The Effects of Vitamin D Supplementation on Lipid and Inflammatory Profile of Healthy Adolescent Boys: A Randomized Controlled Trial
Abstract
1. Introduction
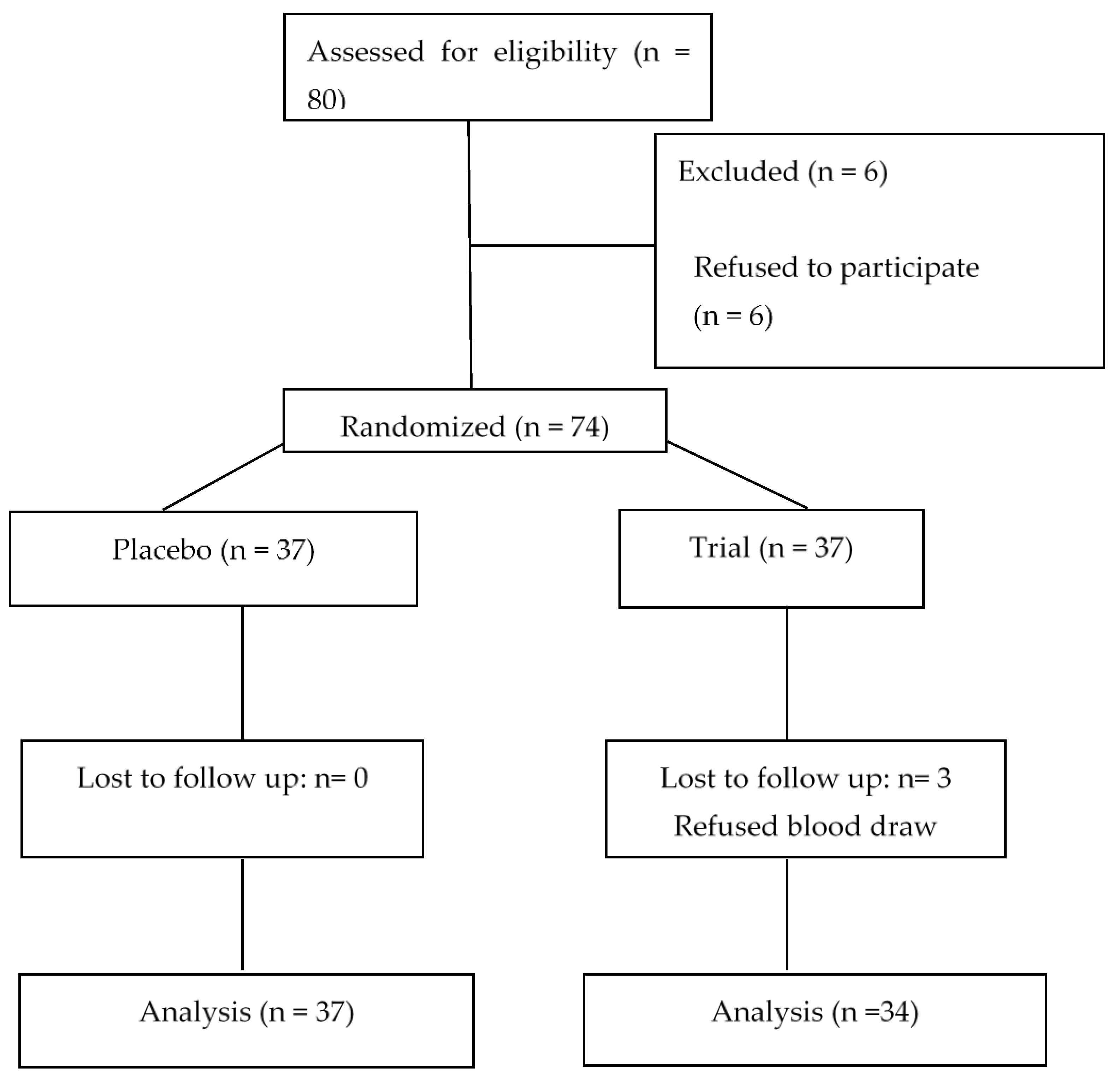
2. Subjects and Methods
3. Laboratory Tests
4. Statistical Analyses
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington DC, USA, 2011. [Google Scholar]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef]
- Rawal, G.; Yadav, S.; Shokeen, P. Health and the vitamin D. Int. J. Health Sci. Res. 2015, 5, 416–423. [Google Scholar]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Green, T.; Skeaff, C.; Rockell, J.; Venn, B.; Lambert, A.; Todd, J.; Khor, G.; Loh, S.; Muslimatun, S.; Agustina, R. Vitamin D status and its association with parathyroid hormone concentrations in women of child-bearing age living in Jakarta and Kuala Lumpur. Eur. J. Clin. Nutr. 2008, 62, 373. [Google Scholar] [CrossRef]
- Al-Sadat, N.; Majid, H.A.; Sim, P.Y.; Su, T.T.; Dahlui, M.; Bakar, M.F.A.; Dzaki, N.; Norbaya, S.; Murray, L.; Cantwell, M.M. Vitamin D deficiency in Malaysian adolescents aged 13 years: Findings from the Malaysian Health and Adolescents Longitudinal Research Team study (MyHeARTs). BMJ Open 2016, 6, e010689. [Google Scholar] [CrossRef]
- Khalaji, N.; Asadzadeh, S.; Neyestani, T.; Hajifaraji, M.; Omidvar, N.; Shariatzadeh, N.; Kalayi, A.; Nikooyeh, B.; Mohammadi, M. High prevalence of vitamin D deficiency in school age children in Tehran, 2008: A red alert. Iran. J. Nutr. Sci. Food Technol. 2013, 7, 389–398. [Google Scholar]
- Ataie-Jafari, A.; Qorbani, M.; Heshmat, R.; Ardalan, G.; Motlagh, M.E.; Asayesh, H.; Arzaghi, S.M.; Tajadini, M.H.; Nejatinamini, S.; Poursafa, P. The association of vitamin D deficiency with psychiatric distress and violence behaviors in Iranian adolescents: The CASPIAN-III study. J. Diabetes Metab. Disord. 2015, 14, 62. [Google Scholar] [CrossRef][Green Version]
