.G.; Xu, S.F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Dev. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Salam, R.A.; Khan, F.R.; Bhutta, Z.A. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst. Rev. 2016, 11, CD008824. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Huang, S. Vitamin D supplementation and risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Scand. J. Infect. Dis. 2013, 45, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Das, R.R.; Singh, M.; Naik, S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst. Rev. 2018, 7, CD011597. [Google Scholar] [CrossRef] [PubMed]
- Slow, S.; Epton, M.; Storer, M.; Thiessen, R.; Lim, S.; Wong, J.; Chin, P.; Tovaranonte, P.; Pearson, J.; Chambers, S.T.; et al. Effect of adjunctive single high-dose vitamin D3 on outcome of community-acquired pneumonia in hospitalised adults: The VIDCAPS randomised controlled trial. Sci. Rep. 2018, 8, 13829. [Google Scholar] [CrossRef]
- Aibana, O.; Huang, C.C.; Aboud, S.; Arnedo-Pena, A.; Becerra, M.C.; Bellido-Blasco, J.B.; Bhosale, R.; Calderon, R.; Chiang, S.; Contreras, C.; et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019, 16, e1002907. [Google Scholar] [CrossRef]
- Balcells, M.E.; Yokobori, N.; Hong, B.Y.; Corbett, J.; Cervantes, J. The lung microbiome, vitamin D, and the tuberculous granuloma: A balance triangle. Microb. Pathog. 2019, 131, 158–163. [Google Scholar] [CrossRef]
- Korf, H.; Decallonne, B.; Mathieu, C. Vitamin D for infections. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 431–436. [Google Scholar] [CrossRef]
- Wu, H.X.; Xiong, X.F.; Zhu, M.; Wei, J.; Zhuo, K.Q.; Cheng, D.Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: A systematic review and meta-analysis. BMC Pulm. Med. 2018, 18, 108. [Google Scholar] [CrossRef]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Ferrari, R.; Caram, L.M.O.; Tanni, S.E.; Godoy, I.; Rupp de Paiva, S.A. The relationship between Vitamin D status and exacerbation in COPD patients—A literature review. Respir. Med. 2018, 139, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Pozzilli, P.; Manfrini, S.; Crino, A.; Picardi, A.; Leomanni, C.; Cherubini, V.; Valente, L.; Khazrai, M.; Visalli, N.; IMDIAB group. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm. Metab. Res. 2005, 37, 680–683. [Google Scholar] [CrossRef]
- Littorin, B.; Blom, P.; Scholin, A.; Arnqvist, H.J.; Blohme, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjornsdottir, S.; Nystrom, L.; et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Cooper, J.D.; Smyth, D.J.; Walker, N.M.; Stevens, H.; Burren, O.S.; Wallace, C.; Greissl, C.; Ramos-Lopez, E.; Hypponen, E.; Dunger, D.B.; et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011, 60, 1624–1631. [Google Scholar] [CrossRef]
- Duan, S.; Lv, Z.; Fan, X.; Wang, L.; Han, F.; Wang, H.; Bi, S. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci. Lett. 2014, 570, 108–113. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum Vitamin D Level and Rheumatoid Arthritis Disease Activity: Review and Meta-Analysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef]
- An, L.; Sun, M.H.; Chen, F.; Li, J.R. Vitamin D levels in systemic sclerosis patients: A meta-analysis. Drug Des. Dev. Ther. 2017, 11, 3119–3125. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, Y.H. Association between Vitamin D level and/or deficiency, and systemic lupus erythematosus: A meta-analysis. Cell. Mol. Biol. 2018, 64, 7–13. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Bro, E.T.; Ho, L.Y.; Singh, R.J.; Jannetto, P.J. Serum 25-hydroxyvitamin D3 levels and flares of systemic lupus erythematosus: A longitudinal cohort analysis. Clin. Rheumatol. 2018, 37, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Munger, K.L.; Koch-Henriksen, N.; Hougaard, D.M.; Magyari, M.; Jorgensen, K.T.; Lundqvist, M.; Simonsen, J.; Jess, T.; Cohen, A.; et al. Neonatal vitamin D status and risk of multiple sclerosis: A population-based case-control study. Neurology 2017, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S., Jr.; Blizzard, L.; Otahal, P.; Van der Mei, I.; Taylor, B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1132–1141. [Google Scholar] [CrossRef]
