Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers
Abstract
1. Introduction
2. Materials and Methods
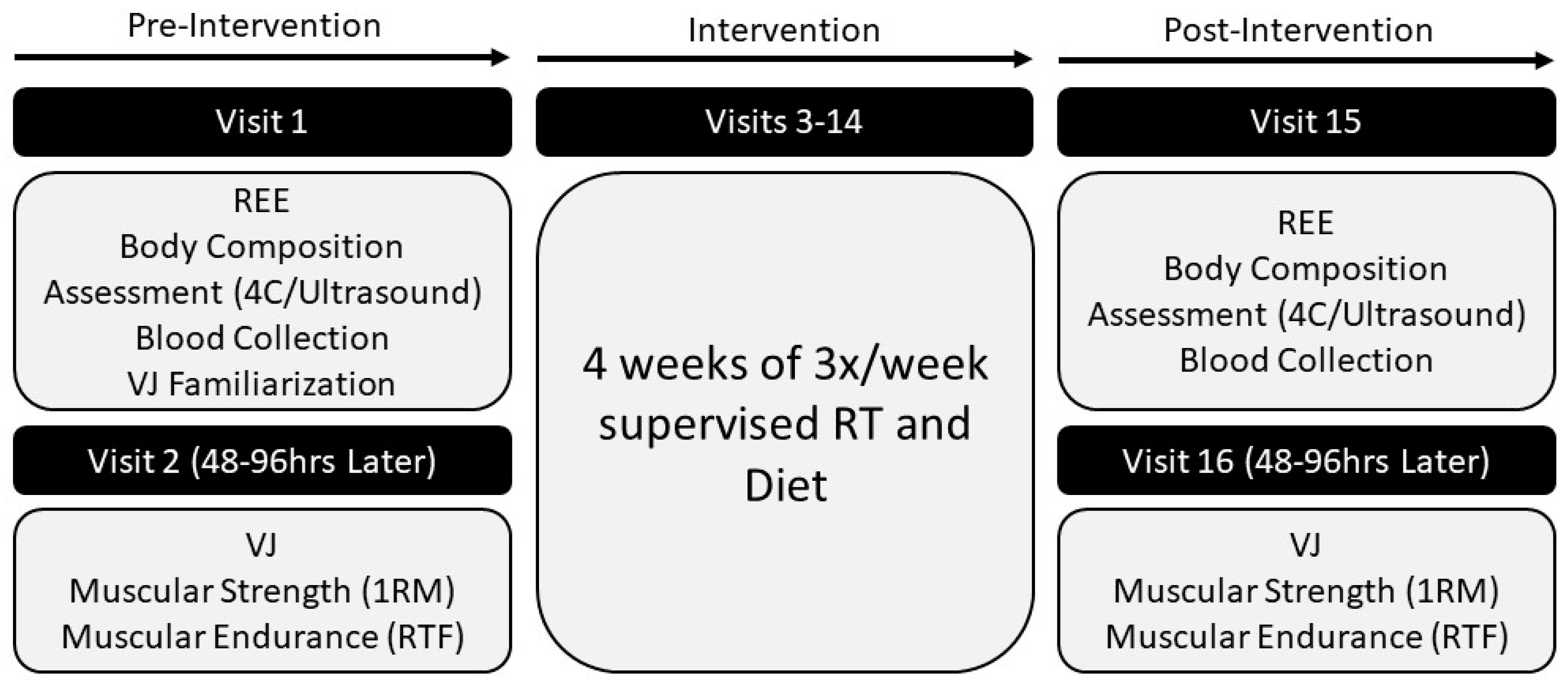
2.1. Experimental Design
2.2. Participants
2.3. Laboratory Assessments
2.3.1. Overview
2.3.2. Anthropometric Measurements
2.3.3. Body Composition
2.3.4. Ultrasound
2.3.5. Resting Energy Expenditure
2.3.6. Blood Sampling and Analysis
2.3.7. Vertical Jump
2.3.8. Estimated One Repetition Maximum
2.3.9. Muscular Endurance
2.3.10. Questionnaires
2.4. Intervention
2.4.1. Dietary Program
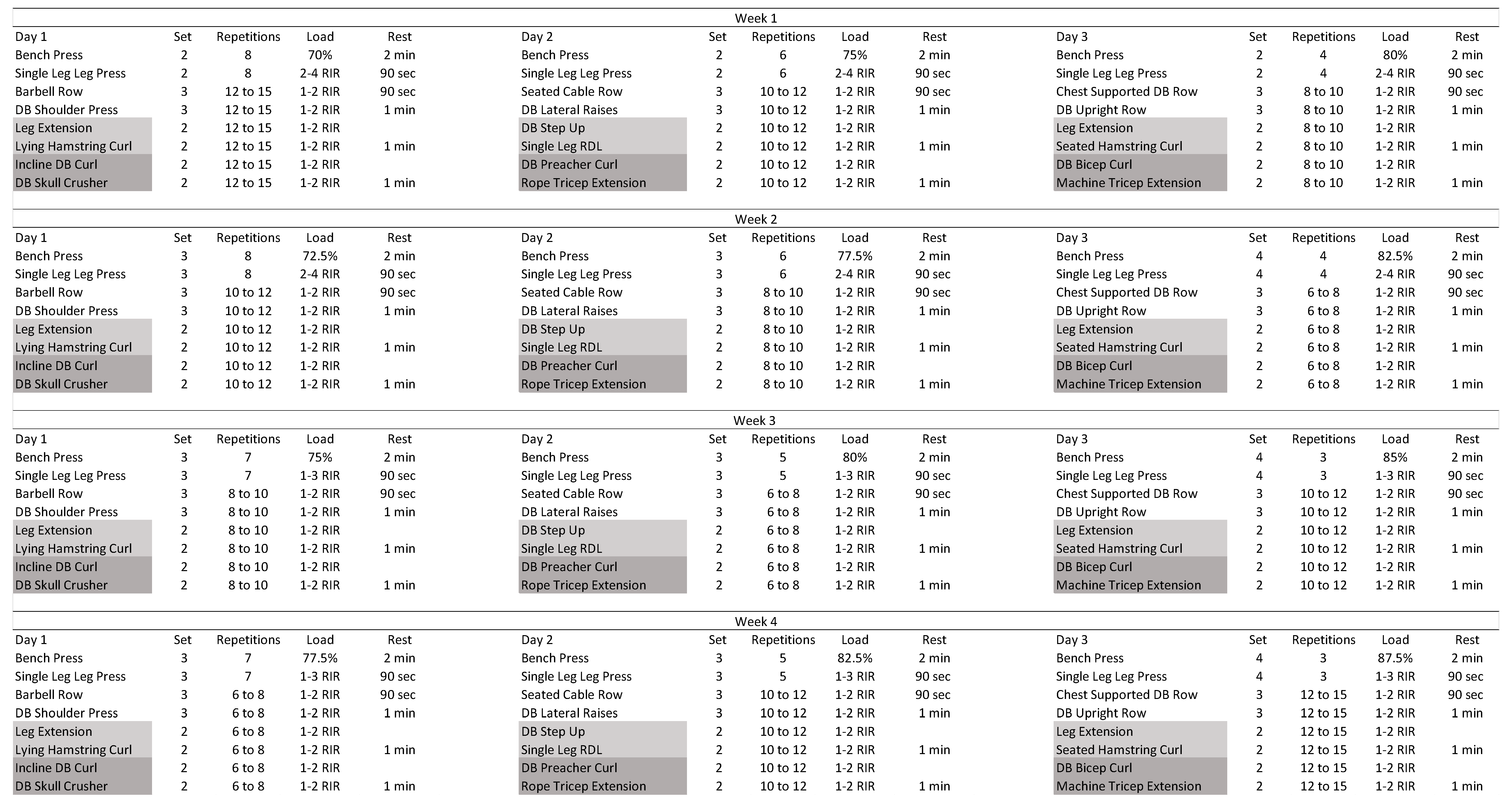
2.4.2. Resistance Training Protocol
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Dietary Intake
3.3. Body Composition and Muscle Morphology
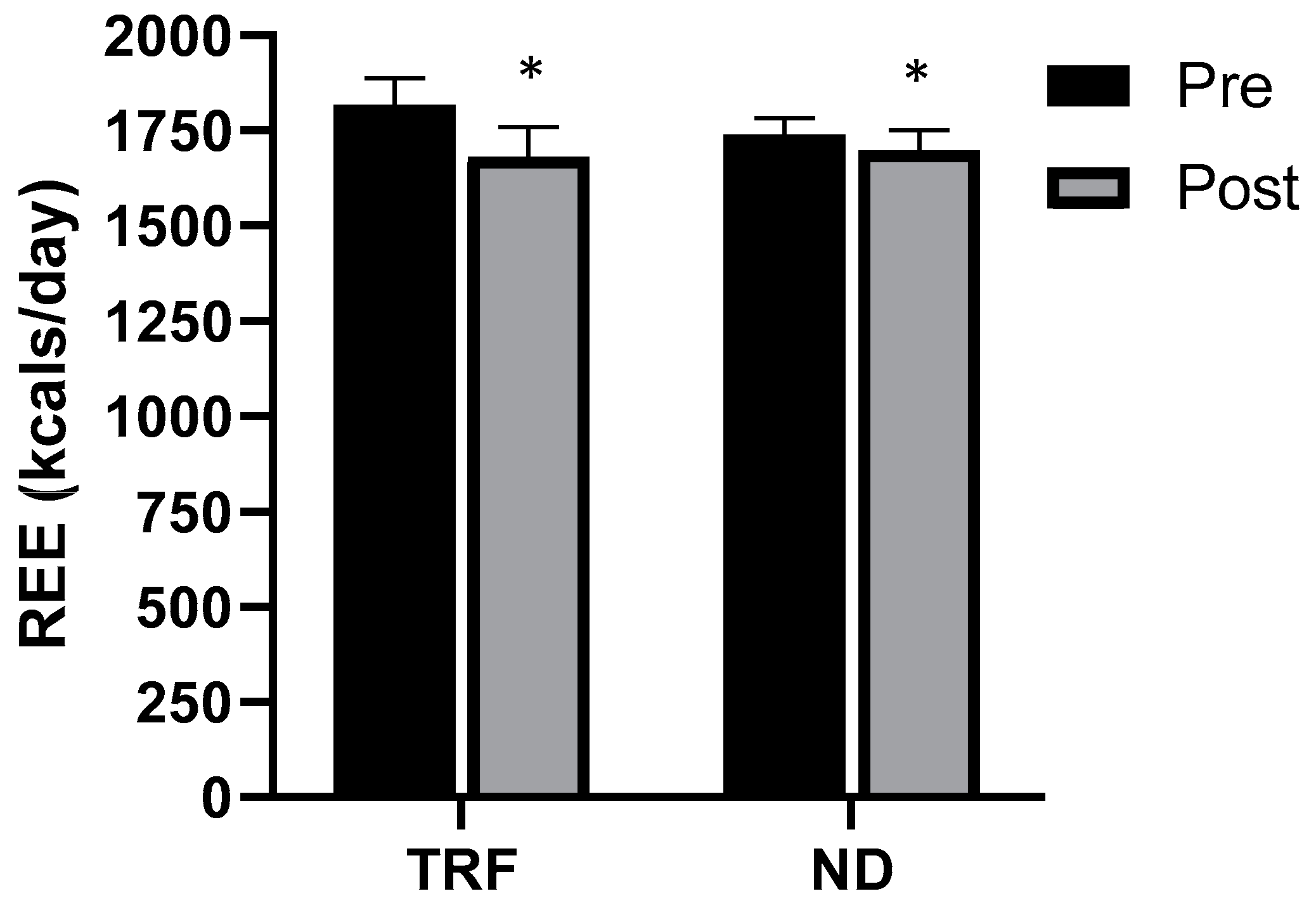
3.4. Resting Energy Expenditure
3.5. Muscular Performance: Vertical Jump
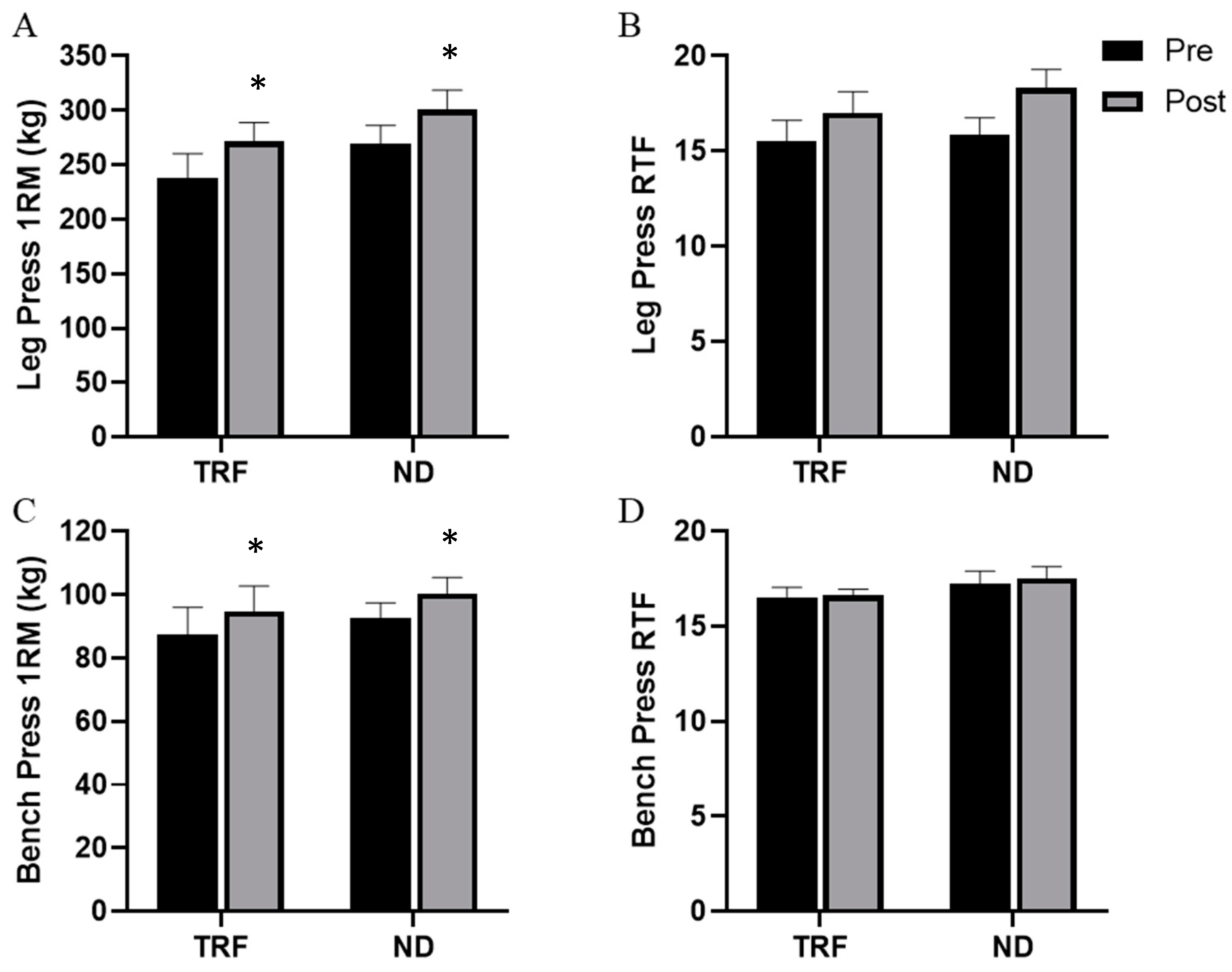
3.6. Muscular Performance: Strength and Endurance
3.7. Psychometric Parameters
3.8. Blood Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.; Wood, R.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Gann, J.G.; La Bounty, P.M. Intermittent Fasting Programs and Their Effects on Body Composition. Strength Cond. J. 2015, 37, 60–71. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2016, 39, 46–58. [Google Scholar] [CrossRef]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, E593–E601. [Google Scholar] [CrossRef]