- Pouraram, H.; Djazayery, A.; Mohammad, K.; Parsaeian, M.; Abdollahi, Z.; Motlagh, A.D.; Djalali, M.; Khodaverdian, K.; Sotoudeh, G.; Yarparvar, A. Second National Integrated Micronutrient Survey in Iran: Study Design and Preliminary Findings. Arch. Iran. Med. 2018, 21, 137. [Google Scholar]
- Chaudhuri, J.R.; Mridula, K.R.; Anamika, A.; Boddu, D.B.; Misra, P.K.; Lingaiah, A.; Balaraju, B.; Bandaru, V.S. Deficiency of 25-hydroxyvitamin d and dyslipidemia in Indian subjects. J. Lipids 2013, 2013, 623420. [Google Scholar] [CrossRef]
- De Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M. Relationship between vitamin D and inflammatory markers in older individuals. Age 2014, 36, 9694. [Google Scholar] [CrossRef]
- Yarparvar, A.; Elmadfa, I.; Djazayery, A.; Abdollahi, Z.; Salehi, F. The association of vitamin d status with lipid profile and inflammation biomarkers in healthy adolescents. Nutrients 2020, 12, 590. [Google Scholar] [CrossRef]
- Hirschler, V.; Maccallini, G.; Sanchez, M.S.; Castaño, L.; Molinari, C.; Group, S.A.d.l.C.S. Improvement in high-density lipoprotein cholesterol levels in argentine Indian school children after vitamin D supplementation. Horm. Res. Paediatr. 2013, 80, 335–342. [Google Scholar] [CrossRef]
- Feroze, U.; Molnar, M.Z.; Dukkipati, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Insights into nutritional and inflammatory aspects of low parathyroid hormone in dialysis patients. J. Ren. Nutr. 2011, 21, 100–104. [Google Scholar] [CrossRef][Green Version]
- Reid, I.R. Effects of calcium supplementation on circulating lipids. Drugs Aging 2004, 21, 7–17. [Google Scholar] [CrossRef]