- Staples, J.A.; Ponsonby, A.L.; Lim, L.L.; McMichael, A.J. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: Latitude, regional ultraviolet radiation, and disease prevalence. Environ. Health Perspect. 2003, 111, 518–523. [Google Scholar] [CrossRef]
- Vieira, V.M.; Hart, J.E.; Webster, T.F.; Weinberg, J.; Puett, R.; Laden, F.; Costenbader, K.H.; Karlson, E.W. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ. Health Perspect. 2010, 118, 957–961. [Google Scholar] [CrossRef]
- Kahn, H.S.; Morgan, T.M.; Case, L.D.; Dabelea, D.; Mayer-Davis, E.J.; Lawrence, J.M.; Marcovina, S.M.; Imperatore, G.; Group SfDiYS. Association of type 1 diabetes with month of birth among U.S. youth: The SEARCH for Diabetes in Youth Study. Diabetes Care 2009, 32, 2010–2015. [Google Scholar] [CrossRef]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008, 51, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Sloka, S.; Grant, M.; Newhook, L.A. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetol. 2010, 47, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, B.N.; Kroehl, M.; Fingerlin, T.E.; Wong, R.; Steck, A.K.; Rewers, M.; Norris, J.M. Association between vitamin D metabolism gene polymorphisms and risk of islet autoimmunity and progression to type 1 diabetes: The diabetes autoimmunity study in the young (DAISY). J. Clin. Endocrinol. Metab. 2013, 98, E1845–E1851. [Google Scholar] [CrossRef] [PubMed]
- Imani, D.; Razi, B.; Motallebnezhad, M.; Rezaei, R. Association between vitamin D receptor (VDR) polymorphisms and the risk of multiple sclerosis (MS): An updated meta-analysis. BMC Neurol. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.Y.; Liu, H.H.; Yin, X.D.; Cao, L.T.; Xu, J.H.; Li, X.M.; Ye, D.Q.; Wang, J. Associations of Vitamin D Receptor Single Nucleotide Polymorphisms with Susceptibility to Systemic Sclerosis. Arch. Med. Res. 2019, 50, 368–376. [Google Scholar] [CrossRef]
- Gisbert-Ferrandiz, L.; Salvador, P.; Ortiz-Masia, D.; Macias-Ceja, D.C.; Orden, S.; Esplugues, J.V.; Calatayud, S.; Hinojosa, J.; Barrachina, M.D.; Hernandez, C. A Single Nucleotide Polymorphism in the Vitamin D Receptor Gene Is Associated with Decreased Levels of the Protein and a Penetrating Pattern in Crohn’s Disease. Inflamm. Bowel. Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef]
- Salimi, S.; Eskandari, F.; Rezaei, M.; Sandoughi, M. Vitamin D Receptor rs2228570 and rs731236 Polymorphisms are Susceptible Factors for Systemic Lupus Erythematosus. Adv. Biomed. Res. 2019, 8, 48. [Google Scholar] [CrossRef]
- Tizaoui, K.; Hamzaoui, K. Association between VDR polymorphisms and rheumatoid arthritis disease: Systematic review and updated meta-analysis of case-control studies. Immunobiology 2015, 220, 807–816. [Google Scholar] [CrossRef]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef]
- Higgins, D.M.; Wischmeyer, P.E.; Queensland, K.M.; Sillau, S.H.; Sufit, A.J.; Heyland, D.K. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J. Parenter. Enteral Nutr. 2012, 36, 713–720. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit. Care Med. 2014, 42, 1365–1371. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Munch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Brown, L.A.S.; Hao, L.; Hebbar, G.; Lee, M. J., Liu S., Ziegler T.R., et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar]
- de Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Wan, Y.D.; Sun, T.W.; Kan, Q.C.; Wang, L.X. Association between vitamin D deficiency and mortality in critically ill adult patients: A meta-analysis of cohort studies. Crit. Care 2014, 18, 684. [Google Scholar] [CrossRef]
