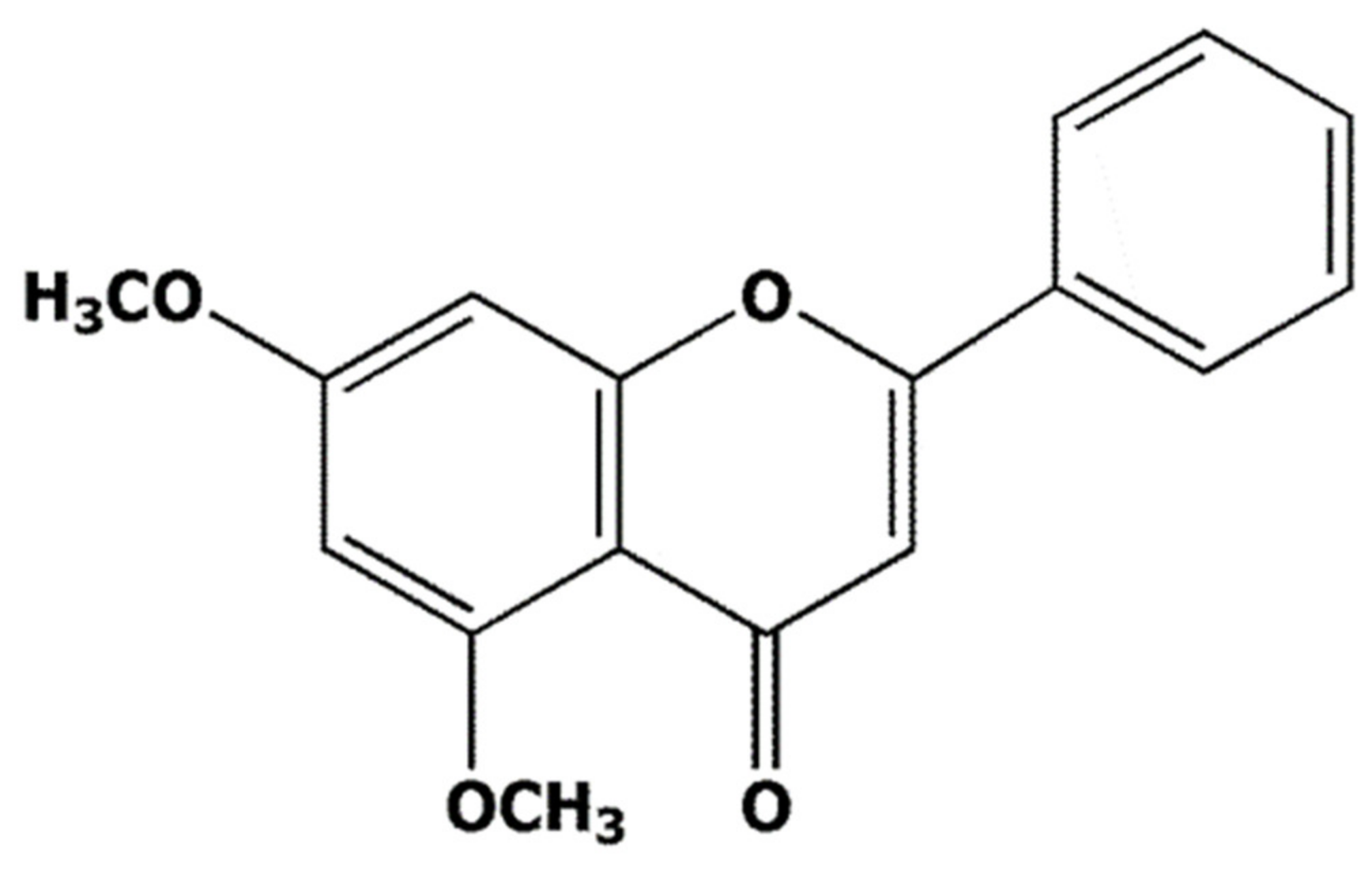
The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Animal Experiments
2.3. Grip Strength Test
2.4. Treadmill Test
2.5. Histological Analysis
2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.7. Western Blot Analysis
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Analysis of Mitochondrial DNA Content
2.10. Statistical Analysis
3. Results
3.1. DMF Enhances Physical Performances in Aged Mice
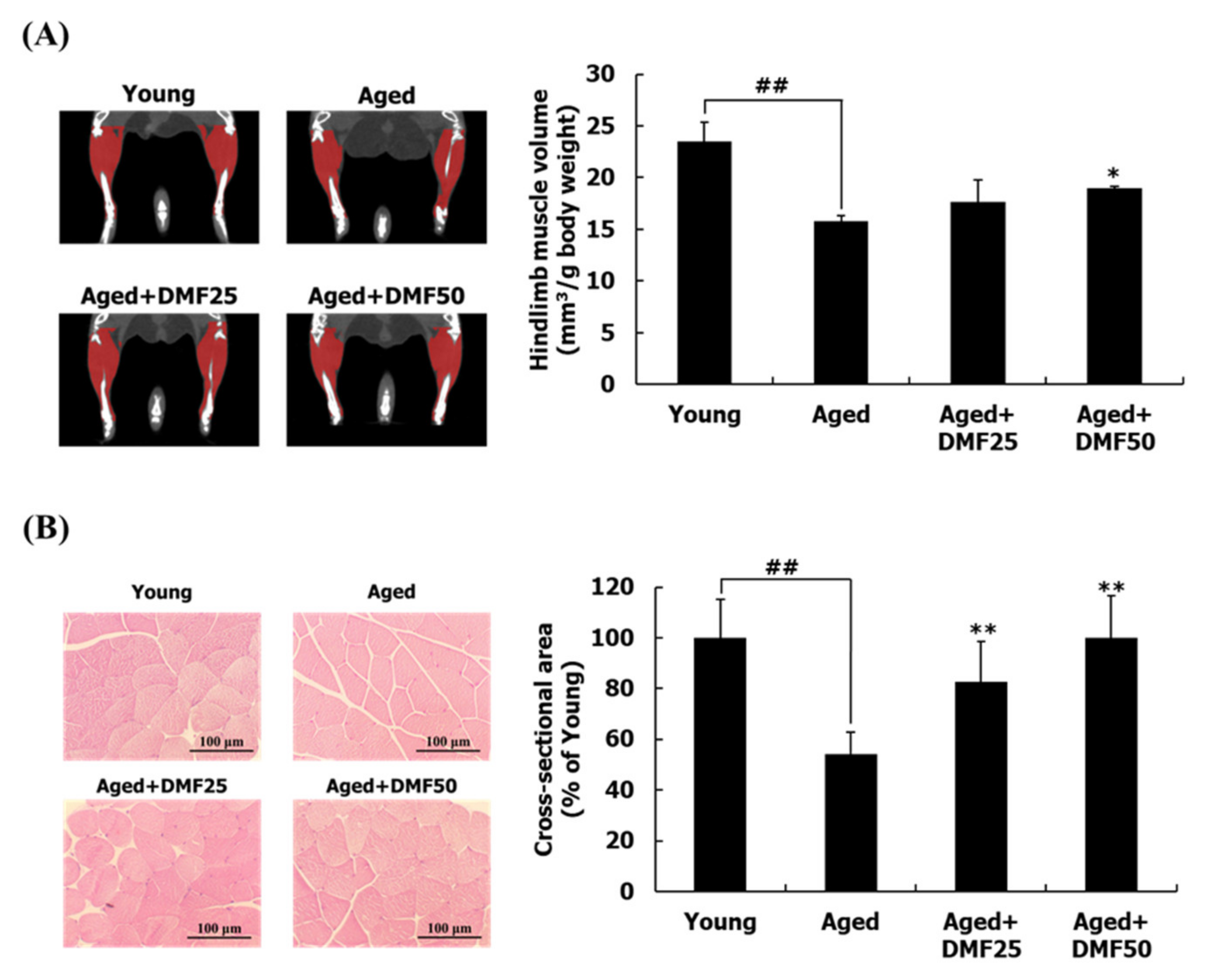
3.2. DMF Increases Muscle Volume, Weights, and Cross-Sectional Area
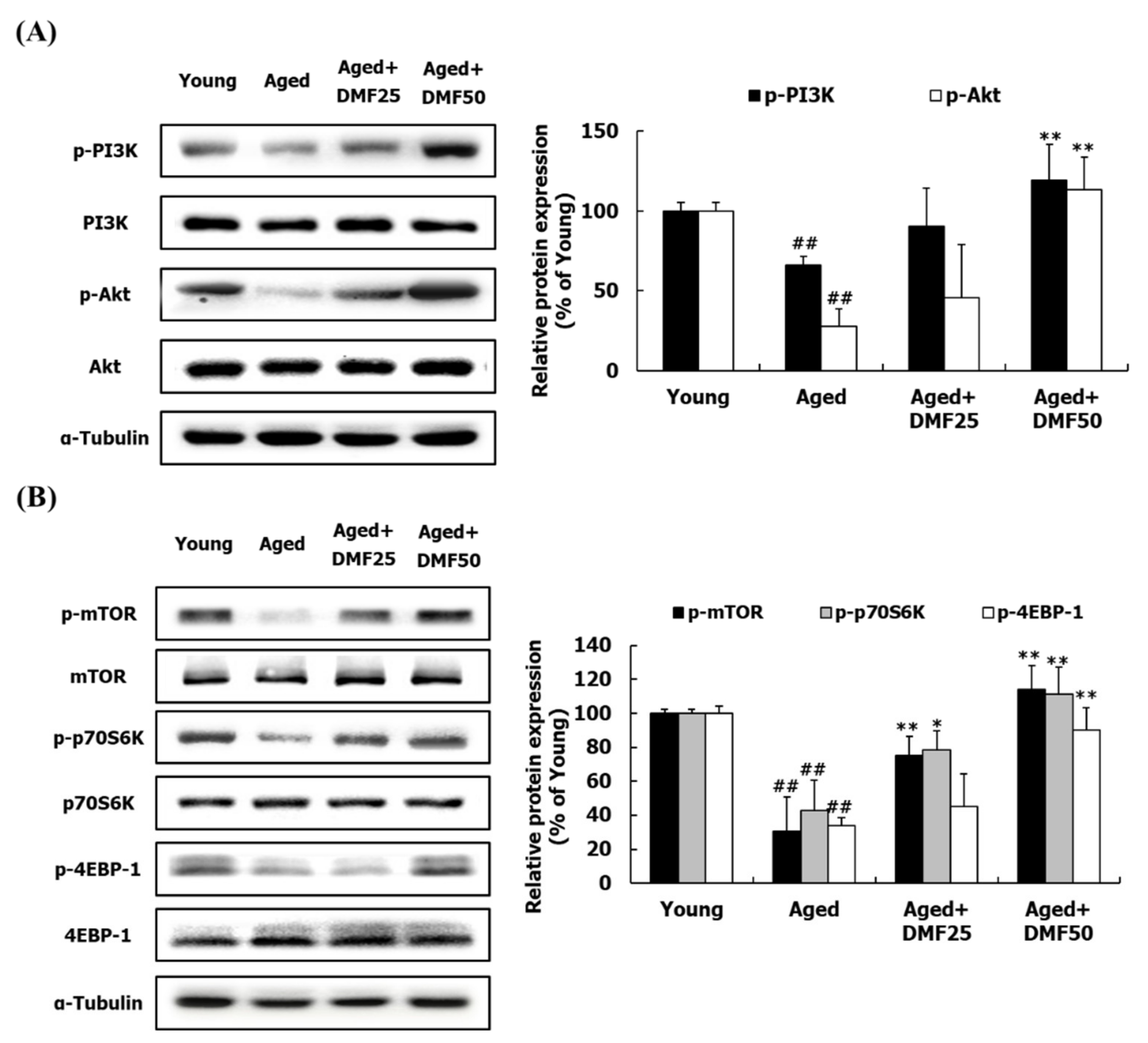
3.3. DMF Increases PI3K-Akt and mTOR Pathways
3.4. DMF Decreases FoxO Pathways
3.5. DMF Increases Mitochondrial Biogenesis
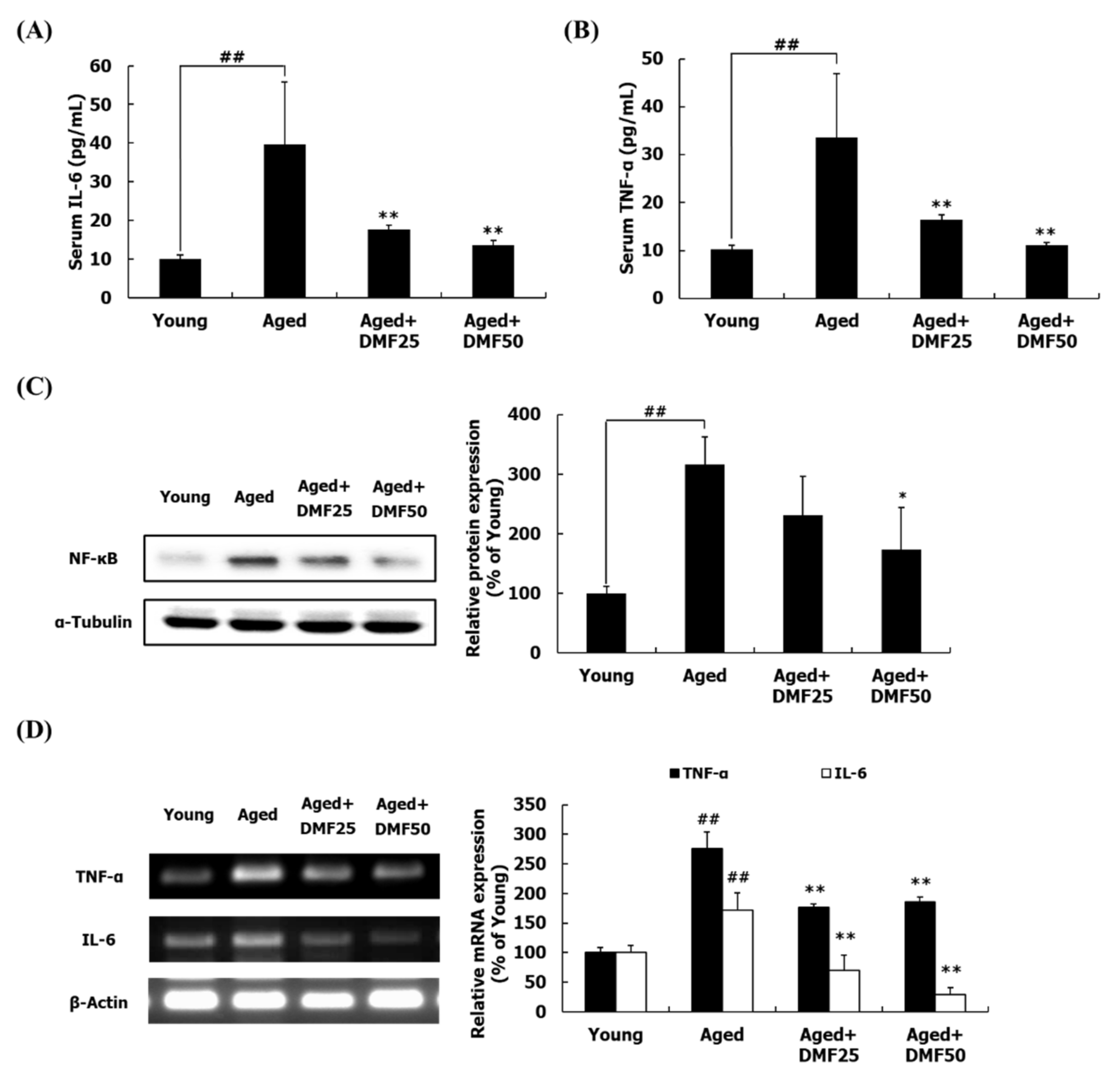