- Kelishadi, R.; Ardalan, G.; Motlagh, M.E.; Shariatinejad, K.; Heshmat, R.; Poursafa, P.; Fakhri, M.; Tajadini, M.; Taslimi, M. National report on the association of serum vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): The CASPIAN-III Study. Nutrition 2014, 30, 33–38. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Mirmoosavi, S.J.; Fazeli, M.; Abasalti, Z.; Avan, A.; Javandoost, A.; Rahmani, F.; Tayefi, M.; Hanachi, P.; Ferns, G.A. High-dose vitamin D supplementation is associated with an improvement in several cardio-metabolic risk factors in adolescent girls: A nine-week follow-up study. Ann. Clin. Biochem. 2018, 55, 227–235. [Google Scholar] [CrossRef]
- Tavakoli, F.; Namakin, K.; Zardast, M. Vitamin D supplementation and high-density lipoprotein cholesterol: A study in healthy school children. Iran. J. Pediatrics 2016, 26. [Google Scholar] [CrossRef]
- Muldowney, S.; Kiely, M. Vitamin D and cardiometabolic health: A review of the evidence. Nutr. Res. Rev. 2011, 24, 1–20. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011, 128 (Suppl. 5), S213–S256. [CrossRef]
- Jiang, H.-M.; Yan, Z.-P.; Zhao, Y.; Hu, X.; Lian, H.-Z. Zincon-immobilized silica-coated magnetic Fe3O4 nanoparticles for solid-phase extraction and determination of trace lead in natural and drinking waters by graphite furnace atomic absorption spectrometry. Talanta 2012, 94, 251–256. [Google Scholar] [CrossRef]
- Plichta, S.B.; Kelvin, E.A.; Munro, B.H. Munro’s Statistical Methods for Health Care Research; Wolters Kluwer Health/Lippincott Williams & Wilkins: London, UK, 2012. [Google Scholar]
- Morris, M.C.; Tangney, C.C. A potential design flaw of randomized trials of vitamin supplements. JAMA 2011, 305, 1348–1349. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef]
