Development and Validation of Two Self-Reported Tools for Insulin Resistance and Hypertension Risk Assessment in A European Cohort: The Feel4Diabetes-Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Background
2.2. Ethics Approval and Consent to Participate
2.3. Study Protocol and Recruitment
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 Risk Factors Collaborators; Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Siu, A.L. Force USPST: Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2015, 163, 778–786. [Google Scholar] [CrossRef]
- Ekoe, J.M.; Goldenberg, R.; Katz, P. Screening for Diabetes in Adults. Can. J. Diabetes 2018, 42 (Suppl. S1), S16–S19. [Google Scholar] [CrossRef]
- Fleming, S.; Atherton, H.; McCartney, D.; Hodgkinson, J.; Greenfield, S.; Hobbs, F.D.; Mant, J.; McManus, R.J.; Thompson, M.; Ward, A.; et al. Self-Screening and Non-Physician Screening for Hypertension in Communities: A Systematic Review. Am. J. Hypertens. 2015, 28, 1316–1324. [Google Scholar] [CrossRef]
- Gilmer, T.P.; O’Connor, P.J. The growing importance of diabetes screening. Diabetes Care 2010, 33, 1695–1697. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef]
- Taylor, J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur. Heart J. 2013, 34, 2108–2109. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: An analysis of 123 nationally representative surveys. Lancet 2019, 394, 639–651. [Google Scholar] [CrossRef]
- Gong, W.; Lu, B.; Yang, Z.; Ye, W.; Du, Y.; Wang, M.; Li, Q.; Zhang, W.; Pan, Y.; Feng, X.; et al. Early-stage atherosclerosis in newly diagnosed, untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Metab. 2009, 35, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N. The burden of diabetes and its complications: Trends and implications for intervention. Diabetes Res. Clin. Pract. 2007, 76 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Balkau, B.; Colagiuri, S.; Zimmet, P.Z.; Tonkin, A.M.; Mitchell, P.; Phillips, P.J.; Shaw, J.E. AUSDRISK: An Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med. J. Aust. 2010, 192, 197–202. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: Cohort study. BMJ 2017, 359, 5019. [Google Scholar] [CrossRef]