- Razavi Khorasani, N.; Moazzami, B.; Zahedi Tajrishi, F.; Mohammadpour, Z.; Rouhi, F.; Alizadeh-Navaei, R.; Ghadimi, R. The Association Between Low Levels of Vitamin D and Clinical Outcomes in Critically-Ill Children: A Systematic Review and Meta-Analysis. Fetal Pediatr. Pathol. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef]
- Overbergh, L.; Decallonne, B.; Waer, M.; Rutgeerts, O.; Valckx, D.; Casteels, K.M.; Laureys, J.; Bouillon, R.; Mathieu, C. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543). Diabetes 2000, 49, 1301–1307. [Google Scholar] [CrossRef]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Gysemans, C.A.; Cardozo, A.K.; Callewaert, H.; Giulietti, A.; Hulshagen, L.; Bouillon, R.; Eizirik, D.L.; Mathieu, C. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 2005, 146, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Mader, J.K.; Hoeller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. High-dose cholecalciferol supplementation significantly increases peripheral CD4(+) Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J. Nutr. 2014, 53, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Treiber, G.; Prietl, B.; Frohlich-Reiterer, E.; Lechner, E.; Ribitsch, A.; Fritsch, M.; Rami-Merhar, B.; Steigleder-Schweiger, C.; Graninger, W.; Borkenstein, M.; et al. Cholecalciferol supplementation improves suppressive capacity of regulatory T-cells in young patients with new-onset type 1 diabetes mellitus—A randomized clinical trial. Clin. Immunol. 2015, 161, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bogdanou, D.; Penna-Martinez, M.; Filmann, N.; Chung, T.L.; Moran-Auth, Y.; Wehrle, J.; Cappel, C.; Huenecke, S.; Herrmann, E.; Koehl, U.; et al. T-lymphocyte and glycemic status after vitamin D treatment in type 1 diabetes: A randomized controlled trial with sequential crossover. Diabetes Metab. Res. Rev. 2017, 33, e2865. [Google Scholar] [CrossRef]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef]
- Di Liberto, D.; Scazzone, C.; La Rocca, G.; Cipriani, P.; Lo Pizzo, M.; Ruscitti, P.; Agnello, L.; Ciaccio, M.; Dieli, F.; Giacomelli, R.; et al. Vitamin D increases the production of IL-10 by regulatory T cells in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 119), 76–81. [Google Scholar]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J.; SOLARIUM study group. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNbeta; the SOLARIUM study. J. Neuroimmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef]
- Brusko, T.; Wasserfall, C.; McGrail, K.; Schatz, R.; Viener, H.L.; Schatz, D.; Haller, M.; Rockell, J.; Gottlieb, P.; Clare-Salzler, M.; et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes 2007, 56, 604–612. [Google Scholar] [CrossRef]
- Marinho, A.; Carvalho, C.; Boleixa, D.; Bettencourt, A.; Leal, B.; Guimaraes, J.; Neves, E.; Oliveira, J.C.; Almeida, I.; Farinha, F.; et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3(+)/IL-17A ratio and clinical course in systemic lupus erythematosus patients: A study in a Portuguese cohort. Immunol. Res. 2017, 65, 197–206. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef]
- 162. Avcioglu, G.; Ozbek Iptec, B.; Akcan, G.; Gorgun, B.; Fidan, K.; Carhan, A.; Yilmaz, G.; Kozaci, L.D. Effects of 1,25-Dihydroxy vitamin D3 on TNF-alpha induced inflammation in human chondrocytes and SW1353 cells: A possible role for toll-like receptors. Mol. Cell. Biochem. 2020, 464, 131–142. [Google Scholar] [CrossRef]
- Luo, J.; Wen, H.; Guo, H.; Cai, Q.; Li, S.; Li, X. 1,25-dihydroxyvitamin D3 inhibits the RANKL pathway and impacts on the production of pathway-associated cytokines in early rheumatoid arthritis. Biomed. Res. Int. 2013, 2013, 101805. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin. Exp. Med. 2014, 14, 275–283. [Google Scholar] [CrossRef]