- Amer, M.; Qayyum, R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006). Am. J. Cardiol. 2012, 109, 226–230. [Google Scholar] [CrossRef]
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 774–781. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Derm. Endocrinol. 2014, 6, e983401. [Google Scholar] [CrossRef]
- Henriksen, V.T.; Rogers, V.E.; Rasmussen, G.L.; Trawick, R.H.; Momberger, N.G.; Aguirre, D.; Barker, T. Pro-inflammatory cytokines mediate the decrease in serum 25(OH)D concentrations after total knee arthroplasty? Med. Hypotheses 2014, 82, 134–137. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Z.; Ma, Q.; Wu, Z.; Fan, P.; Zhou, X.; Chen, L.; Zhou, S.; Goltzman, D.; Miao, D.; et al. 1, 25(OH)(2)D(3) inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr. Med. Chem. 2013, 20, 4131–4141. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Padilla, N.; Alvarez, A.; Linetsky, E.; Lanzoni, G.; Mattina, A.; Bertuzzi, F.; Fabbri, A.; Baidal, D.; et al. The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation. Nutrients 2019, 11, 2937. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Medler, J.; Wajant, H. Tumor necrosis factor receptor-2 (TNFR2): An overview of an emerging drug target. Expert Opin. Ther. Targets 2019, 23, 295–307. [Google Scholar] [CrossRef]
- Wang, Y.; Si, S.; Liu, J.; Wang, Z.; Jia, H.; Feng, K.; Sun, L.; Song, S.J. The Associations of Serum Lipids with Vitamin D Status. PLoS ONE 2016, 11, e0165157. [Google Scholar] [CrossRef]
- Nouri Saeidlou, S.; Vahabzadeh, D.; Babaei, F.; Vahabzadeh, Z. Seasonal variations of vitamin D and its relation to lipid profile in Iranian children and adults. J. Health Popul. Nutr. 2017, 36, 21. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Taheri, E.; Djalali, M.; Moghadam, A.M.; Qorbani, M. Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. J. Diabetes Metab. Disord. 2014, 13, 7. [Google Scholar] [CrossRef]