- Parikh, N.I.; Pencina, M.J.; Wang, T.J.; Benjamin, E.J.; Lanier, K.J.; Levy, D.; D’Agostino, R.B.; Kannel, W.B., Sr.; Vasan, R.S. A risk score for predicting near-term incidence of hypertension: The Framingham Heart Study. Ann. Intern. Med. 2008, 148, 102–110. [Google Scholar] [CrossRef]
- Kshirsagar, A.V.; Chiu, Y.L.; Bomback, A.S.; August, P.A.; Viera, A.J.; Colindres, R.E.; Bang, H. A hypertension risk score for middle-aged and older adults. J. Clin. Hypertens. 2010, 12, 800–808. [Google Scholar] [CrossRef]
- Gray, L.J.; Taub, N.A.; Khunti, K.; Gardiner, E.; Hiles, S.; Webb, D.R.; Srinivasan, B.T.; Davies, M.J. The Leicester Risk Assessment score for detecting undiagnosed Type 2 diabetes and impaired glucose regulation for use in a multiethnic UK setting. Diabetic Med. J. Br. Diabetic Assoc. 2010, 27, 887–895. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Bang, H.; Pankow, J.S.; Ballantyne, C.M.; Golden, S.H.; Folsom, A.R.; Chambless, L.E. Atherosclerosis Risk in Communities I: Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005, 28, 2013–2018. [Google Scholar] [CrossRef]
- Xiang, A.H.; Wang, C.; Peters, R.K.; Trigo, E.; Kjos, S.L.; Buchanan, T.A. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006, 55, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.K.; Meigs, J.B.; Sullivan, L.M.; D’Agostino, R.B., Sr.; Wilson, P.W. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005, 54, 3252–3257. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, E.T.; Fabsitz, R.R.; Devereux, R.; Best, L.; Welty, T.K.; Howard, B.V. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: The Strong Heart Study. Hypertension 2006, 47, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Manios, Y.; Androutsos, O.; Lambrinou, C.P.; Cardon, G.; Lindstrom, J.; Annemans, L.; Mateo-Gallego, R.; de Sabata, M.S.; Iotova, V.; Kivela, J.; et al. A school- and community-based intervention to promote healthy lifestyle and prevent type 2 diabetes in vulnerable families across Europe: Design and implementation of the Feel4Diabetes-study. Public Health Nutr. 2018, 21, 3281–3290. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group: [2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)]. G. Ital. Cardiol. 2018, 19 (Suppl. S1), 3S–73S. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of optimal cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Robinson, C.A.; Agarwal, G.; Nerenberg, K. Validating the CANRISK prognostic model for assessing diabetes risk in Canada’s multi-ethnic population. Chronic Dis. Inj. Can. 2011, 32, 19–31. [Google Scholar] [PubMed]
- Costa, B.; Barrio, F.; Pinol, J.L.; Cabre, J.J.; Mundet, X.; Sagarra, R.; Salas-Salvado, J.; Sola-Morales, O.; DE-PLAN-CAT/PREDICE Research Group. Shifting from glucose diagnosis to the new HbA1c diagnosis reduces the capability of the Finnish Diabetes Risk Score (FINDRISC) to screen for glucose abnormalities within a real-life primary healthcare preventive strategy. BMC Med. 2013, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, M.; De Berardis, G.; Rossi, M.C.; Sacco, M.; Belfiglio, M.; Pellegrini, F.; Tognoni, G.; Valentini, M.; Nicolucci, A. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: The IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care 2005, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Makrilakis, K.; Liatis, S.; Grammatikou, S.; Perrea, D.; Stathi, C.; Tsiligros, P.; Katsilambros, N. Validation of the Finnish diabetes risk score (FINDRISC) questionnaire for screening for undiagnosed type 2 diabetes, dysglycaemia and the metabolic syndrome in Greece. Diabetes Metab. 2011, 37, 144–151. [Google Scholar] [CrossRef]
- Tankova, T.; Chakarova, N.; Atanassova, I.; Dakovska, L. Evaluation of the Finnish Diabetes Risk Score as a screening tool for impaired fasting glucose, impaired glucose tolerance and undetected diabetes. Diabetes Res. Clin. Pract. 2011, 92, 46–52. [Google Scholar] [CrossRef]
- Mavrogianni, C.; Lambrinou, C.P.; Androutsos, O.; Lindstrom, J.; Kivela, J.; Cardon, G.; Huys, N.; Tsochev, K.; Iotova, V.; Chakarova, N.; et al. Evaluation of the Finnish Diabetes Risk Score as a screening tool for undiagnosed type 2 diabetes and dysglycaemia among early middle-aged adults in a large-scale European cohort. The Feel4Diabetes-study. Diabetes Res. Clin. Pract. 2019, 150, 99–110. [Google Scholar] [CrossRef]
