Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Determination of Sample Size
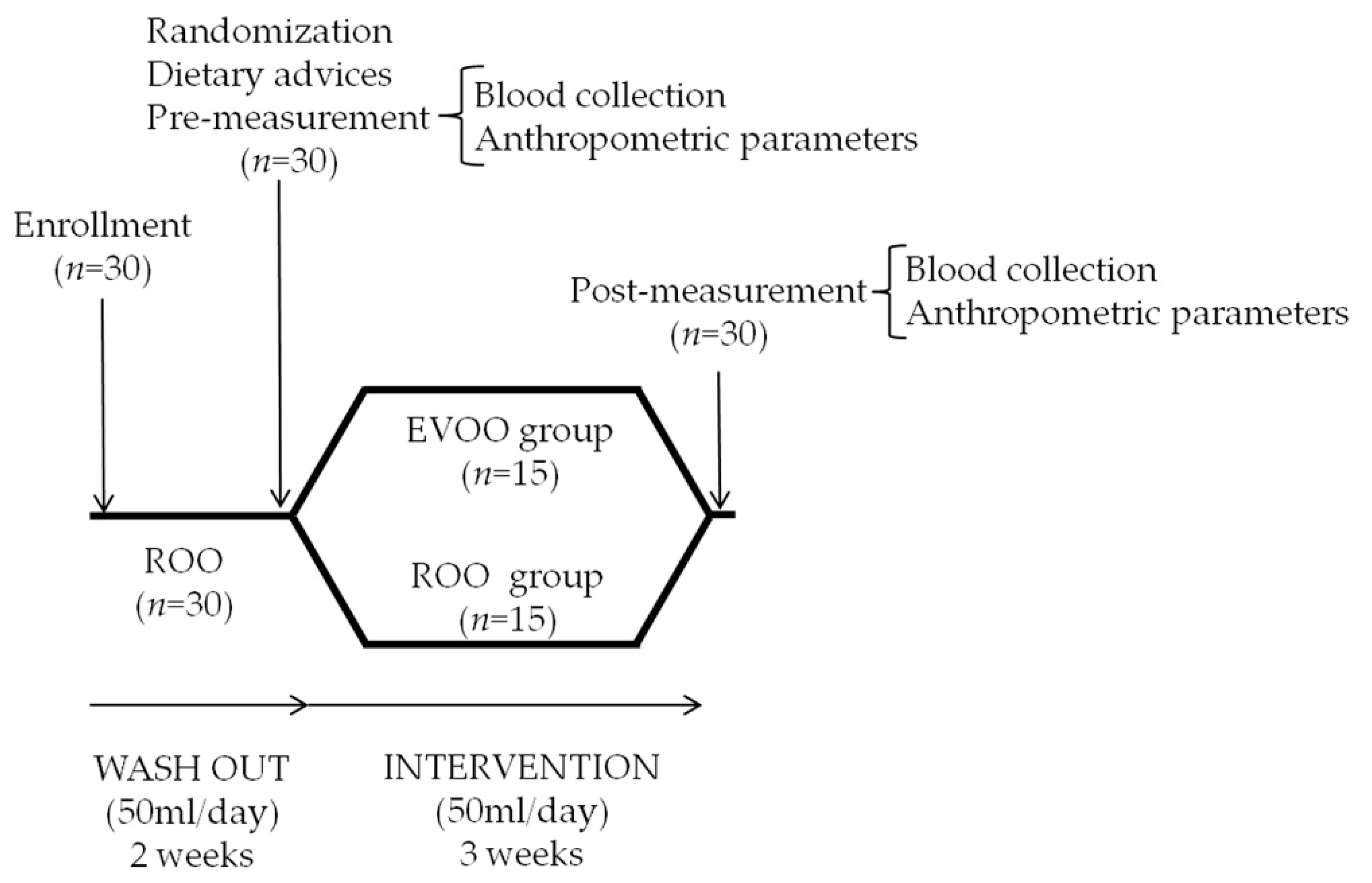
2.3. Study Design
2.4. Blood Collection and Preparation of Blood Samples
2.5. Determination of Thrombosis-Related Parameters
2.6. Determination of Inflammatory Markers
2.7. Determination of Lipid Profile
2.8. NO Measurement
2.9. Cortisol Measurement
2.10. Statistical Analysis
3. Results
3.1. Participants
3.2. Effects of Each Olive Oil Type on Laboratory Parameters in Patients with FM
3.3. Effects of Type of Olive Oil on Laboratory Parameters in Patients with FM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Häuser, W.; Sarzi-Puttini, P.; Fitzcharles, M.A. Fibromyalgia syndrome: Under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 116), 90–97. [Google Scholar]
- Macfarlane, G.J. Chronic widespread pain and fibromyalgia: Should reports of increased mortality influence management? Curr. Rheumatol. Rep. 2005, 7, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Coskun Benlidayi, I. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef] [PubMed]
- La Rubia, M.; Rus, A.; Molina, F.; Del Moral, M.L. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin. Exp. Rheumatol. 2013, 31, S121–S127. [Google Scholar]
- Talotta, R.; Bazzichi, L.; Di Franco, M.; Casale, R.; Batticciotto, A.; Gerardi, M.C.; Sarzi-Puttini, P. One year in review 2017: Fibromyalgia. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 105), 6–12. [Google Scholar]
- Molina, F.; del Moral, M.L.; La Rubia, M.; Blanco, S.; Carmona, R.; Rus, A. Are patients with Fibromyalgia in a prothrombotic state? Biol. Res. Nurs. 2019, 21, 224–230. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Summerhill, V.; Karagodin, V.; Grechko, A.; Myasoedova, V.; Orekhov, A. Vasculoprotective role of olive oil compounds via modulation of oxidative stress in atherosclerosis. Front. Cardiovasc. Med. 2018, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet 2014, 27, 1–21. [Google Scholar] [CrossRef]
- Mas, A.J.; Carmona, L.; Valverde, M.; Ribas, B. EPISER study group. Prevalence and impact of fibromialgia on function and quality of life in individuals from the general population: Results from a nationwide study in Spain. Clin. Exp. Rheumatol. 2008, 26, 519–526. [Google Scholar]
- Collado, A.; Gómez, E.; Coscolla, R.; Sunyol, R.; Solé, E.; Rivera, J.; Altarriba, E.; Carbonell, J.; Castells, X. Work, family and social environment in patients with Fibromyalgia in Spain: An epidemiological study: EPIFFAC study. BMC Health Serv. Res. 2014, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, W.; Vorster, H.H.; Jerling, J.C.; Barnard, H.C.; Smuts, C.M.; Silvis, N.; Kruger, A.; Venter, C.S. Both fish oil and olive oil lowered plasma fibrinogen in women with high baseline fibrinogen levels. Thromb. Haemost. 1994, 72, 557–562. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association (WMA). Declaration of Helsinki. (2008, October). Ethical principles for medical research involving human subjects. In Proceedings of the 59th WMA General Assembly, Seoul, Korea, October 2008. [Google Scholar]