- Wang, J.H.; Keisala, T.; Solakivi, T.; Minasyan, A.; Kalueff, A.V.; Tuohimaa, P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J. Steroid Biochem. Mol. Biol. 2009, 113, 222–226. [Google Scholar] [CrossRef]
- Christensen, R.; Lorenzen, J.K.; Svith, C.R.; Bartels, E.M.; Melanson, E.L.; Saris, W.H.; Tremblay, A.; Astrup, A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: A meta-analysis of randomized controlled trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 475–486. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef]
- Ni, Z.; Smogorzewski, M.; Massry, S.G. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology 1994, 135, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Hovsepian, S.; Kelishadi, R.; Djalalinia, S.; Farzadfar, F.; Naderimagham, S.; Qorbani, M. Prevalence of dyslipidemia in Iranian children and adolescents: A systematic review. J. Res. Med Sci. Off. J. Isfahan Univ. Med Sci. 2015, 20, 503–521. [Google Scholar] [CrossRef]
| Variable | The Study Groups | p-Value | |||
|---|---|---|---|---|---|
| Placebo (n = 37) | Vitamin D (n = 34) | ||||
| Anthropometric variables | Height (cm) | 163.22 ± 2.97 | 165.01 ± 3.05 | 0.167 * | |
| Weight (kg) | 64.02 ± 4.97 | 63.21 ± 5.11 | 0.394 * | ||
| BMI (kg/m2) | 23.43.00 ± 1.64 | 22.45 ± 1.45 | 0.680 * | ||
| BMI | Normal | 33 (89.18) | 28 (82.35) | 0.402 ** | |
| Over-weight | 4 (10.81) | 6 (17.64) | |||
| Variable | The Study Groups | MMD | p-Value ** | ||
|---|---|---|---|---|---|
| Placebo (n = 37) | Vitamin D (n = 34) | ||||
| Energy (kcal) | Before | 1896.76 ± 151.35 | 1920.24 ± 194.77 | 24.66 | 0.535 |