- Gomez-Arbelaez, D.; Alvarado-Jurado, L.; Ayala-Castillo, M.; Forero-Naranjo, L.; Camacho, P.A.; Lopez-Jaramillo, P. Evaluation of the Finnish Diabetes Risk Score to predict type 2 diabetes mellitus in a Colombian population: A longitudinal observational study. World J. Diabetes 2015, 6, 1337–1344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Zhang, Y.; Hu, G.; Chen, L. Evaluation of Finnish Diabetes Risk Score in screening undiagnosed diabetes and prediabetes among U.S. adults by gender and race: NHANES 1999–2010. PLoS ONE 2014, 9, e97865. [Google Scholar] [CrossRef]
- Fonseca, V.A. Early identification and treatment of insulin resistance: Impact on subsequent prediabetes and type 2 diabetes. Clin. Cornerstone 2007, 8 (Suppl. S7), S7–S18. [Google Scholar] [CrossRef]
- Hanley, A.J.; Williams, K.; Stern, M.P.; Haffner, S.M. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio Heart Study. Diabetes Care 2002, 25, 1177–1184. [Google Scholar] [CrossRef]
- Schafer, S.; Kantartzis, K.; Machann, J.; Venter, C.; Niess, A.; Schick, F.; Machicao, F.; Haring, H.U.; Fritsche, A.; Stefan, N. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur. J. Clin. Investig. 2007, 37, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. Indian Diabetes Prevention P: The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20. [Google Scholar] [CrossRef]
- Liu, A.Y.; Silvestre, M.P.; Poppitt, S.D. Prevention of type 2 diabetes through lifestyle modification: Is there a role for higher-protein diets? Adv. Nutr. 2015, 6, 665–673. [Google Scholar] [CrossRef]
- Herman, W.H.; Hoerger, T.J.; Brandle, M.; Hicks, K.; Sorensen, S.; Zhang, P.; Hamman, R.F.; Ackermann, R.T.; Engelgau, M.M.; Ratner, R.E. Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann. Intern. Med. 2005, 142, 323–332. [Google Scholar] [CrossRef]