- Rus, A.; Castro, L.; Del Moral, M.L.; Peinado, A. Inducible NOS inhibitor 1400W reduces hypoxia/re-oxygenation injury in rat lung. Redox Rep. 2010, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pifarre, R. Thrombosis and cardiovascular disease. Med. Clin. N. Am. 1998, 82, 511–522. [Google Scholar] [CrossRef]
- Aleman, M.M.; Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and red blood cells in venous thrombosis. Thromb. Res. 2014, 133, S38–S40. [Google Scholar] [CrossRef]
- Batista, T.R.; Figueiredo, R.C.; Rios, D.R.A. Platelets volume indexes and cardiovascular risk factors. Rev. Assoc. Med. Bras. 2018, 64, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Vagdatli, E.; Gounari, E.; Lazaridou, E.; Katsibourlia, E.; Tsikopoulou, F.; Labrianou, I. Platelet distribution width: A simple, practical and specific marker of activation of coagulation. Hippokratia 2010, 14, 28–32. [Google Scholar] [PubMed]
- Levin, J.; Bessman, J.D. The inverse relation between platelet volume and platelet number. Abnormalities in hematologic disease and evidence that platelet size does not correlate with platelet age. J. Lab. Clin. Med. 1983, 101, 295–307. [Google Scholar] [PubMed]
- Delgado-Lista, J.; Garcia-Rios, A.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Olive oil and haemostasis: Platelet function, thrombogenesis and fibrinolysis. Curr. Pharm. Des. 2011, 17, 778–785. [Google Scholar] [CrossRef]
- Widmer, R.J.; Freund, M.A.; Flammer, A.J.; Sexton, J.; Lennon, R.; Romani, A.; Mulinacci, N.; Vinceri, F.F.; Lerman, L.O.; Lerman, A. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur. J. Nutr. 2013, 52, 1223–1231. [Google Scholar] [CrossRef]
- Mezzano, D.; Leighton, F. Haemostatic cardiovascular risk factors: Differential effects of red wine and diet on healthy young. Pathophysiol. Haemost. Thromb. 2003, 33, 472–478. [Google Scholar] [CrossRef]
- Kouli, G.M.; Panagiotakos, D.B.; Kyrou, I.; Magriplis, E.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Olive oil consumption and 10-year (2002–2012) cardiovascular disease incidence: The ATTICA study. Eur. J. Nutr. 2019, 58, 131–138. [Google Scholar] [CrossRef]
- Miller, M.A. Association of inflammatory markers with cardiovascular risk and sleepiness. J. Clin. Sleep Med. 2011, 7, S31–S33. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Martínez-Lara, E.; Rus, A.; Camacho, M.V.; Del Moral, M.L.; Siles, E. Insight into the biological pathways underlying fibromyalgia by a proteomic approach. J. Proteom. 2018, 186, 47–55. [Google Scholar] [CrossRef]