- Wen, H.Y.; Luo, J.; Li, X.F.; Wei, D.D.; Liu, Y. 1,25-Dihydroxyvitamin D3 modulates T cell differentiation and impacts on the production of cytokines from Chinese Han patients with early rheumatoid arthritis. Immunol. Res. 2019, 67, 48–57. [Google Scholar] [CrossRef]
- Alhassan Mohammed, H.; Mirshafiey, A.; Vahedi, H.; Hemmasi, G.; Moussavi Nasl Khameneh, A.; Parastouei, K.; Saboor-Yaraghi, A.A. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D3 in Patients with Inflammatory Bowel Diseases. Scand. J. Immunol. 2017, 85, 386–394. [Google Scholar] [CrossRef]
- Ashtari, F.; Toghianifar, N.; Zarkesh-Esfahani, S.H.; Mansourian, M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation 2015, 22, 400–404. [Google Scholar] [CrossRef]
- da Costa, D.S.; Hygino, J.; Ferreira, T.B.; Kasahara, T.M.; Barros, P.O.; Monteiro, C.; Oliveira, A.; Tavares, F.; Vasconcelos, C.C.; Alvarenga, R.; et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J. Neuroimmunol. 2016, 299, 8–18. [Google Scholar] [CrossRef]
- Bendix-Struve, M.; Bartels, L.E.; Agnholt, J.; Dige, A.; Jorgensen, S.P.; Dahlerup, J.F. Vitamin D3 treatment of Crohn’s disease patients increases stimulated T cell IL-6 production and proliferation. Aliment. Pharmacol. Ther. 2010, 32, 1364–1372. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Longhi, M.S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018, 103, 38–45. [Google Scholar] [CrossRef]
- Shahin, D.; El-Farahaty, R.M.; Houssen, M.E.; Machaly, S.A.; Sallam, M.; ElSaid, T.O.; Neseem, N.O. Serum 25-OH vitamin D level in treatment-naive systemic lupus erythematosus patients: Relation to disease activity, IL-23 and IL-17. Lupus 2017, 26, 917–926. [Google Scholar] [CrossRef]
- Zerr, P.; Vollath, S.; Palumbo-Zerr, K.; Tomcik, M.; Huang, J.; Distler, A.; Beyer, C.; Dees, C.; Gela, K.; Distler, O.; et al. Vitamin D receptor regulates TGF-beta signalling in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, e20. [Google Scholar] [CrossRef]
- Mauf, S.; Penna-Martinez, M.; Jentzsch, T.; Ackermann, H.; Henrich, D.; Radeke, H.H.; Bruck, P.; Badenhoop, K.; Ramos-Lopez, E. Immunomodulatory effects of 25-hydroxyvitamin D3 on monocytic cell differentiation and influence of vitamin D3 polymorphisms in type 1 diabetes. J. Steroid Biochem. Mol. Biol. 2015, 147, 17–23. [Google Scholar] [CrossRef]
- Gregori, S.; Tomasoni, D.; Pacciani, V.; Scirpoli, M.; Battaglia, M.; Magnani, C.F.; Hauben, E.; Roncarolo, M.G. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 2010, 116, 935–944. [Google Scholar] [CrossRef]
- Nikolic, T.; Woittiez, N.J.C.; van der Slik, A.; Laban, S.; Joosten, A.; Gysemans, C.; Mathieu, C.; Zwaginga, J.J.; Koeleman, B.; Roep, B.O. Differential transcriptome of tolerogenic versus inflammatory dendritic cells points to modulated T1D genetic risk and enriched immune regulation. Genes Immun. 2017, 18, 176–183. [Google Scholar] [CrossRef]
- Wu, H.J.; Lo, Y.; Luk, D.; Lau, C.S.; Lu, L.; Mok, M.Y. Alternatively activated dendritic cells derived from systemic lupus erythematosus patients have tolerogenic phenotype and function. Clin. Immunol. 2015, 156, 43–57. [Google Scholar] [CrossRef][Green Version]
- Baeke, F.; van Etten, E.; Gysemans, C.; Overbergh, L.; Mathieu, C. Vitamin D signaling in immune-mediated disorders: Evolving insights and therapeutic opportunities. Mol. Aspects Med. 2008, 29, 376–387. [Google Scholar] [CrossRef]
- Mathieu, C.; Laureys, J.; Sobis, H.; Vandeputte, M.; Waer, M.; Bouillon, R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992, 41, 1491–1495. [Google Scholar] [CrossRef]
- Giulietti, A.; Gysemans, C.; Stoffels, K.; van Etten, E.; Decallonne, B.; Overbergh, L.; Bouillon, R.; Mathieu, C. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia 2004, 47, 451–462. [Google Scholar] [CrossRef]