- Howard, B.V.; Lee, E.T.; Yeh, J.L.; Go, O.; Fabsitz, R.R.; Devereux, R.B.; Welty, T.K. Hypertension in adult American Indians. The Strong Heart Study. Hypertension 1996, 28, 256–264. [Google Scholar] [CrossRef]
- Jolly, S.E.; Koller, K.R.; Metzger, J.S.; Day, G.M.; Silverman, A.; Hopkins, S.E.; Austin, M.A.; Boden-Albala, B.; Ebbesson, S.O.; Boyer, B.B.; et al. Prevalence of Hypertension and Associated Risk Factors in Western Alaska Native People: The Western Alaska Tribal Collaborative for Health (WATCH) Study. J. Clin. Hypertens. 2015, 17, 812–818. [Google Scholar] [CrossRef]
- Franceschini, N.; Tao, R.; Liu, L.; Rutherford, S.; Haack, K.; Almasy, L.; Goring, H.H.; Laston, S.; Lee, E.T.; Best, L.G.; et al. Mapping of a blood pressure QTL on chromosome 17 in American Indians of the strong heart family study. BMC Cardiovasc. Disord. 2014, 14, 158. [Google Scholar] [CrossRef][Green Version]
- Schmidt, M.I.; Duncan, B.B.; Vigo, A.; Pankow, J.; Ballantyne, C.M.; Couper, D.; Brancati, F.; Folsom, A.R. Detection of undiagnosed diabetes and other hyperglycemia states: The Atherosclerosis Risk in Communities Study. Diabetes Care 2003, 26, 1338–1343. [Google Scholar] [CrossRef][Green Version]
| Development Cohort Mean ± SD | Validation Cohort Mean ± SD | p Value | |
|---|---|---|---|
| European IR Risk Index (n = 1581) | n = 1076 | n = 505 | |
| Age (years) | 40.7 ± 5.29 | 40.6 ± 5.15 | 0.666 |
| BMI (kg/m2) | |||
| male | 29.7 ± 3.97 | 29.2 ± 4.39 | 0.172 |
| female | 27.3 ± 5.69 | 27.3 ± 5.67 | 0.985 |
| Waist circumference (cm) | |||
| male | 102.7 ± 9.93 | 101.0 ± 11.64 | 0.068 |
| female | 88.7 ± 13.31 | 89.1 ± 12.95 | 0.619 |
| HOMA-IR | 2.0 ± 2.40 | 1.9 ± 1.39 | 0.340 |
| SBP (mmHg) | 116.8 ± 16.20 | 116.4 ± 15.47 | 0.673 |
| DBP (mmHg) | 77.7 ± 11.39 | 77.0 ± 10.37 | 0.242 |
| European HTN Risk Index (n = 1350) | n = 906 | n = 444 | |
| Age (years) | 40.1 ± 5.34 | 40.3 ± 5.47 | 0.590 |
| BMI (kg/m2) | |||
| male | 29.2 ± 3.58 | 29.1 ± 3.89 | 0.224 |
| female | 27.1 ± 5.04 | 27.2 ± 5.48 | 0.930 |
| Waist circumference (cm) | |||
| male | 102.8 ± 10.77 | 101.7 ± 12.07 | 0.330 |
| female | 87.6 ± 13.17 | 88.7 ± 13.41 | 0.268 |
| HOMA-IR | 2.2 ± 2.80 | 2.0 ± 1.46 | 0.145 |