- Kozić-Dokmanović, S.; Kolovrat, K.; Laškaj, R.; Jukić, V.; Vrkić, N.; Begovac, J. Effect of extra virgin olive oil on biomarkers of inflammation in HIV-infected patients: A randomized, crossover, controlled clinical trial. Med. Sci. Monit. 2015, 16, 2406–2413. [Google Scholar] [CrossRef][Green Version]
- Mena, M.P.; Sacanella, E.; Vazquez-Agell, M.; Morales, M.; Fitó, M.; Escoda, R.; Serrano-Martínez, M.; Salas-Salvadó, J.; Benages, N.; Casas, R.; et al. Inhibition of circulating immune cell activation: A molecular antiinflammatory effect of the Mediterranean diet. Am. J. Clin. Nutr. 2009, 89, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, T.; Fitó, M.; de la Torre, R.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; Albaladejo, M.F.; Abanades, S.; Schroder, H.; et al. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr. 2004, 134, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; de la Torre, R.; Fitó, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015, 113 (Suppl. 2), S19–S28. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; Sánchez, M.J.; Molina-Montes, E.; Chirlaque, M.D.; Huerta, J.M.; Navarro, C.; et al. Olive oil intake and CHD in the European Prospective Investigation into Cancer and Nutrition Spanish cohort. Br. J. Nutr. 2012, 108, 2075–2082. [Google Scholar] [CrossRef]
- Buckland, G.; Mayén, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef]
- Venturini, D.; Simao, A.N.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Llorente-Cortes, V.; Estruch, R.; Mena, M.P.; Ros, E.; González, M.A.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef]
- Crawford, A.A.; Söderberg, S.; Kirschbaum, C.; Murphy, L.; Eliasson, M.; Ebrahim, S.; Davey-Smith, G.; Olsson, T.; Sattar, N.; Lawlor, D.A.; et al. Morning plasma cortisol as a cardiovascular risk factor: Findings from prospective cohort and Mendelian randomization studies. Eur. J. Endocrinol. 2019, 181, 429–438. [Google Scholar] [CrossRef]
- Iob, E.; Steptoe, A. Cardiovascular disease and Hair cortisol: A novel biomarker of chronic stress. Curr. Cardiol. Rep. 2019, 21, 116. [Google Scholar] [CrossRef]
- Medeiros-de-Moraes, I.M.; Gonçalves-de-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; De Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Francini, A.; Domenici, V.; Cifelli, M.; Belardinelli, E.; Sebastiani, L.; Cantini, C.; Di Stefano, R. Effects of extra virgin olive oil and apples enriched-dark chocolate on endothelial progenitor cells in patients with cardiovascular risk factors: A randomized cross-over trial. Antioxidants 2019, 8, 88. [Google Scholar] [CrossRef]
- Zamora-Zamora, F.; Martínez-Galiano, J.M.; Gaforio-Martínez, J.J.; Delgado-Rodríguez, M. Olive oil and body weight. Systematic review and meta-analysis of randomized controlled trials. Rev. Esp. Salud Publica 2018, 92, e201811083. [Google Scholar] [PubMed]