- Harvie, M.N.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, D.G.R.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, D.G.R.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2010, 35, 714–727. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, N.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults123. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Donahoo, W.; McCall, K.; Brannon, S.; Melanson, E.L.; Gozansky, W.S. Effect of 8 Weeks of Intermittent Fasting on Weight Loss, Body Composition, and Insulin Sensitivity in Obese Individuals. Obesity 2009, 17, S258. [Google Scholar]
- Klempel, M.C.; Kroeger, C.M.; Varady, K.A. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metab. Clin. Exp. 2013, 62, 137–143. [Google Scholar] [CrossRef]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, N.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.R.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Boil. Med. 2007, 43, 1348. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011, 10, 119. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; Van Dusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2016, 17, 200–207. [Google Scholar] [CrossRef]
- Toombs, R.J.; Ducher, G.; Shepherd, J.A.; De Souza, M.J. The Impact of Recent Technological Advances on the Trueness and Precision of DXA to Assess Body Composition. Obesity 2011, 20, 30–39. [Google Scholar] [CrossRef]
- Wang, Z.; Pi-Sunyer, F.X.; Kotler, D.P.; Wielopolski, L.; Withers, R.T.; Pierson, R.N.; Heymsfield, S.B. Multicomponent methods: Evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. Am. J. Clin. Nutr. 2002, 76, 968–974. [Google Scholar] [CrossRef]
- Thomaes, T.; Thomis, M.; Onkelinx, S.; Coudyzer, W.; Cornelissen, V.; Vanhees, L. Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD-patients. BMC Med. Imaging 2012, 12, 7. [Google Scholar] [CrossRef]