| SBP (mmHg) | 117.5 ± 17.06 | 116.7 ± 16.51 | 0.466 |
| DBP (mmHg) | 77.9 ± 12.13 | 76.8 ± 11.06 | 0.092 |
| HOMA-IR Model | b | p Value | Cut-Offs | Points Allocated |
|---|---|---|---|---|
| BMI | 0.001 | |||
| - | <25 kg/m2 | 0 | ||
| 0.340 | 25–30 kg/m2 | 9 | ||
| 0.680 | >30 kg/m2 | 19 | ||
| Waist Circumference (women and men respectively) | 0.003 | |||
| - | <80 cm or <94 cm | 0 | ||
| 0.118 | 80–88 cm or 94–102 cm | 3 | ||
| 0.236 | >88 cm or >102 cm | 7 | ||
| Screen time | 0.001 | |||
| - | <2 h/day | 0 | ||
| 0.113 | ≥2 h/day | 3 | ||
| Sex | 0.023 | |||
| - | female | 0 | ||
| 0.066 | male | 2 | ||
| Breakfast | 0.001 | |||
| - | ≥5 times/week | 0 | ||
| 0.095 | <5 times/week | 3 | ||
| Sugary drinks (1 portion = 250 mL) | 0.018 | |||
| - | <1 portion/week | 0 | ||
| 0.063 | ≥1 portion/week | 2 | ||
| Walking (3 days/ week for at least 30 min) | 0.033 | |||
| - | Yes | 0 | ||
| 0.057 | No | 2 | ||
| Vigorous physical activity (3 days/ week for at least 10 min) | 0.002 | |||
| - | Yes | 0 | ||
| 0.084 | No | 2 | ||
| Maximum total points | 40 |
| Hypertension Model | b | p Value | Cut-Offs | Points Allocated |
|---|---|---|---|---|
| BMI | 0.001 | |||
| - | <25 kg/m2 | 0 | ||
| 0.308 | 25–30 kg/m2 | 10 | ||
| 0.616 | >30 kg/m2 | 20 | ||
| Sex | 0.001 | |||
| - | female | 0 | ||
| 0.204 | male | 6 | ||
| Vigorous physical activity (3 days/ week for at least 10 min) | 0.091 | |||
| - | Yes | 0 | ||
| 0.048 | No | 2 | ||
| Legumes | 0.001 | |||
| - | ≥1 cup/week | 0 | ||
| 0.254 | <1 cup/week | 8 | ||
| Alcohol (1 portion = 125 mL of wine, 330 mL of beer or 40mL of hard liquor) | 0.020 | |||
| - | <3 portions/week | 0 | ||
| 0.069 | ≥3 portions/week | 2 | ||
| Age | 0.099 | |||
| - | <40 years | 0 | ||
| 0.047 | ≥40 years | 2 | ||
| Maximum total points | 40 |
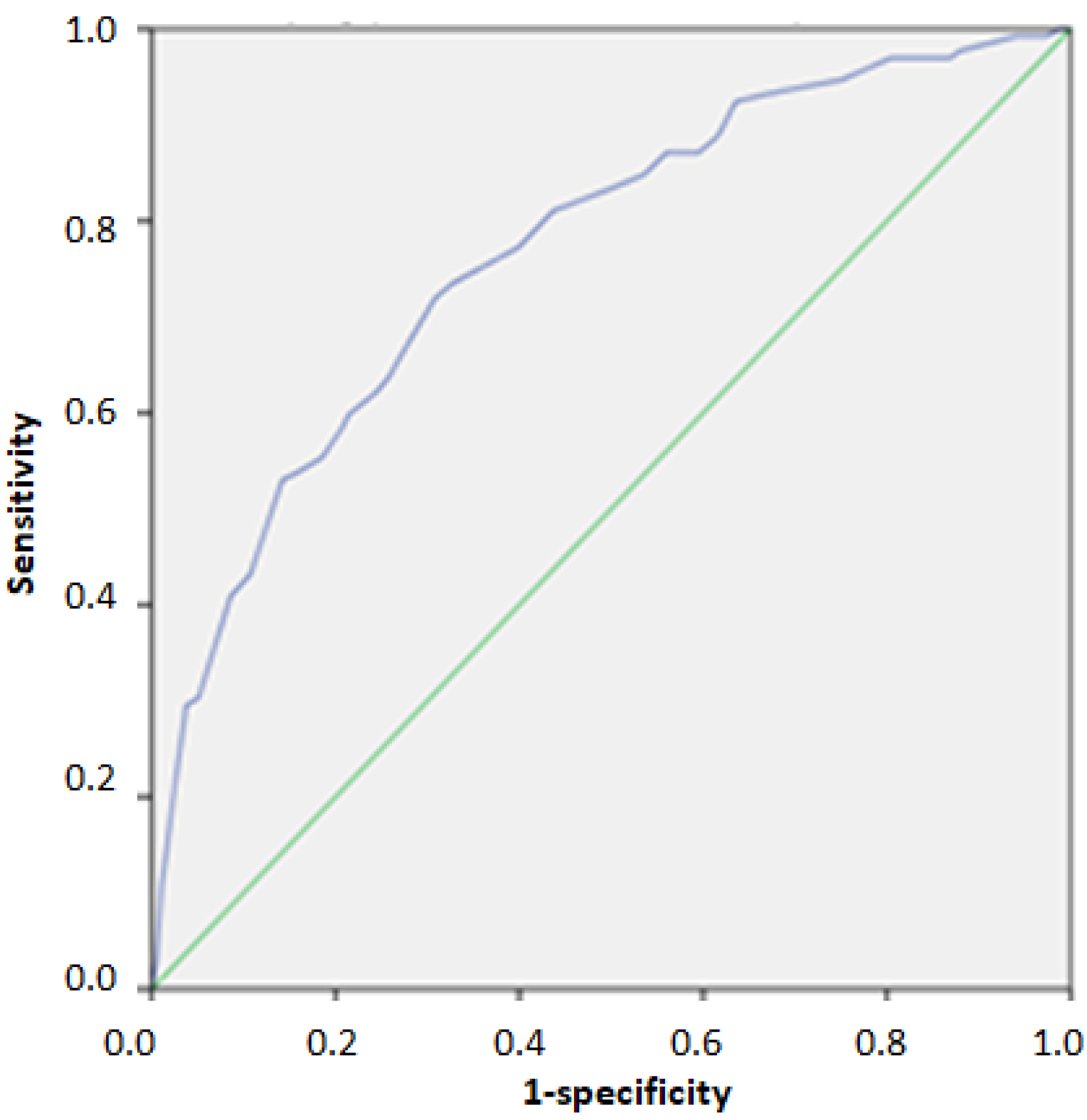
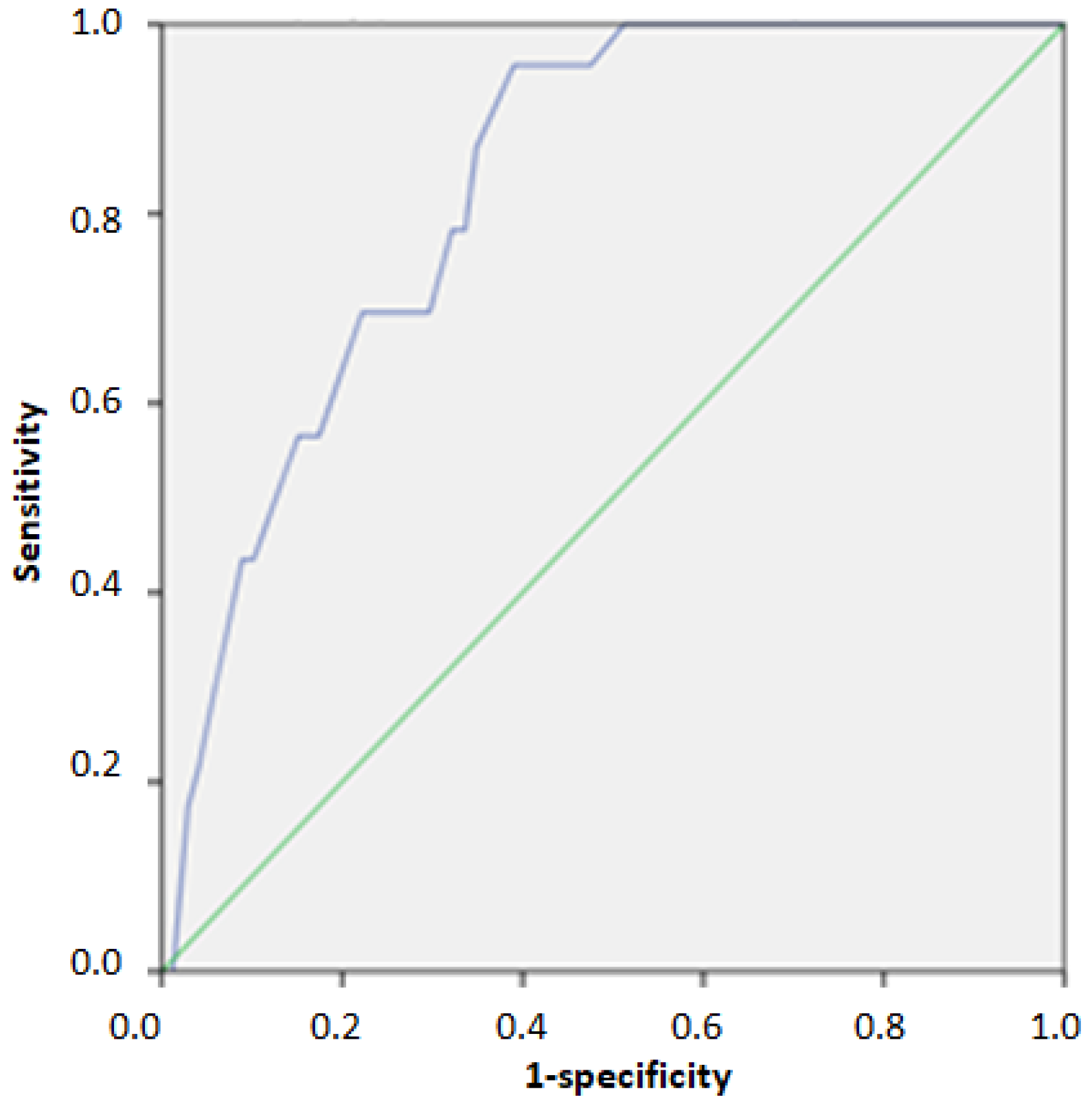
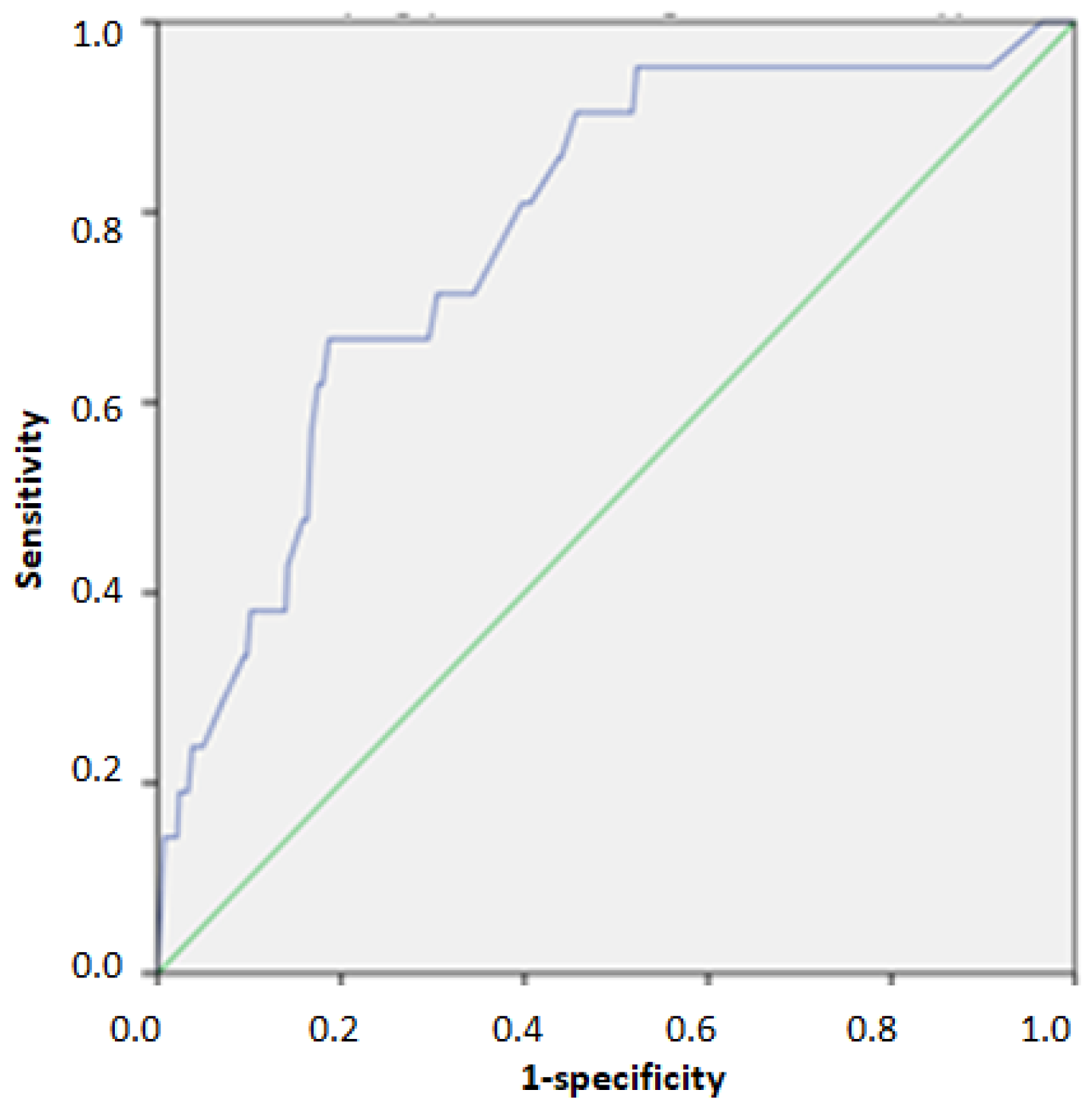
| Score | AUC | 95% Confidence Interval | n of TP | n of Un | PPV % | NPV % | Se | Sp | |
|---|---|---|---|---|---|---|---|---|---|
| European IR Risk Index (n = 505) | |||||||||