- Okifuji, A.; Bradshaw, D.H.; Olson, C. Evaluating obesity in fibromyalgia: Neuroendocrine biomarkers, symptoms, and functions. Clin. Rheumatol. 2009, 28, 475–478. [Google Scholar] [CrossRef] [PubMed]
| COMPOSITION | EVOO | ROO |
|---|---|---|
| Polyunsaturated fatty acids (%) | 4.3 | 3.8 |
| Linoleic acid (%) | 3.9 | 3.5 |
| Linolenic acid (%) | 0.4 | 0.3 |
| Monounsaturated fatty acids (%) | 81.7 | 81.7 |
| Oleic acid (%) | 80.7 | 80.7 |
| Eicosenoic acid (%) | 0.3 | 0.3 |
| Palmitoleic acid (%) | 0.7 | 0.7 |
| Saturated fatty acids (%) | 14 | 14.5 |
| Palmitic acid (%) | 9.6 | 10.9 |
| Stearic acid (%) | 3.6 | 2.8 |
| Arachidic acid (%) | 0.4 | 0.4 |
| Behenic acid (%) | 0.1 | 0.1 |
| Others (%) | 0.3 | 0.3 |
| Tocopherols (mg/Kg) | 203 | 75 |
| Polyphenols (mg/Kg) | 248 | 152 |
| Anthropometric characteristics | EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size |
| Weight (kg) | 62.57 ± 11.48 | 62.54 ± 10.85 | 0.994 | 0.003 | 66.59 ± 8.59 | 66.30 ± 8.83 | 0.935 | 0.033 | 0.709 | 0.158 |
| Body mass index (kg/m2) | 25.67 ± 4.71 | 25.62 ± 4.25 | 0.928 | 0.011 | 26.74 ± 2.24 | 26.55 ± 2.15 | 0.852 | 0.087 | 0.669 | 0.188 |
| Waist circumference (cm) | 90.32 ± 10.93 | 87.77 ± 10.28 | 0.580 | 0.240 | 92.88 ± 7.88 | 88.71 ± 6.84 | 0.003* | 0.560 | 0.328 | 0.417 |
| Blood-coagulation parameters | EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size |
| Prothrombin time (sec) | 11.01 ± 0.40 | 11.06 ± 0.52 | 0.812 | 0.108 | 11.07 ± 0.59 | 11.35 ± 0.50 | 0.243 | 0.512 | 0.402 | 0.372 |
| Cephaline time (sec) | 30.16 ± 3.61 | 30.56 ± 3.15 | 0.795 | 0.118 | 30.54 ± 3.03 | 30.59 ± 3.11 | 0.967 | 0.016 | 0.689 | 0.177 |
| Fibrinogen (g/L) | 3.71 ± 0.71 | 3.47 ± 0.51 | 0.398 | 0.388 | 3.20 ± 1.05 | 2.47 ± 0.34 | 0.016*# | 0.935 | 0.152# | 0.072 |
| Platelet indices | EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size |
| Platelet count (× 109/L) | 250.73 ± 85.14 | 254.09 ± 56.71 | 0.914 | 0.046 | 299.91 ± 66.20 | 306.64 ± 77.59 | 0.829 | 0.093 | 0.748# | 0.0009 |
| MPV (fL) | 8.25 ± 0.90 | 8.90 ± 1.65 | 0.319 | 0.489 | 7.55 ± 0.46 | 8.65 ± 1.02 | 0.007* | 1.39 | 0.474 | 0.346 |
| PDW (%) | 61.29 ± 12.67 | 62.25 ± 13.46 | 0.864 | 0.073 | 59.95 ± 11.38 | 48.41 ± 10.08 | 0.016* | 1.074 | 0.035* | 0.967 |
| EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size | |
| Red blood cell count (× 1012/L) | 5.03 ± 0.26 | 4.78 ± 0.21 | 0.001* | 1.058 | 4.87 ± 0.35 | 4.68 ± 0.40 | 0.063 | 0.506 | 0.610 | 0.221 |
| Inflammatory markers | EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size |
| IL-6 (pg/mL) | 2.28 ± 2.35 | 2.17 ± 0.94 | 0.505# | 0.0009 | 2.26 ± 2.53 | 2.22 ± 0.97 | 0.328# | 0.0001 | 0.654# | 0.0002 |
| IL-10 (pg/mL) | 1.53 ± 1.14 | 1.99 ± 0.44 | 0.235 | 0.532 | 1.54 ± 1.47 | 2.04 ± 0.87 | 0.325 | 0.414 | 0.525# | 0.0002 |