- Bemben, M.G. Use of diagnostic ultrasound for assessing muscle size. J. Strength Cond. Res. 2002, 16, 103–108. [Google Scholar]
- Jenkins, N.D.; Miller, J.M.; Buckner, S.L.; Cochrane, K.C.; Bergstrom, H.C.; Hill, E.C.; Smith, C.; Housh, T.; Cramer, J.T. Test–Retest Reliability of Single Transverse versus Panoramic Ultrasound Imaging for Muscle Size and Echo Intensity of the Biceps Brachii. Ultrasound Med. Boil. 2015, 41, 1584–1591. [Google Scholar] [CrossRef]
- Young, H.-J.; Jenkins, N.T.; Zhao, Q.; McCully, K.K. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef]
- Pardo, E.; El Behi, H.; Boizeau, P.; Verdonk, F.; Alberti, C.; Lescot, T. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol. 2018, 18, 205. [Google Scholar] [CrossRef]
- Wells, A.J.; Fukuda, D.H.; Hoffman, J.R.; Gonzalez, A.M.; Jajtner, A.R.; Townsend, J.R.; Mangine, G.T.; Fragala, M.S.; Stout, J.R. Vastus lateralis exhibits non-homogenous adaptation to resistance training. Muscle Nerve 2014, 50, 785–793. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Zourdos, M.C.; Klemp, A.; Dolan, C.; Quiles, J.M.; Schau, K.A.; Jo, E.; Helms, E.; Esgro, B.; Duncan, S.; Merino, S.G.; et al. Novel Resistance Training–Specific Rating of Perceived Exertion Scale Measuring Repetitions in Reserve. J. Strength Cond. Res. 2016, 30, 267–275. [Google Scholar] [CrossRef]
- Laurent, C.M.; Green, J.M.A.; Bishop, P.; Sjökvist, J.E.; Schumacker, R.; Richardson, M.T.; Curtner-Smith, M. A Practical Approach to Monitoring Recovery: Development of a Perceived Recovery Status Scale. J. Strength Cond. Res. 2011, 25, 620–628. [Google Scholar] [CrossRef]