| After | 1963.35 ± 160.97 | 1989.62 ± 169.90 | |||
| PC | 4.26 | 4.40 | |||
| p-value * | 0.098 | 0.078 | |||
| CHO (gr) | Before | 241.04 ± 37.33 | 251.29 ± 46.99 | 11.94 | 0.339 |
| After | 251.22 ± 40.19 | 256.93 ± 63.62 | |||
| PC | 6.69 | 8.34 | |||
| p-value * | 0.262 | 0.201 | |||
| Fat (gr) | Before | 79.67 ± 14.42 | 79.69 ± 19.20 | −4.89 | 0.264 |
| After | 83.96 ± 16.41 | 79.06 ± 20.33 | |||
| PC | 9.46 | 7.10 | |||
| p-value * | 0.236 | 0.908 | |||
| Protein (gr) | Before | 59.27 ± 12.36 | 57.27 ± 13.17 | 4.07 | 0.284 |
| After | 57.62 ± 15.61 | 61.80 ± 15.88 | |||
| PC | 2.43 | 12.26 | |||
| p-value * | 0.646 | 0.183 | |||
| Vitamin D (µg) | Before | 0.35 ± 0.24 | 0.36 ± 0.24 | −0.59 | 0.366 |
| After | 0.46 ± 0.27 | 0.40 ± 0.27 | |||
| PC | 136.70 | 99.48 | |||
| p-value * | 0.071 | 0.531 | |||
| Vitamin A (µg) | Before | 615.73 ± 556.07 | 625.55 ± 473.41 | −105.30 | 0.267 |
| After | 560.77 ± 450.42 | 456.49 ± 329.51 | |||
| PC | 97.42 | 2.48 | |||
| p-value * | 0.610 | 0.087 | |||
| Variable | The Study Groups | MMD | p-Value ** | ||
|---|---|---|---|---|---|
| Placebo (n = 37) | Vitamin D (n = 34) | ||||
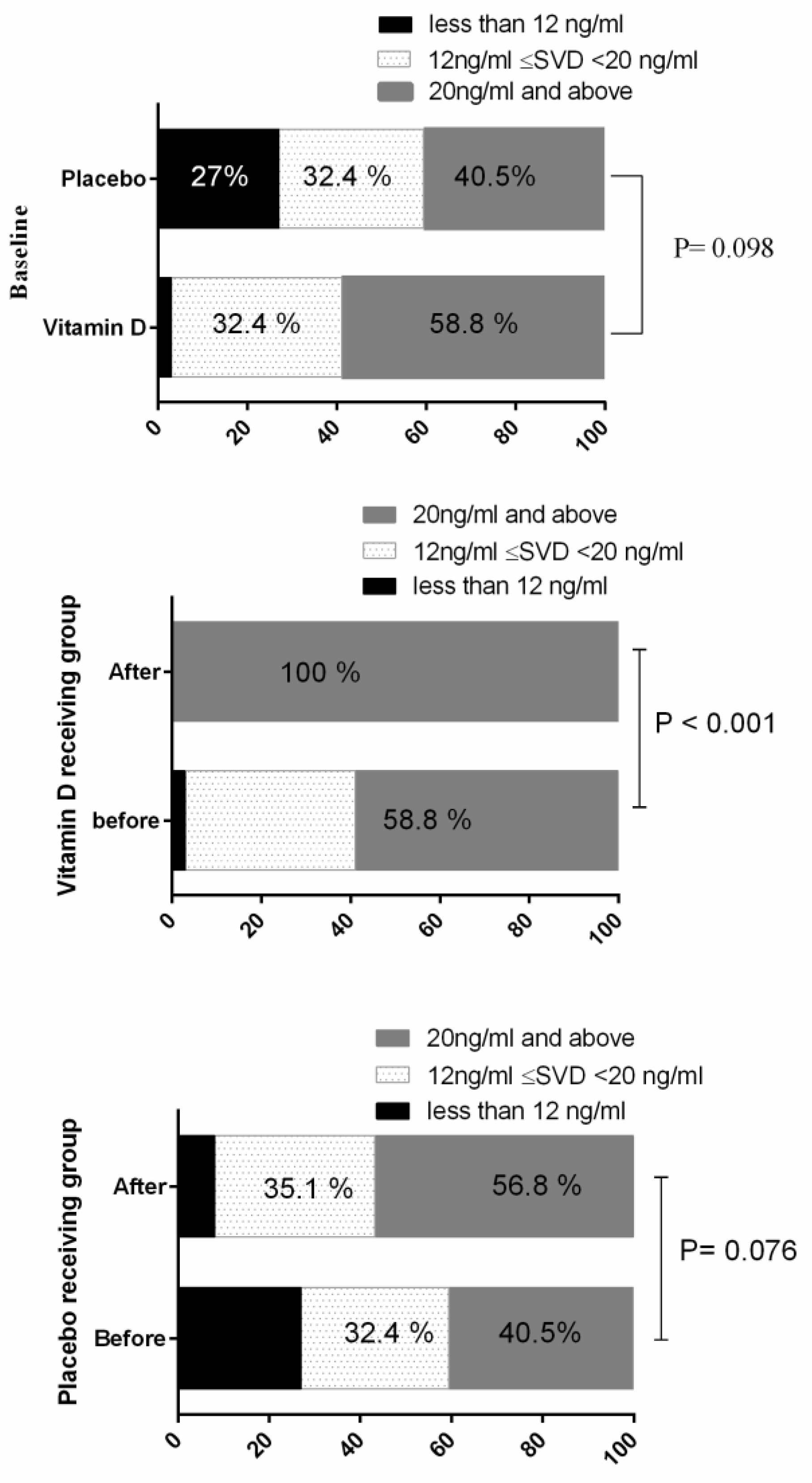
| Vitamin D (ng/mL) | Before | 22.15 ± 12.33 | 24.16 ± 10.16 | 17.08 | <0.001 |
| After | 23.45 ± 10.10 | 41.65 ± 6.82 | |||
| PC | 16.51 | 97.66 | |||
| p-value * | 0.275 | <0.001 | |||
| Vitamin A (mg/l) | Before | 0.485 ± 0.099 | 0.464 ± 0.072 | −0.006 | 0.731 |
| After | 0.484 ± 0.069 | 0.470 ± 0.081 | |||
| PC | 2.78 | 2.45 | |||
| p-value * | 0.913 | 0.677 | |||
| PB (µg/dL) | Before | 1.54 ± 1.77 | 1.25 ± 1.24 | 0.012 | 0.953 |
| After | 1.17 ± 0.746 | 1.13 ± 0.995 | |||
| PC | 90.25 | 114.52 | |||
| p-value * | 0.133 | 0.661 | |||
