Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotics
2.2. Experimental Design
2.3. Aerobic Exercise Training
2.4. Physical Activities
2.5. Peripheral Fatigue-Associated Biochemical Variables
2.6. Short Chain Fatty Acid Analysis
2.7. Clinical Biochemical Profiles
2.8. Body Composition, Histology, and Glycogen Analysis
2.9. Statistical Analysis
3. Results
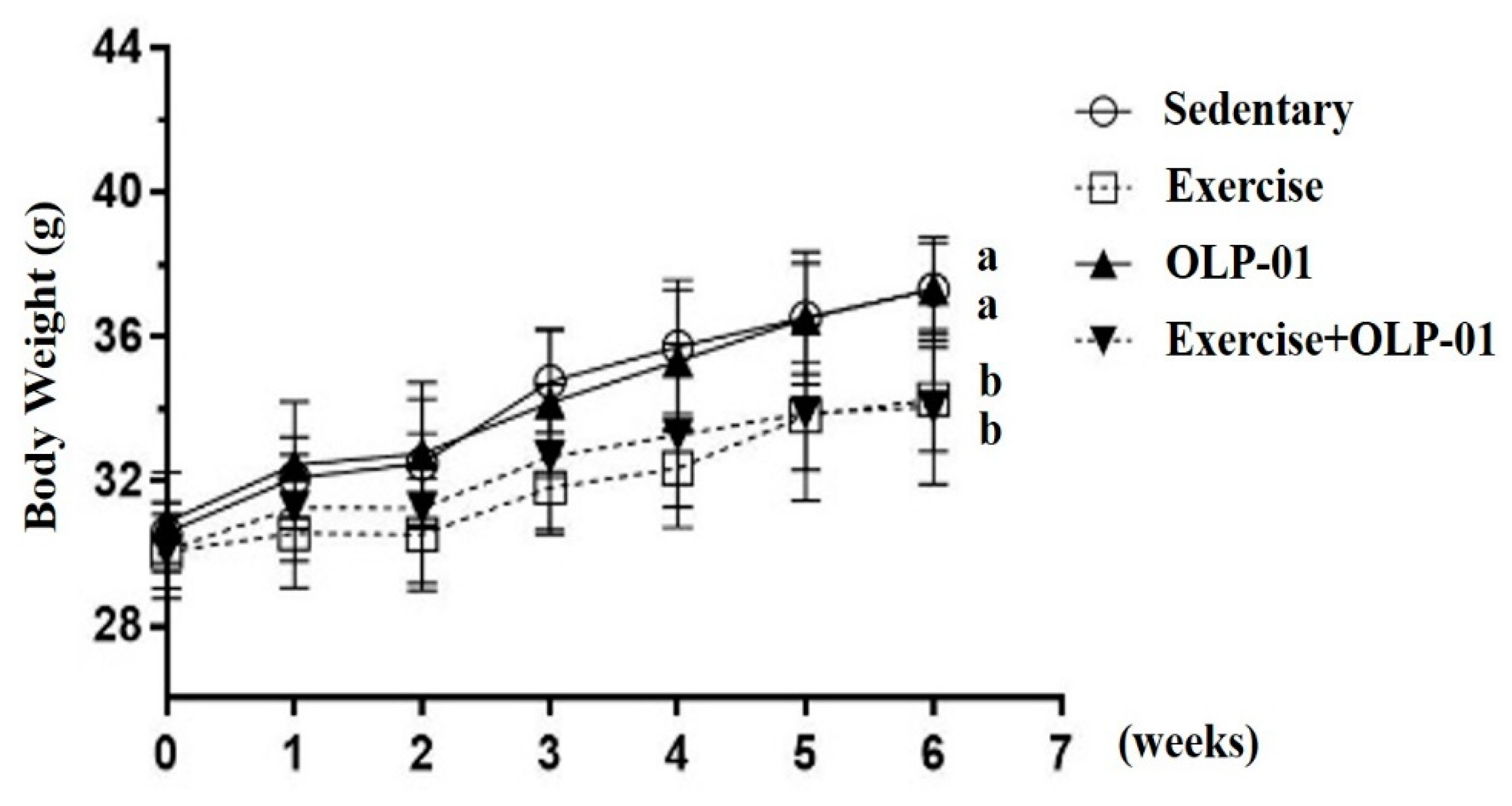
3.1. Effects of Exercise and Probiotic Intervention on Growth Curve and Body Composition
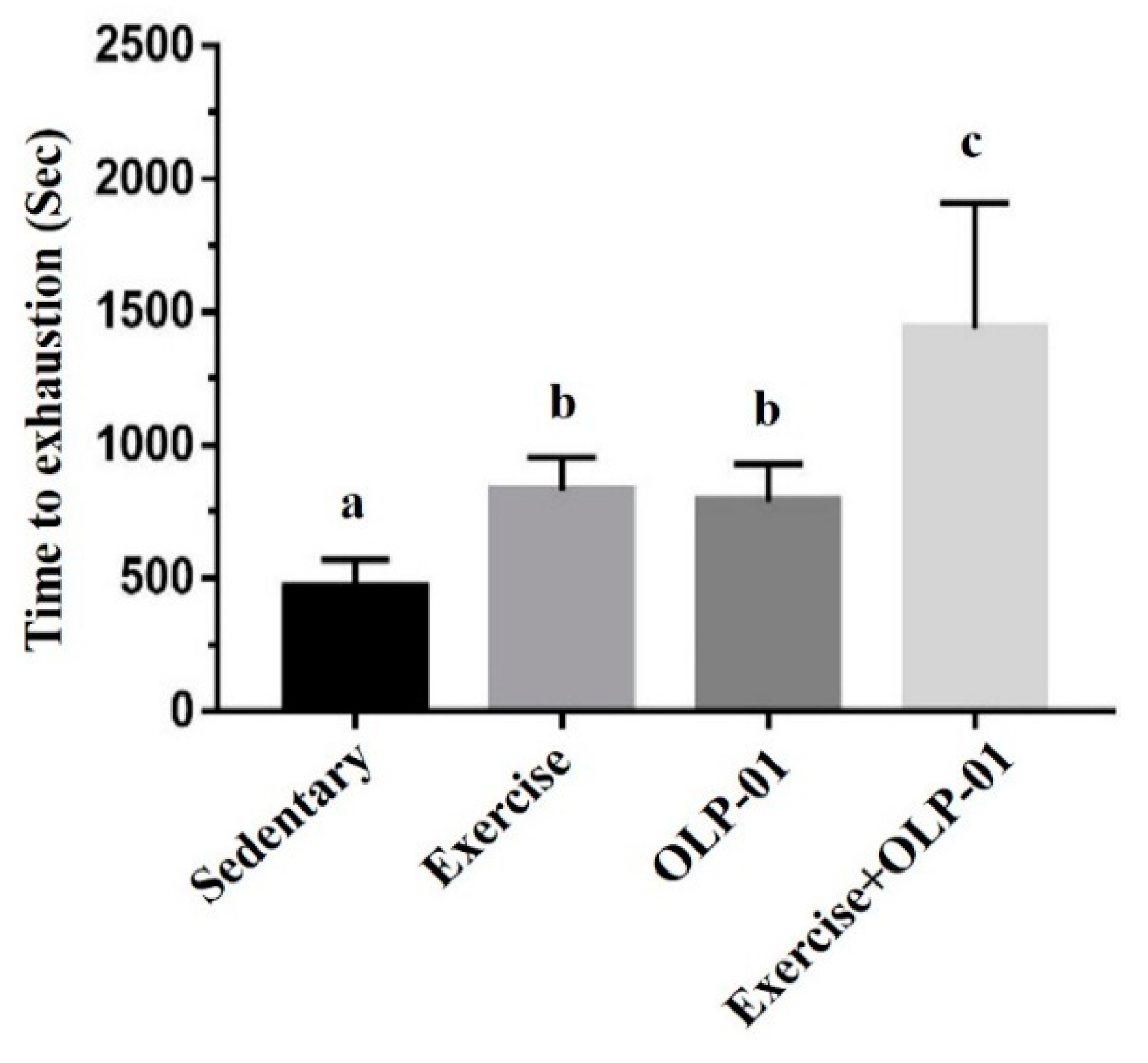
3.2. Effects of Exercise and Probiotics on the Performance of Exhaustive Exercise
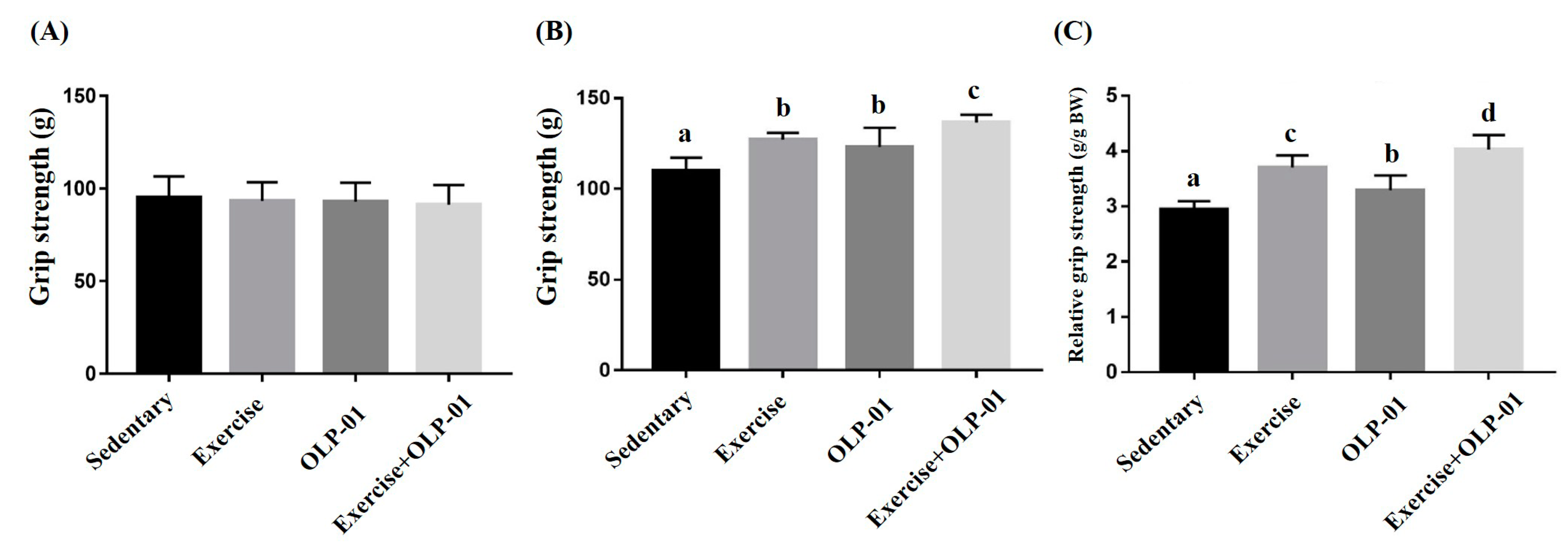
3.3. Effects of Exercise and Probiotic Intervention on Forelimb Grip Strength
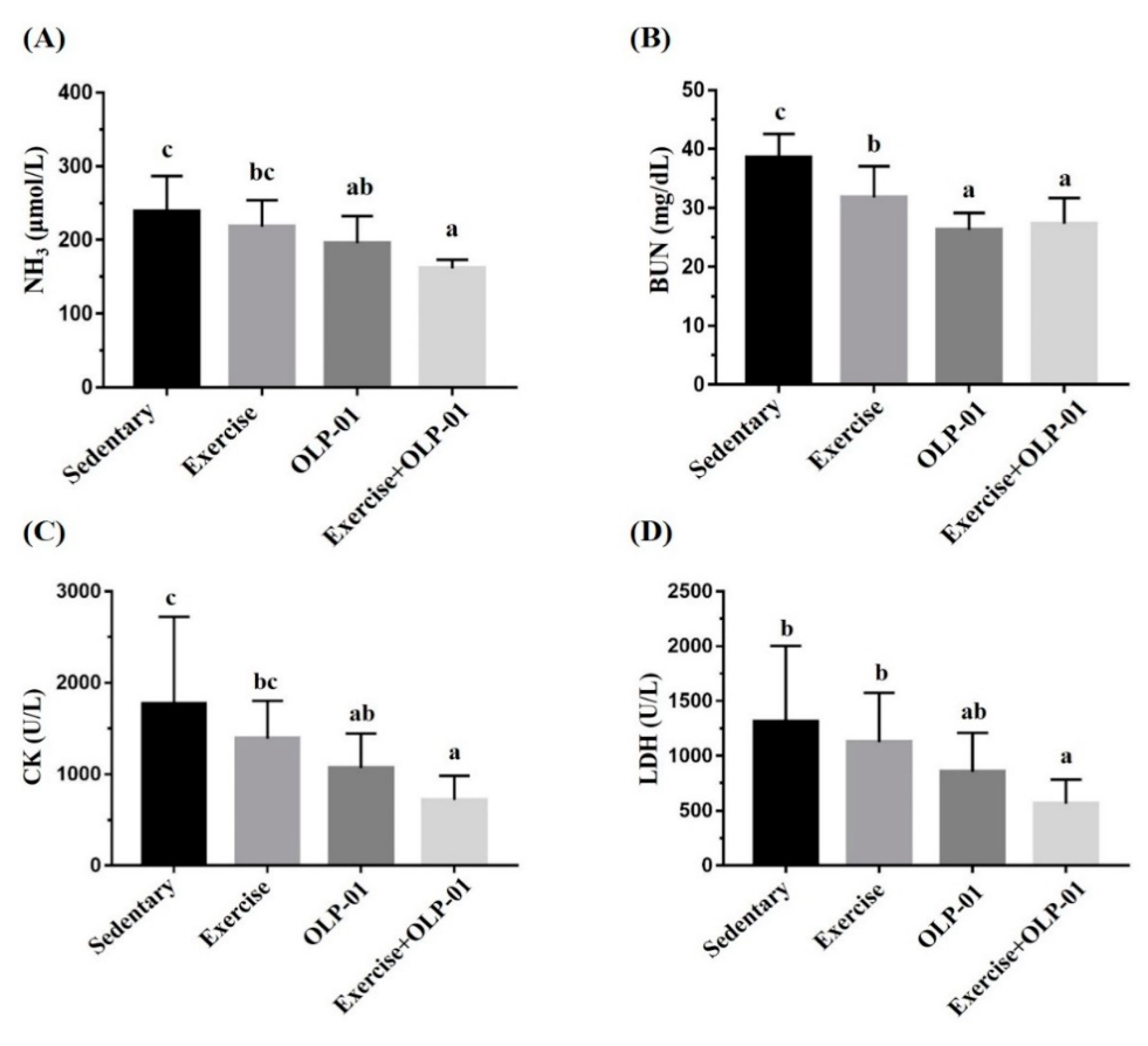
3.4. Effects of Exercise and Probiotic Intervention on Fatigue-Associated Biochemistry
3.5. Effects of Exercise and Probiotic Intervention on Clinical Biochemistry and CBC
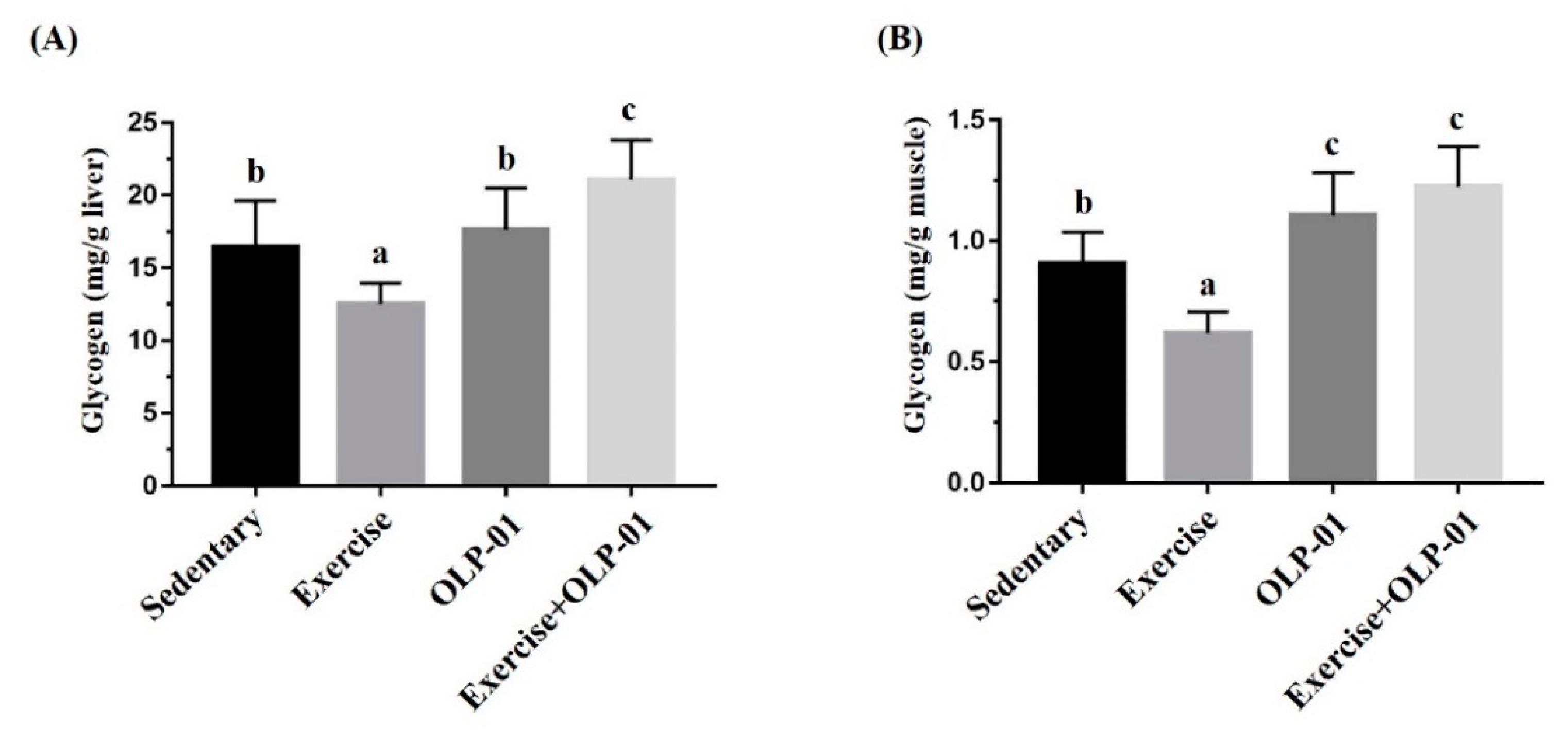
3.6. Effects of Exercise and Probiotic Intervention on Tissue Glycogen Content
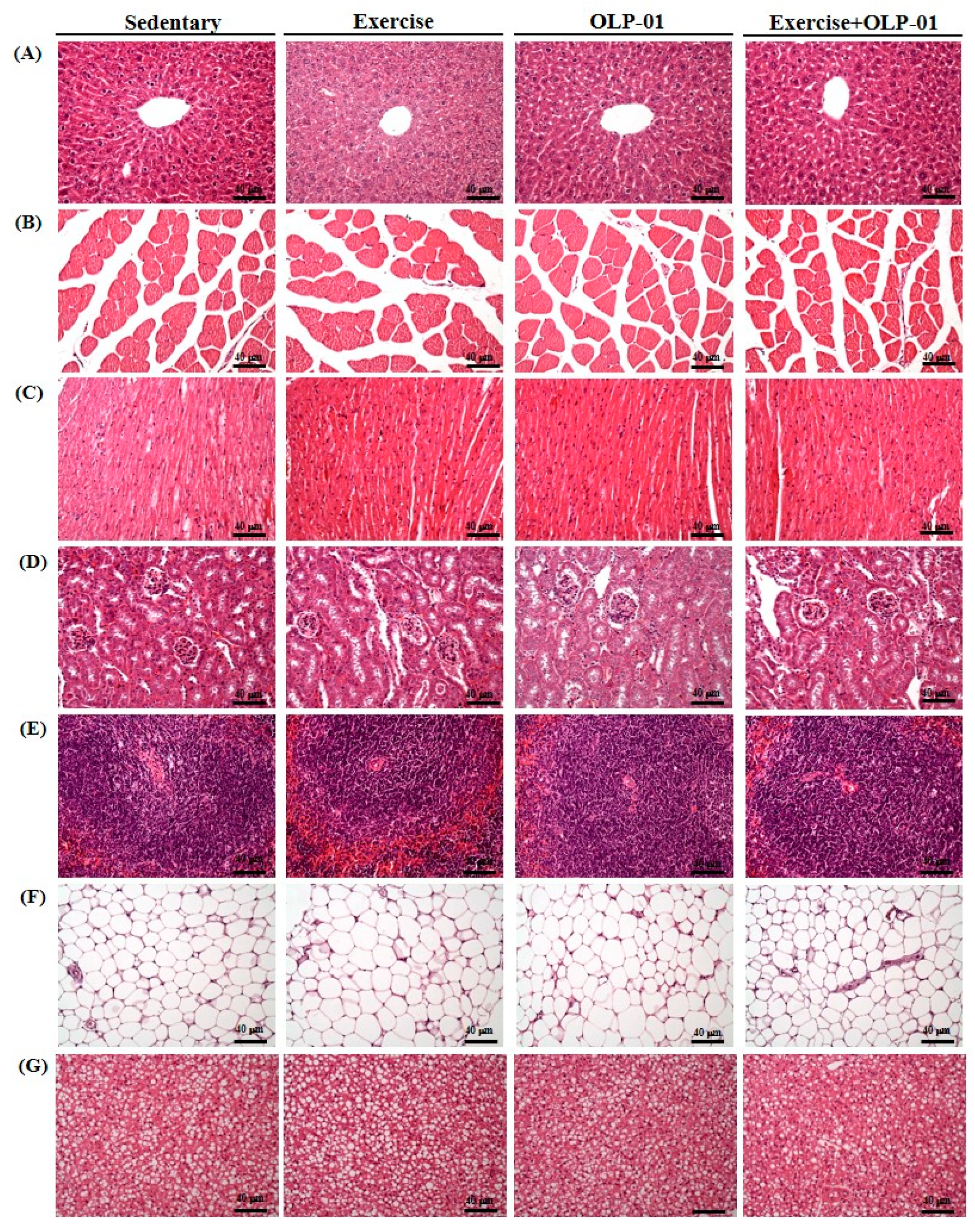
3.7. Effects of Exercise and Probiotics on Histology
3.8. Effects of Exercise and Probiotic Intervention on SCFAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kujala, U.M. Benefits of exercise therapy for chronic diseases. Br. J. Sports Med. 2006, 40, 3–4. [Google Scholar] [CrossRef]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of exercise physiology: Responses to acute exercise and long-term adaptations to training. Pm R. 2012, 4, 797–804. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.C.X.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrients 2019, 63–64, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Hajishafiee, M.; Ghiasvand, R.; Hariri, M.; Darvishi, L.; Ghassemi, S.; Iraj, B.; Hovsepian, V. Quercetin and vitamin C supplementation: Effects on lipid profile and muscle damage in male athletes. Int. J. Prev. Med. 2013, 1, S58–S62. [Google Scholar]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrients 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond Res. 2015, 29, 552–558. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrients 2018, 45, 125–134. [Google Scholar] [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr Pr. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Hunsche, C.; Cruces, J.; De la Fuente, M. Improvement of Redox State and Functions of Immune Cells as Well as of Behavioral Response in Aged Mice After Two-Week Supplementation of Fermented Milk with Probiotics. Curr. Microbiol. 2019, 76, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Hong, G.; Huang, C.; Qian, W.; Bai, T.; Song, J.; Song, Y.; Hou, X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2019, 117089. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lee, M.C.; Lee, C.C.; Ng, K.S.; Hsu, Y.J.; Tsai, T.Y.; Young, S.L.; Lin, J.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, H.G.; Lee, Y.R.; Lee, W.L. Moderate exercise training is more effective than resveratrol supplementation for ameliorating lipid metabolic complication in skeletal muscle of high fat diet-induced obese mice. J. Exerc. Nutr. Biochem. 2015, 19, 131–137. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-Rich Extract from Antrodia camphorata Improves Physical Fatigue and Exercise Performance in Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef]
- Chamberland, V.; Rioux, P. Not only students can express alcohol dehydrogenase: Goldfish can too! Adv. Physiol. Educ. 2010, 34, 222–227. [Google Scholar] [CrossRef]
- Musa, T.H.; Li, W.; Xiaoshan, L.; Guo, Y.; Wenjuan, Y.; Xuan, Y.; YuePu, P.; Pingmin, W. Association of normative values of grip strength with anthropometric variables among students, in Jiangsu Province. Homo 2018, 69, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M. Central and peripheral factors in fatigue. J. Sports Sci. 1995, 13, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Z.P.; Zhu, T.; Fung, H.Y.; Wong, T.L.; Wen, X.; Ma, D.L.; Leung, C.H.; Han, Q.B. Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice. Molecules 2017, 22, 155. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Yang, Y.; Gao, Y.; Sun, Q.; Liu, J.; Yang, X.; Wang, J.; Zhang, J. β-glucan Salecan Improves Exercise Performance and Displays Anti-Fatigue Effects through Regulating Energy Metabolism and Oxidative Stress in Mice. Nutrients 2018, 10, 858. [Google Scholar] [CrossRef]