| CRP (mg/l) | 4.15 ± 4.47 | 2.81 ± 2.32 | 0.259# | 0.034 | 3.21 ± 5.02 | 1.55 ± 1.86 | 0.123# | 0.046 | 0.898# | 0.001 |
| ESR (mm) | 22.00 ± 10.38 | 12.91 ± 7.05 | 0.019* | 1.024 | 16.45 ± 11.14 | 8.27 ± 3.94 | 0.028* | 0.979 | 0.699# | 0.001 |
| NLR | 1.82 ± 0.78 | 1.58 ± 0.51 | 0.399 | 0.364 | 2.51 ± 0.79 | 1.76 ± 0.45 | 0.027* | 1.167 | 0.197 | 0.569 |
| PLR | 155.42 ± 43.93 | 136.07 ± 34.25 | 0.287 | 0.491 | 191.98 ± 42.54 | 176.24 ± 60.37 | 0.509 | 0.301 | 0.898 | 0.058 |
| Lipid profile | EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size |
| Total cholesterol (mg/dl) | 217.11 ± 26.29 | 235.67 ± 25.95 | 0.07 | 0.711 | 209.70 ± 24.15 | 219.00 ± 30.38 | 0.458 | 0.339 | 0.455 | 0.643 |
| HDL-cholesterol (mg/dl) | 63.40 ± 9.38 | 61.20 ± 9.68 | 0.612 | 0.231 | 63.50 ± 16.51 | 61.80 ± 17.35 | 0.825 | 0.1 | 0.892 | 0.062 |
| LDL-cholesterol (mg/dl) | 134.44 ± 28.53 | 154.22 ± 25.38 | 0.07 | 0.733 | 128.40 ± 21.53 | 139.40 ± 27.82 | 0.336 | 0.442 | 0.423 | 0.372 |
| Triglycerides (mg/dl) | 96.50 ± 42.95 | 102.70 ± 51.37 | 0.959# | 0.004 | 87.00 ± 37.84 | 86.30 ± 36.79 | 0.957 | 0.019 | 0.690 | 0.181 |
| Apolipoprotein A1 (mg/dl) | 159.59 ± 21.45 | 155.50 ± 16.43 | 0.638 | 0.214 | 160.10 ± 27.96 | 151.90 ± 28.42 | 0.524 | 0.291 | 0.532 | 0.285 |
| Apolipoprotein B (mg/dl) | 99.20 ± 11.04 | 98.70 ± 13.27 | 0.928 | 0.041 | 93.80 ± 14.09 | 87.10 ± 16.52 | 0.342 | 0.463 | 0.199 | 0.597 |
| EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size | |
| NO (μmol/mg protein) | 30.21 ± 21.56 | 45.48 ± 43.78 | 0.612# | 0.443 | 27.15 ± 20.81 | 37.84 ± 14.99 | 0.300 | 0.589 | 0.803 | 0.125 |
| EVOO Pre | EVOO Post | p1 | Effect Size | ROO Pre | ROO Post | p2 | Effect Size | p3 | Effect Size | |
| Cortisol (μg/dl) | 11.18 ± 4.14 | 8.45 ± 4.44 | 0.152 | 0.636 | 10.36 ± 3.75 | 12.55 ± 3.88 | 0.067 | 0.574 | 0.010* | 1.205 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, A.; Molina, F.; Martínez-Ramírez, M.J.; Aguilar-Ferrándiz, M.E.; Carmona, R.; del Moral, M.L. Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia. Nutrients 2020, 12, 918. https://doi.org/10.3390/nu12040918
Rus A, Molina F, Martínez-Ramírez MJ, Aguilar-Ferrándiz ME, Carmona R, del Moral ML. Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia. Nutrients. 2020; 12(4):918. https://doi.org/10.3390/nu12040918
Chicago/Turabian StyleRus, Alma, Francisco Molina, María Josefa Martínez-Ramírez, María Encarnación Aguilar-Ferrándiz, Ramón Carmona, and María Luisa del Moral. 2020. "Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia" Nutrients 12, no. 4: 918. https://doi.org/10.3390/nu12040918
APA StyleRus, A., Molina, F., Martínez-Ramírez, M. J., Aguilar-Ferrándiz, M. E., Carmona, R., & del Moral, M. L. (2020). Effects of Olive Oil Consumption on Cardiovascular Risk Factors in Patients with Fibromyalgia. Nutrients, 12(4), 918. https://doi.org/10.3390/nu12040918