- Takiishi, T.; Ding, L.; Baeke, F.; Spagnuolo, I.; Sebastiani, G.; Laureys, J.; Verstuyf, A.; Carmeliet, G.; Dotta, F.; Van Belle, T.L.; et al. Dietary supplementation with high doses of regular vitamin D3 safely reduces diabetes incidence in NOD mice when given early and long term. Diabetes 2014, 63, 2026–2036. [Google Scholar] [CrossRef]
- Niino, M.; Fukazawa, T.; Kikuchi, S.; Sasaki, H. Therapeutic potential of vitamin D for multiple sclerosis. Curr. Med. Chem. 2008, 15, 499–505. [Google Scholar] [CrossRef]
- Correa Freitas, E.; Evelyn Karnopp, T.; de Souza Silva, J.M.; Cavalheiro do Espirito Santo, R.; da Rosa, T.H.; de Oliveira, M.S.; da Costa Goncalves, F.; de Oliveira, F.H.; Guilherme Schaefer, P.; Andre Monticielo, O. Vitamin D supplementation ameliorates arthritis but does not alleviates renal injury in pristane-induced lupus model. Autoimmunity 2019, 52, 69–77. [Google Scholar] [CrossRef]
- Fan, P.; He, L.; Hu, N.; Luo, J.; Zhang, J.; Mo, L.F.; Wang, Y.H.; Pu, D.; Lv, X.H.; Hao, Z.M.; et al. Effect of 1,25-(OH)2D3 on Proliferation of Fibroblast-Like Synoviocytes and Expressions of Pro-Inflammatory Cytokines through Regulating MicroRNA-22 in a Rat Model of Rheumatoid Arthritis. Cell. Physiol. Biochem. 2017, 42, 145–155. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Nguyen, J.K.; Liu, J.; Keller, E.; Campbell, N.; Zhang, C.J.; Smith, H.R.; Li, X.; Jorgensen, T.N. Low Levels of Vitamin D Promote Memory B Cells in Lupus. Nutrients 2020, 12, 291. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, J.; Li, T.; Chen, Y.; June, R.R.; Zheng, S.G. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells Through miR-124 Mediated Inhibition of IL-6 Signaling. Front. Immunol. 2019, 10, 178. [Google Scholar] [CrossRef]
- The EURODIAB Substudy 2 Study Group. Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. Diabetologia 1999, 42, 51–54. [Google Scholar] [CrossRef]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Jasper, E.A.; Nidey, N.L.; Schweizer, M.L.; Ryckman, K.K. Gestational vitamin D and offspring risk of multiple sclerosis: A systematic review and meta-analysis. Ann. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Thorsen, S.U.; Marild, K.; Olsen, S.F.; Holst, K.K.; Tapia, G.; Granstrom, C.; Halldorsson, T.I.; Cohen, A.S.; Haugen, M.; Lundqvist, M.; et al. Lack of Association Between Maternal or Neonatal Vitamin D Status and Risk of Childhood Type 1 Diabetes: A Scandinavian Case-Cohort Study. Am. J. Epidemiol. 2018, 187, 1174–1181. [Google Scholar] [CrossRef]
- Jacobsen, R.; Thorsen, S.U.; Cohen, A.S.; Lundqvist, M.; Frederiksen, P.; Pipper, C.B.; Pociot, F.; Thygesen, L.C.; Ascherio, A.; Svensson, J.; et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: Results from two large Danish population-based studies. Diabetologia 2016, 59, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Zhang, W.G.; Chen, J.J.; Zhang, Z.L.; Han, S.F.; Qin, L.Q. Vitamin D intake and risk of type 1 diabetes: A meta-analysis of observational studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef]
- Silvis, K.; Aronsson, C.A.; Liu, X.; Uusitalo, U.; Yang, J.; Tamura, R.; Lernmark, A.; Rewers, M.; Hagopian, W.; She, J.X.; et al. Maternal dietary supplement use and development of islet autoimmunity in the offspring: TEDDY study. Pediatr. Diabetes 2019, 20, 86–92. [Google Scholar] [CrossRef]
- Stene, L.C.; Joner, G.; Norwegian Childhood Diabetes Study Group. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: A large, population-based, case-control study. Am. J. Clin Nutr. 2003, 78, 1128–1134. [Google Scholar] [CrossRef]
- Brekke, H.K.; Ludvigsson, J. Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatr. Diabetes 2007, 8, 11–14. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Simpson, M.; Brady, H.; Yin, X.; Seifert, J.; Barriga, K.; Hoffman, M.; Bugawan, T.; Baron, A.E.; Sokol, R.J.; Eisenbarth, G.; et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: The Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 2011, 54, 2779–2788. [Google Scholar] [CrossRef]