- Yuan, T.; Wu, D.; Sun, K.; Tan, X.; Wang, J.; Zhao, T.; Ren, B.; Zhao, B.; Liu, Z.; Liu, X. Anti-Fatigue Activity of Aqueous Extracts of Sonchus arvensis L. in Exercise Trained Mice. Molecules 2019, 24, 1168. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, C.C.; Chuang, H.L.; Chiu, C.C.; Chen, W.C.; Hsu, M.C. Cornu cervi pantotrichum supplementation improves physiological adaptions during intensive endurance training. J. Vet. Med. Sci. 2017, 79, 674–682. [Google Scholar] [CrossRef]
- Stallknecht, B.; Vissing, J.; Galbo, H. Lactate production and clearance in exercise. Effects of training. A mini-review. Scand. J. Med. Sci. Sports 1998, 8, 127–131. [Google Scholar] [CrossRef]
- Lin, Q.Q.; Lin, R.; Ji, Q.L.; Zhang, J.Y.; Wang, W.R.; Yang, L.N.; Zhang, K.F. Effect of exercise training on renal function and renal aquaporin-2 expression in rats with chronic heart failure. Clin. Exp. Pharm. Physiol. 2011, 38, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Machida, M.; Kohara, A.; Omi, N.; Takemasa, T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J. Nutr. Sci. Vitam. 2011, 57, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kwak, Y.S. Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes. J. Exerc. Rehabil. 2016, 12, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta Physiol. 2018, 222. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Castell, L.M.; Nieman, D.C.; Bermon, S.; Peeling, P. Exercise-Induced Illness and Inflammation: Can Immunonutrition and Iron Help? Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 181–188. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharm. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Joisten, N.; Walzik, D.; Schenk, A.; Bloch, W.; Zimmer, P.; Wahl, P. Aqua cycling for immunological recovery after intensive, eccentric exercise. Eur. J. Appl. Physiol. 2019, 119, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Rejec, A.; Butinar, J.; Gawor, J.; Petelin, M. Evaluation of Complete Blood Count Indices (NLR, PLR, MPV/PLT, and PLCRi) in Healthy Dogs, Dogs With Periodontitis, and Dogs With Oropharyngeal Tumors as Potential Biomarkers of Systemic Inflammatory Response. J. Vet. Dent. 2017, 34, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Effect of Probiotics Supplementations on Health Status of Athletes. Int. J. Environ. Res. Public Health 2019, 16, 4469. [Google Scholar] [CrossRef] [PubMed]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Gollisch, K.S.; Holton, L.; Kim, Y.B.; Brandauer, J.; Fujii, N.L.; Hirshman, M.F.; Goodyear, L.J. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013, 280, 916–926. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhao, T.T.; Gui, D.K.; Gao, C.L.; Gu, J.L.; Gan, W.J.; Huang, W.; Xu, Y.; Zhou, H.; Chen, W.N.; et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Yamamura, R.; Nakamura, K.; Kitada, N.; Aizawa, T.; Shimizu, Y.; Nakamura, K.; Ayabe, T.; Kimura, T.; Tamakoshi, A. Associations of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci. Microbiota Food Health 2020, 39, 11–17. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, 956–966. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Sedentary | Exercise | OLP-01 | Exercise + OLP-01 |
|---|---|---|---|---|
| Liver (g) | 2.13 ± 0.29 | 2.08 ± 0.20 | 2.12 ± 0.28 | 2.13 ± 0.25 |
| Muscle (g) | 0.36 ± 0.04 | 0.34 ± 0.03 | 0.36 ± 0.04 | 0.35 ± 0.03 |
| Kidney (g) | 0.65 ± 0.08 | 0.66 ± 0.07 | 0.66 ± 0.09 | 0.65 ± 0.04 |
| Heart (g) | 0.20 ± 0.03 | 0.21 ± 0.02 | 0.21 ± 0.03 | 0.20 ± 0.03 |
| Spleen (g) | 0.26 ± 0.03 | 0.25 ± 0.11 | 0.26 ± 0.06 | 0.25 ± 0.06 |
| Cecum (g) | 0.72± 0.15 | 0.73± 0.11 | 0.69± 0.08 | 0.75± 0.18 |
| Perirenal Fat (g) | 0.124 ± 0.02 b | 0.094 ± 0.03 a | 0.123 ± 0.03 b | 0.086 ± 0.02 a |
| Water intake (mL/mouse/day) | 8.1 ± 0.9 | 8.2 ± 1.1 | 8.4 ± 1.2 | 8.3 ± 0.9 |
| Diet intake (g/mouse/day) | 7.5 ± 1.2 | 7.6 ± 1.5 | 7.7 ± 1.1 | 7.5± 1.3 |
| Time Point | Sedentary | Exercise | OLP-01 | Exercise + OLP-01 |
|---|---|---|---|---|
| Lactate (mmol/L) | ||||
| Before swimming (A) | 2.3 ± 0.3 a | 2.3 ± 0.3 a | 2.4 ± 0.2 a | 2.4 ± 0.3 a |
| After swimming (B) | 6.8 ± 0.8 c | 6.2 ± 0.3 b | 6.1 ± 0.2 b | 5.2 ± 0.5 a |
| After a 20 min rest(C) | 3.9 ± 0.5 b | 3.7 ± 0.6 a,b | 3.3± 0.8 a,b | 3.1 ± 0.5 a |
| Rate of lactate production and clearance | ||||