- Rushall, B.S. A tool for measuring stress tolerance in elite athletes. J. Appl. Sport Psychol. 1990, 2, 51–66. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Mettler, S.; Mitchell, N.; Tipton, K. Increased Protein Intake Reduces Lean Body Mass Loss during Weight Loss in Athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef]
- Phillips, S.M. A brief review of higher dietary protein diets in weight loss: A focus on athletes. Sports Med. 2014, 44, S149–S153. [Google Scholar] [CrossRef]
- Morton, R.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.A.; Aragon, A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2017, 52, 376–384. [Google Scholar] [CrossRef]
- Schwartz, A.; Doucet, E. Relative changes in resting energy expenditure during weight loss: A systematic review. Obes. Rev. 2009, 11, 531–547. [Google Scholar] [CrossRef]
- Fukunaga, T.; Miyatani, M.; Tachi, M.; Kouzaki, M.; Kawakami, Y.; Kanehisa, H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol. Scand. 2001, 172, 249–255. [Google Scholar] [CrossRef]
- Castro, M.J.; McCann, D.J.; Shaffrath, J.D.; Adams, W.C. Peak torque per unit cross-sectional area differs between strength-trained and untrained young adults. Med. Sci. Sports Exerc. 1995, 27, 397–403. [Google Scholar] [CrossRef]
- Maughan, R.J.; Watson, J.S.; Weir, J. Relationships between muscle strength and muscle cross-sectional area in male sprinters and endurance runners. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 309–318. [Google Scholar] [CrossRef]
- Alway, S.E.; Stray-Gundersen, J.; Grumbt, W.H.; Gonyea, W.J. Muscle cross-sectional area and torque in resistance-trained subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 86–90. [Google Scholar] [CrossRef]