| Cut off score for Identifying individuals above 75th percentile of HOMA-IR | 23/40 | 0.768 | 0.721–0.815 | 95 | 37 | 45.5% | 87.5% | 0.720 | 0.691 |
| Cut off score for Identifying individuals above 95th percentile of HOMA-IR | 31/40 | 0.828 | 0.766–0.890 | 16 | 7 | 13.0% | 98.2% | 0.696 | 0.778 |
| European HTN Risk Index (n = 444) | |||||||||
| Cut off for detecting 2nd and 3rd grade hypertension | 26/40 | 0.778 | 0.680–0.876 | 14 | 7 | 14.0% | 97.8% | 0.667 | 0.797 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellakis, S.; Mavrogianni, C.; Karatzi, K.; Lindstrom, J.; Cardon, G.; Iotova, V.; Wikström, K.; Shadid, S.; Moreno, L.A.; Tsochev, K.; et al. Development and Validation of Two Self-Reported Tools for Insulin Resistance and Hypertension Risk Assessment in A European Cohort: The Feel4Diabetes-Study. Nutrients 2020, 12, 960. https://doi.org/10.3390/nu12040960
Kanellakis S, Mavrogianni C, Karatzi K, Lindstrom J, Cardon G, Iotova V, Wikström K, Shadid S, Moreno LA, Tsochev K, et al. Development and Validation of Two Self-Reported Tools for Insulin Resistance and Hypertension Risk Assessment in A European Cohort: The Feel4Diabetes-Study. Nutrients. 2020; 12(4):960. https://doi.org/10.3390/nu12040960
Chicago/Turabian StyleKanellakis, Spyridon, Christina Mavrogianni, Kalliopi Karatzi, Jaana Lindstrom, Greet Cardon, Violeta Iotova, Katja Wikström, Samyah Shadid, Luis A. Moreno, Kaloyan Tsochev, and et al. 2020. "Development and Validation of Two Self-Reported Tools for Insulin Resistance and Hypertension Risk Assessment in A European Cohort: The Feel4Diabetes-Study" Nutrients 12, no. 4: 960. https://doi.org/10.3390/nu12040960
APA StyleKanellakis, S., Mavrogianni, C., Karatzi, K., Lindstrom, J., Cardon, G., Iotova, V., Wikström, K., Shadid, S., Moreno, L. A., Tsochev, K., Bíró, É., Dimova, R., Antal, E., Liatis, S., Makrilakis, K., Manios, Y., & on behalf of the Feel4Diabetes-study group. (2020). Development and Validation of Two Self-Reported Tools for Insulin Resistance and Hypertension Risk Assessment in A European Cohort: The Feel4Diabetes-Study. Nutrients, 12(4), 960. https://doi.org/10.3390/nu12040960