| PTH (pg/mL) | Before | 25.44 ± 7.42 | 26.80 ± 7.94 | −5.47 | 0.003 |
| After | 28.59 ± 9.53 | 23.43 ± 4.91 | |||
| PC | 20.70 | −8.37 | |||
| p-value * | 0.111 | 0.004 | |||
| Calcium(mg/dl) | Before | 10.80 ± 0.380 | 10.74 ± 0.400 | −0.021 | 0.788 |
| After | 10.63 ± 0.358 | 10.59 ± 0.314 | |||
| PC | −1.47 | −1.26 | |||
| p-value * | 0.017 | 0.054 | |||
| Albumin (g/dl) *** | Before | 5.15 ± 0.308 | 5.22 ± 0.322 | −0.001 | 0.992 |
| After | 5.26 ± 0.314 | 5.29 ± 0.297 | |||
| PC | 2.17 | 1.40 | |||
| p-value * | 0.071 | 0.265 | |||
| Variable | The Study Groups | MMD | p-Value ** | ||
|---|---|---|---|---|---|
| Placebo (n = 37) | Vitamin D (n = 34) | ||||
| VLDL (mg/dl) | Before | 25.67 ± 12.28 | 22.94 ± 9.28 | −4.75 | 0.095 |
| After | 29.00 ± 15.33 | 23.02 ± 8.85 | |||
| PC | 18.83 | 13.81 | |||
| p-value * | 0.154 | 0.969 | |||
| LDL (mg/dl) | Before | 87.83 ± 19.08 | 84.97 ± 17.62 | −5.17 | 0.092 |
| After | 86.86 ± 20.17 | 79.44 ± 17.92 | |||
| PC | 0.06 | −6.24 | |||
| p-value * | 0.707 | 0.003 | |||
| TG (mg/dl) | Before | 130.67 ± 60.21 | 115.26 ± 45.89 | −30.31 | 0.001 |
| After | 136.34 ± 54.67 | 99.26 ± 29.30 | |||
| PC | 9.52 | −1.02 | |||
| p-value * | 0.383 | 0.108 | |||
| HDL (mg/dl) | Before | 43.48 ± 7.79 | 44.32 ± 8.75 | 2.93 | 0.021 |
| After | 42.10 ± 5.89 | 45.58 ± 8.85 | |||
| PC | −2.06 | 4.34 | |||
| p-value * | 0.116 | 0.282 | |||
| TC (mg/dl) | Before | 169.62 ± 32.17 | 164.08 ± 23.31 | −6.32 | 0.213 |
| After | 163.78 ± 32.14 | 153.47 ± 25.68 | |||
| PC | −2.39 | −6.18 | |||
| p-value * | 0.171 | 0.002 | |||
| The Study Groups | MMD | p-Value ** | |||
|---|---|---|---|---|---|
| Placebo (n = 37) | Vitamin D (n = 34) | ||||
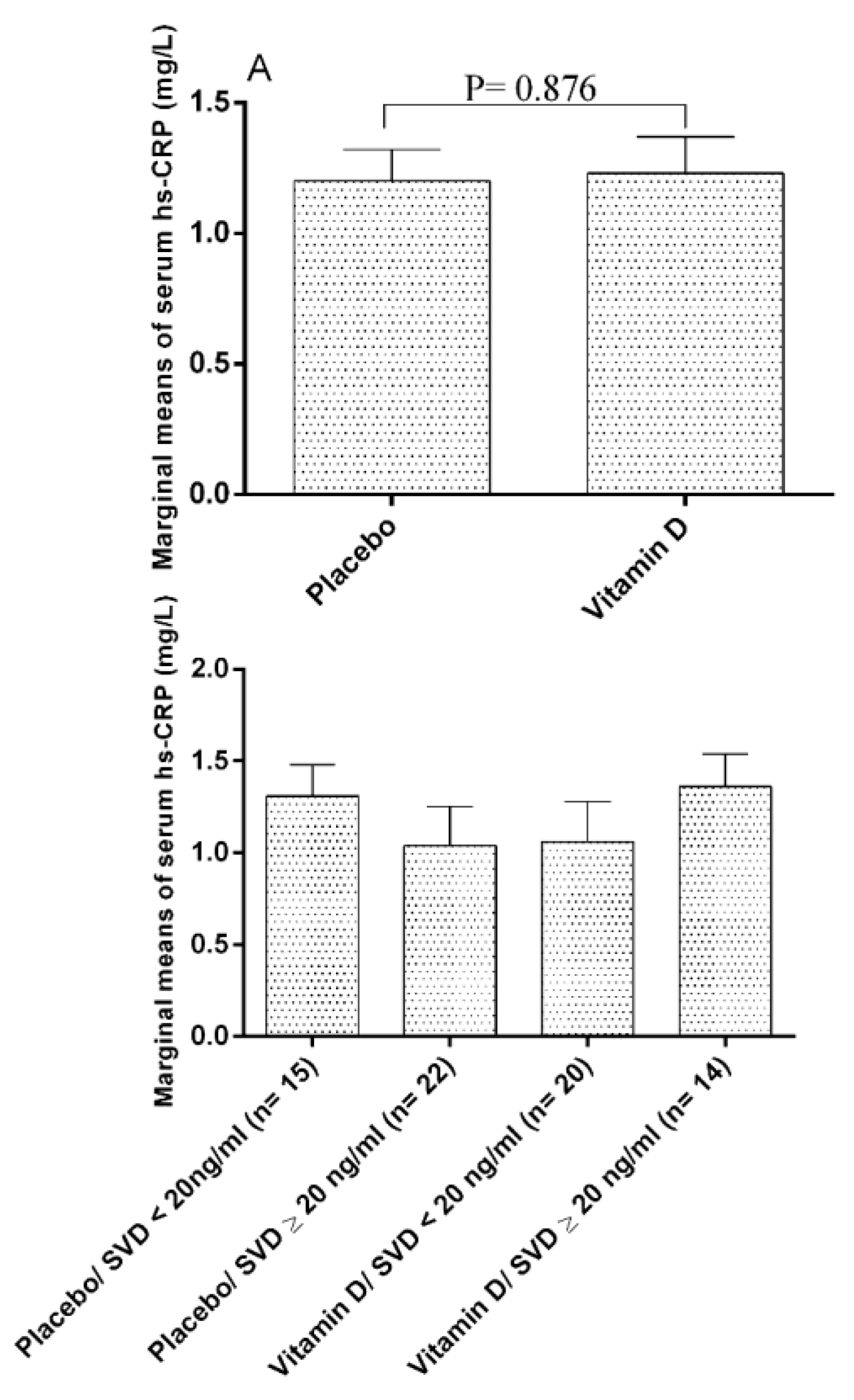
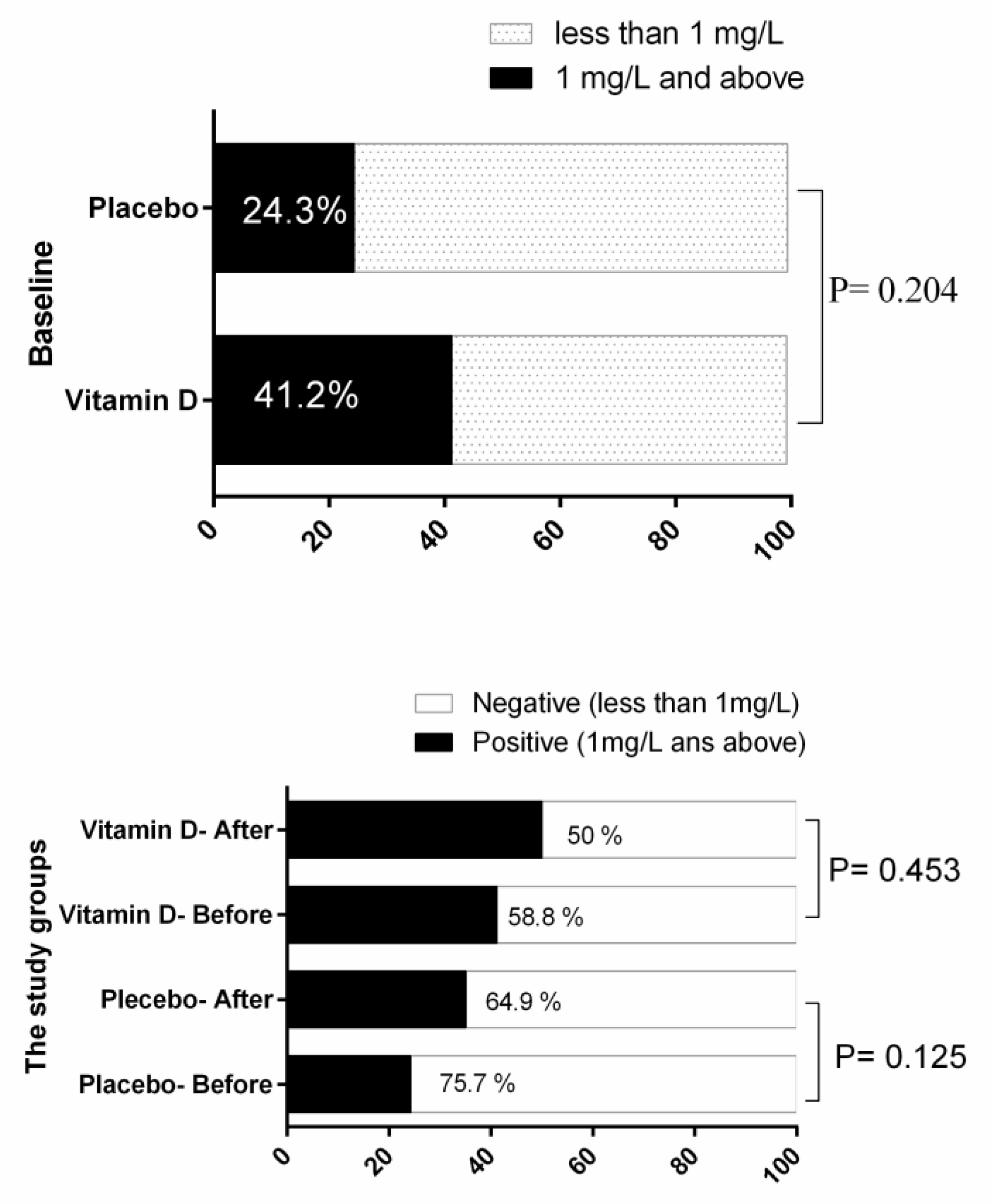