- Folland, J.; Williams, A. The adaptations to strength training: Morphological and neurological contributions to increased strength. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef]
- Jones, E.J.; Bishop, P.A.; Woods, A.K.; Green, J.M. Cross-Sectional Area and Muscular Strength. Sports Med. 2008, 38, 987–994. [Google Scholar] [CrossRef]
- DeFreitas, J.M.; Beck, T.W.; Stock, M.S.; Dillon, M.A.; Kasishke, P.R. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur. J. Appl. Physiol. Occup. Physiol. 2011, 111, 2785–2790. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; Stone, M.H.; Koziris, L.P.; Thrush, J.T.; Fleck, S.J. Relationships Between Serum Testosterone, Cortisol, and Weightlifting Performance. J. Strength Cond. Res. 2000, 14, 338. [Google Scholar] [CrossRef]
- Hakkinen, A.; Pakarinen, A.; Alen, M.; Kauhanen, H.; Komi, P. Relationships between Training Volume, Physical Performance Capacity, and Serum Hormone Concentrations during Prolonged Training in Elite Weight Lifters. Int. J. Sports Med. 1987, 8, 61–65. [Google Scholar] [CrossRef]
- Diver, M.J.; Imtiaz, K.E.; Ahmad, A.M.; Vora, J.P.; Fraser, W.D. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin. Endocrinol. 2003, 58, 710–717. [Google Scholar] [CrossRef]
- Axelsson, J.; Ingre, M.; Åkerstedt, T.; Holmbäck, U. Effects of Acutely Displaced Sleep on Testosterone. J. Clin. Endocrinol. Metab. 2005, 90, 4530–4535. [Google Scholar] [CrossRef]
- Penev, P.D. Association between sleep and morning testosterone levels in older men. Sleep 2007, 30, 427–432. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; Dejager, J.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef]