| Production rate = B/A | 2.96 ± 0.2 c | 2.72 ± 0.3 b, c | 2.51 ± 0.2 b | 2.14 ± 0.1 a |
| Clearance rate = (B − C)/B | 0.43 ± 0.06 | 0.41 ± 0.09 | 0.45 ± 0.15 | 0.40 ± 0.06 |
| Parameter | Sedentary | Exercise | OLP-01 | Exercise + OLP-01 |
|---|---|---|---|---|
| AST (U/L) | 64 ± 9 a | 92 ± 24 b | 69 ± 10 a | 62 ± 10 a |
| ALT (U/L) | 38 ± 5 | 43± 9 | 37 ± 4 | 37 ± 2 |
| CK (U/L) | 136 ± 41 a | 210 ± 69 b | 143 ± 47 a | 141 ± 37 a |
| GLU (mg/dL) | 147 ± 24 | 144 ± 23 | 148 ± 22 | 151 ± 29 |
| CREA (mg/dL) | 0.41± 0.03 | 0.40 ± 0.04 | 0.40 ± 0.03 | 0.41 ± 0.02 |
| BUN (mg/dL) | 23.1 ± 1.6 | 22.9 ± 1.5 | 23.0 ± 1.4 | 23.1 ± 1.4 |
| UA (mg/dL) | 2.41 ± 0.4 | 2.35 ± 0.6 | 2.41 ± 0.5 | 2.48 ± 0.4 |
| TC (mg/dL) | 136 ± 22 | 133 ± 27 | 130 ± 20 | 128 ± 13 |
| TG (mg/dL) | 147 ± 30 | 164 ± 26 | 140 ± 19 | 143 ± 19 |
| ALB (g/dL) | 3.2 ± 0.1 | 3.1 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.1 |
| TP (g/dL) | 5.5 ± 0.3 | 5.4 ± 0.4 | 5.3 ± 0.2 | 5.3 ± 0.3 |
| Parameter | Sedentary | Exercise | OLP-01 | Exercise + OLP-01 |
|---|---|---|---|---|
| WBC (103/μL) | 12.2 ± 4.5 | 12.4 ± 2.4 | 12.8 ± 5.3 | 11.5 ± 3.1 |
| Neu (%) | 17.7 ± 8.1 | 17.3± 7.5 | 18.1 ± 10.1 | 17.2 ± 5.2 |
| Lym (%) | 81.2 ± 9.2 | 80.4 ± 9.1 | 81.4 ± 11 | 81.2 ± 5.5 |
| Mono (%) | 0.74 ± 1.3 | 1.34 ± 1.7 | 0.2 ± 0.1 | 0.54 ± 0.4 |
| Eosi (%) | 0.14 ± 0.2 a | 0.64± 0.8 a,b | 0.21 ± 0.2 a,b | 0.78 ± 0.8 b |
| Baso (%) | 0.19 ± 0.1 | 0.28 ± 0.2 | 0.16 ± 0.1 | 0.27 ± 0.3 |