| IL−6 (ng/L) | Before | 129.40 ± 49.60 | 118.29 ± 40.19 | −10.44 | 0.083 |
| After | 133.64 ± 63.04 | 112.30 ± 32.75 | |||
| PC | 4.22 | −3.34 | |||
| p-value * | 0.425 | 0.008 | |||
| IL−10 (ng/L) | Before | 111.11 ± 26.84 | 118.85 ± 26.83 | 6.56 | 0.001 |
| After | 109.46 ± 31.78 | 124.22 ± 26.62 | |||
| PC | −2.22 | 4.75 | |||
| p-value * | 0.332 | <0.001 | |||
| TNFR−2 (ng/L) | Before | 18.37 ± 1.33 | 19.36 ± 5.21 | −1.64 | 0.019 |
| After | 16.78 ± 1.63 | 15.44 ± 4.07 | |||
| PC | −8.01 | −17.38 | |||
| p-value * | <0.001 | <0.001 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarparvar, A.; Elmadfa, I.; Djazayery, A.; Abdollahi, Z.; Salehi, F.; Heshmat, R. The Effects of Vitamin D Supplementation on Lipid and Inflammatory Profile of Healthy Adolescent Boys: A Randomized Controlled Trial. Nutrients 2020, 12, 1213. https://doi.org/10.3390/nu12051213
Yarparvar A, Elmadfa I, Djazayery A, Abdollahi Z, Salehi F, Heshmat R. The Effects of Vitamin D Supplementation on Lipid and Inflammatory Profile of Healthy Adolescent Boys: A Randomized Controlled Trial. Nutrients. 2020; 12(5):1213. https://doi.org/10.3390/nu12051213
Chicago/Turabian StyleYarparvar, Amirhossein, Ibrahim Elmadfa, Abolghasem Djazayery, Zahra Abdollahi, Forouzan Salehi, and Ramin Heshmat. 2020. "The Effects of Vitamin D Supplementation on Lipid and Inflammatory Profile of Healthy Adolescent Boys: A Randomized Controlled Trial" Nutrients 12, no. 5: 1213. https://doi.org/10.3390/nu12051213
APA StyleYarparvar, A., Elmadfa, I., Djazayery, A., Abdollahi, Z., Salehi, F., & Heshmat, R. (2020). The Effects of Vitamin D Supplementation on Lipid and Inflammatory Profile of Healthy Adolescent Boys: A Randomized Controlled Trial. Nutrients, 12(5), 1213. https://doi.org/10.3390/nu12051213