- Galvão-Teles, A.; Graves, L.; Burke, C.W.; Fotherby, K.; Fraser, R. Free cortisol in obesity; effect of fasting. Eur. J. Endocrinol. 1976, 81, 321–329. [Google Scholar] [CrossRef]
- Rideout, C.A.; Linden, W.; Barr, S.I. High cognitive dietary restraint is associated with increased cortisol excretion in postmenopausal women. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2006, 61, 628–633. [Google Scholar] [CrossRef]
- Degoutte, F.; Jouanel, P.; Bègue, R.J.; Colombier, M.; Lac, G.; Pequignot, J.M.; Filaire, E. Food Restriction, Performance, Biochemical, Psychological, and Endocrine Changes in Judo Athletes. Int. J. Sports Med. 2006, 27, 9–18. [Google Scholar] [CrossRef]
- Anderson, D.A.; Shapiro, J.R.; Lundgren, J.D.; Spataro, L.E.; Frye, C.A. Self-reported dietary restraint is associated with elevated levels of salivary cortisol. Appetite 2002, 38, 13–17. [Google Scholar] [CrossRef]
- Vance, M.L.; Thorner, M.O. Fasting Alters Pulsatile and Rhythmic Cortisol Release in Normal Man. J. Clin. Endocrinol. Metab. 1989, 68, 1013–1018. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | TRF (n = 13) | ND (n = 13) | p-Value |
|---|---|---|---|
| Age (yr) | 22.9 ± 3.6 | 22.5 ± 2.2 | 0.743 |
| Height (cm) | 178.1 ± 7.3 | 177.5 ± 8.8 | 0.862 |
| Body Mass (kg) | 82.0 ± 10.6 | 83.3 ± 15.0 | 0.804 |
| Body Fat (%) | 19.9 ± 8.3 | 18.9 ± 7.4 | 0.742 |
| Fat Mass (kg) | 16.8 ± 8.6 | 16.3 ± 8.5 | 0.886 |
| Fat Free Mass (kg) | 65.2 ± 6.6 | 67.0 ± 9.5 | 0.588 |
| Vertical Jump Height (cm) | 30.5 ± 6.4 | 34.2 ± 9.4 | 0.241 |
| Bench Press 1RM (kg) | 87.6 ± 30.4 | 92.6 ± 17.2 | 0.606 |