| Platelet (103/μL) | 1216 ± 283 b | 1646 ± 332 c | 957 ± 124 a | 1009 ± 145 a,b |
| PLR | 123 ± 22 a | 187 ± 37 b | 108 ± 18 a | 117 ± 17 a |
| NLR | 0.21 ± 0.08 | 0.26 ± 0.12 | 0.18 ± 0.06 | 0.20 ± 0.07 |
| SCFAs | Sedentary | Exercise | OLP-01 | Exercise + OLP-01 |
|---|---|---|---|---|
| acetic acid | 3.12 ± 0.34 a | 3.75± 0.54 a,b | 3.18 ± 0.41 a | 4.22 ± 0.39 b |
| propionic acid | 0.49 ± 0.08 a | 0.54± 0.07 a | 0.52 ± 0.08 a | 0.71 ± 0.11 b |
| isobutyric acid | 0.02 ± 0.003 a | 0.02 ± 0.004 a | 0.02 ± 0.006 a | 0.03 ± 0.006 a |
| butyric acid | 0.53 ± 0.15 a | 0.85 ± 0.28 b, c | 0.59 ± 0.24 a,b | 1.16 ± 0.18 c |
| valeric acid | 0.053 ± 0.02 | 0.029 ± 0.01 | 0.025 ± 0.02 | 0.048 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Hsu, Y.-J.; Huang, C.-C.; Liu, H.-C.; Lee, M.-C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. https://doi.org/10.3390/nu12041145
Huang W-C, Hsu Y-J, Huang C-C, Liu H-C, Lee M-C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients. 2020; 12(4):1145. https://doi.org/10.3390/nu12041145
Chicago/Turabian StyleHuang, Wen-Ching, Yi-Ju Hsu, Chi-Chang Huang, Hsuan-Chen Liu, and Mon-Chien Lee. 2020. "Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance" Nutrients 12, no. 4: 1145. https://doi.org/10.3390/nu12041145
APA StyleHuang, W.-C., Hsu, Y.-J., Huang, C.-C., Liu, H.-C., & Lee, M.-C. (2020). Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients, 12(4), 1145. https://doi.org/10.3390/nu12041145