- Stene, L.C.; Ulriksen, J.; Magnus, P.; Joner, G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia 2000, 43, 1093–1098. [Google Scholar] [CrossRef]
- Marjamaki, L.; Niinisto, S.; Kenward, M.G.; Uusitalo, L.; Uusitalo, U.; Ovaskainen, M.L.; Kronberg-Kippila, C.; Simell, O.; Veijola, R.; Ilonen, J.; et al. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia 2010, 53, 1599–1607. [Google Scholar] [CrossRef]
- Norris, J.M.; Lee, H.S.; Frederiksen, B.; Erlund, I.; Uusitalo, U.; Yang, J.; Lernmark, A.; Simell, O.; Toppari, J.; Rewers, M.; et al. Plasma 25-Hydroxyvitamin D Concentration and Risk of Islet Autoimmunity. Diabetes 2018, 67, 146–154. [Google Scholar] [CrossRef]
- Tapia, G.; Marild, K.; Dahl, S.R.; Lund-Blix, N.A.; Viken, M.K.; Lie, B.A.; Njolstad, P.R.; Joner, G.; Skrivarhaug, T.; Cohen, A.S.; et al. Maternal and Newborn Vitamin D-Binding Protein, Vitamin D Levels, Vitamin D Receptor Genotype, and Childhood Type 1 Diabetes. Diabetes Care 2019, 42, 553–559. [Google Scholar] [CrossRef]
- van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef]
- Moran-Auth, Y.; Penna-Martinez, M.; Badenhoop, K. VDR FokI polymorphism is associated with a reduced T-helper cell population under vitamin D stimulation in type 1 diabetes patients. J. Steroid Biochem. Mol. Biol. 2015, 148, 184–186. [Google Scholar] [CrossRef]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef]
- Becklund, B.R.; Hansen, D.W., Jr.; Deluca, H.F. Enhancement of 1,25-dihydroxyvitamin D3-mediated suppression of experimental autoimmune encephalomyelitis by calcitonin. Proc. Natl. Acad. Sci. USA 2009, 106, 5276–5281. [Google Scholar] [CrossRef]
- Malihi, Z.; Wu, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Mallya, S.M.; Corrado, K.R.; Saria, E.A.; Yuan, F.F.; Tran, H.Q.; Saucier, K.; Atti, E.; Tetradis, S.; Arnold, A. Modeling vitamin D insufficiency and moderate deficiency in adult mice via dietary cholecalciferol restriction. Endocr. Res. 2016, 41, 290–299. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Matsui, I.; Hamano, T.; Shimomura, A.; Mori, D.; Yonemoto, S.; Takabatake, Y.; Tsubakihara, Y.; St-Arnaud, R.; Isaka, Y.; et al. Excess 25-hydroxyvitamin D3 exacerbates tubulointerstitial injury in mice by modulating macrophage phenotype. Kidney Int. 2015, 88, 1013–1029. [Google Scholar] [CrossRef]
- Tabasi, N.; Rastin, M.; Mahmoudi, M.; Ghoryani, M.; Mirfeizi, Z.; Rabe, S.Z.; Reihani, H. Influence of vitamin D on cell cycle, apoptosis, and some apoptosis related molecules in systemic lupus erythematosus. Iran. J. Basic Med. Sci. 2015, 18, 1107–1111. [Google Scholar]
- Chen, L.; Eapen, M.S.; Zosky, G.R. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci. Rep. 2017, 7, 45172. [Google Scholar] [CrossRef]
- Baeke, F.; Van Belle, T.L.; Takiishi, T.; Ding, L.; Korf, H.; Laureys, J.; Gysemans, C.; Mathieu, C. Low doses of anti-CD3, ciclosporin A and the vitamin D analogue, TX527, synergise to delay recurrence of autoimmune diabetes in an islet-transplanted NOD mouse model of diabetes. Diabetologia 2012, 55, 2723–2732. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. https://doi.org/10.3390/nu12051248
Martens P-J, Gysemans C, Verstuyf A, Mathieu C. Vitamin D’s Effect on Immune Function. Nutrients. 2020; 12(5):1248. https://doi.org/10.3390/nu12051248
Chicago/Turabian StyleMartens, Pieter-Jan, Conny Gysemans, Annemieke Verstuyf, and Chantal Mathieu. 2020. "Vitamin D’s Effect on Immune Function" Nutrients 12, no. 5: 1248. https://doi.org/10.3390/nu12051248
APA StyleMartens, P.-J., Gysemans, C., Verstuyf, A., & Mathieu, C. (2020). Vitamin D’s Effect on Immune Function. Nutrients, 12(5), 1248. https://doi.org/10.3390/nu12051248