| Leg Press 1RM (kg) | 238.1 ± 80.0 | 269.5 ± 60.8 | 0.271 |
| Bench Press RTF | 16.5 ± 1.9 | 17.2 ± 2.4 | 0.421 |
| Leg Press RTF | 15.5 ± 3.9 | 15.8 ± 3.3 | 0.830 |
| Dietary Component | TRF (n = 13) | ND (n = 13) | p-Value |
|---|---|---|---|
| Protein (g/kg) | 1.83 ± 0.10 | 1.83 ± 0.07 | 0.956 |
| Carbohydrate (g/kg) | 2.28 ± 0.71 | 2.25 ± 0.51 | 0.889 |
| Fat (g/kg) | 0.84 ± 0.26 | 0.85 ±0.22 | 0.917 |
| Total Kcal | 1946 ± 310 | 1939 ± 260 | 0.950 |
| Relative Kcal (g/kg) | 23.81 ± 3.25 | 23.64 ± 3.07 | 0.892 |
| Muscle | TRF Pre | TRF Post | ND Pre | ND Post | p-Value | ηp2 |
|---|---|---|---|---|---|---|
| VL CSA (cm2) | 31.0 ± 7.8 | 33.7 ± 5.3 | 36.0 ± 7.3 | 37.5 ± 5.3 | 0.002* | 0.329 |
| VL MT (cm) | 1.8 ± 0.4 | 2.0 ± 0.4 | 2.1 ± 0.4 | 2.2 ± 0.4 | 0.065 | 0.135 |
| VL EI (AU) | 57.4 ± 12.8 | 53.1 ± 12.4 | 49.2 ± 8.1 | 46.6 ± 7.4 | 0.001* | 0.368 |
| RF CSA (cm2) | 12.6 ± 2.4 | 12.7 ± 2.1 | 14.6 ± 4.3 | 15.0 ± 4.5 | 0.228 | 0.060 |
| RF MT (cm) | 2.39 ± 0.4 | 2.35 ± 0.4 | 2.71 ± 0.5 | 2.70 ± 0.5 | 0.462 | 0.023 |
| RF EI (AU) | 63.8 ± 16.7 | 63.8 ± 18.2 | 58.2 ± 12 | 55.3 ± 13.8 | 0.182 | 0.073 |
| BB CSA (cm2) | 11.9 ± 2.2 | 12.7 ± 1.9 | 12.6 ± 2.1 | 13.3 ± 2.5 | 0.013* | 0.229 |
| BB MT (cm) | 2.3 ± 0.4 | 2.6 ± 0.2 | 2.3 ± 0.4 | 2.6 ± 0.3 | <0.001* | 0.534 |
| BB EI (AU) | 93.3 ± 12.4 | 88.1 ± 11.9 | 88.2 ± 13.6 | 87.9 ± 7.3 | 0.177 | 0.074 |
| Biomarker | TRF Pre | TRF Post | Inter-Assay CV (%) | ND Pre | ND Post | Inter-Assay CV (%) | p-Value (Main Effect for Time) | ηp2 (Main Effect for Time) | p-Value (Group × Time Interaction) | ηp2 (Group × Time Interaction) |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum Testosterone (ng/dL) | 587.8 ± 8.7 | 581.2 ± 8.7 | 1.3 | 557.2 ± 8.7 | 556.1 ± 10.3 | 1.1 | 0.041 * | 0.163 | 0.140 | 0.088 |
| Plasma Cortisol (ng/mL) | 118.3 ± 31.9 | 106.1 ± 49.7 | 6.6 | 119.2 ± 37.7 | 150.7 ± 39.1 | 4.3 | 0.348 | 0.037 | 0.040 *† | 0.165 |
| Serum Adiponectin (µg/mL) | 5.5 ± 2.3 | 4.7 ± 1.8 | 3.2 | 5.1 ± 2.0 | 4.4 ± 1.9 | 4.7 | <0.001 * | 0.417 | 0.721 | 0.005 |
| Plasma Leptin (pg/mL) | 4680 ± 4566 | 3689 ± 4512 | 4.2 | 4773 ± 3884 | 3629 ± 3105 | 5.6 | 0.001 * | 0.351 | 0.800 | 0.003 |
| Plasma Ghrelin (pg/mL) | 2226 ± 1205 | 2296 ± 945 | 1.2 | 3107 ± 1172 | 3217 ± 1216 | 2.8 | 0.427 | 0.027 | 0.856 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients 2020, 12, 1126. https://doi.org/10.3390/nu12041126
Stratton MT, Tinsley GM, Alesi MG, Hester GM, Olmos AA, Serafini PR, Modjeski AS, Mangine GT, King K, Savage SN, et al. Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients. 2020; 12(4):1126. https://doi.org/10.3390/nu12041126
Chicago/Turabian StyleStratton, Matthew T., Grant M. Tinsley, Michaela G. Alesi, Garrett M. Hester, Alex A. Olmos, Paul R. Serafini, Andrew S. Modjeski, Gerald T. Mangine, Kelsey King, Shelby N. Savage, and et al. 2020. "Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers" Nutrients 12, no. 4: 1126. https://doi.org/10.3390/nu12041126
APA StyleStratton, M. T., Tinsley, G. M., Alesi, M. G., Hester, G. M., Olmos, A. A., Serafini, P. R., Modjeski, A. S., Mangine, G. T., King, K., Savage, S. N., Webb, A. T., & VanDusseldorp, T. A. (2020). Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients, 12(4), 1126. https://doi.org/10.3390/nu12041126