3.6. DMF Attenuates Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, D.; Vidal, H. TNF-α-and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Yu, H.; Gan, L.; Ye, Y.; Lin, L. Natural constituents from food sources: Potential therapeutic agents against muscle wasting. Food Funct. 2019, 10, 6967–6986. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Zhang, X.; Fielding, R.A.; Lanza, I.; LeBrasseur, N.K. The clinical impact and biological mechanisms of skeletal muscle aging. Bone 2019, 127, 26–36. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Ma, J.; Kang, S.Y.; Meng, X.; Kang, A.N.; Park, J.H.; Park, Y.-K.; Jung, H.W. Effects of rhizome extract of Dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in C2C12 myotubes. Molecules 2018, 23, 2023. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, Y.T.; Liu, H.W.; Chan, Y.C.; Liu, M.Y.; Hu, S.H.; Tseng, W.T.; Wu, H.L.; Wang, M.F.; Chang, S.J. Oligonol alleviates sarcopenia by regulation of signaling pathways involved in protein turnover and mitochondrial quality. Mol. Nutr. Food Res. 2019, 63, 1801102. [Google Scholar] [CrossRef]
- Choi, W.H.; Son, H.J.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin ameliorates the obesity-induced skeletal muscle atrophy by attenuating mitochondrial dysfunction in the muscle of obese mice. Mol. Nutr. Food Res. 2017, 61, 1700218. [Google Scholar] [CrossRef]
- Wang, D.-T.; Yin, Y.; Yang, Y.-J.; Lv, P.-J.; Shi, Y.; Lu, L.; Wei, L.-B. Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Sa, B.-K.; Kim, C.; Kim, M.-B.; Hwang, J.-K. Panduratin A prevents tumor necrosis factor-alpha-induced muscle atrophy in L6 rat skeletal muscle cells. J. Med. Food 2017, 20, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.D.; Selvadurai, H.; Tein, I. Bioenergetic provision of energy for muscular activity. Paediatr. Respir. Rev. 2009, 10, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-B.; Kim, T.; Kim, C.; Hwang, J.-K. Standardized Kaempferia parviflora extract enhances exercise performance through activation of mitochondrial biogenesis. J. Med. Food 2018, 21, 30–38. [Google Scholar] [CrossRef]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflügers Arch. 2017, 469, 573–591. [Google Scholar] [CrossRef]
- Curcio, F.; Ferro, G.; Basile, C.; Liguori, I.; Parrella, P.; Pirozzi, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Tocchetti, C.G. Biomarkers in sarcopenia: A multifactorial approach. Exp. Gerontol. 2016, 85, 1–8. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim. Biophys. Acta 2012, 1820, 532–541. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Comprehensive approach to sarcopenia treatment. Curr. Clin. Pharmacol. 2014, 9, 171–180. [Google Scholar] [CrossRef]
- Ono, Y.; Sakamoto, K. Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-κB signaling pathway and myoblast-derived tumor necrosis factor-α. PLoS One 2017, 12, e0182040. [Google Scholar] [CrossRef]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S. Isoflavones derived from soy beans prevent MuRF1-mediated muscle atrophy in C2C12 myotubes through SIRT1 activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef]
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharmacol. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in raw 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Song, Y.; Kim, M.-B.; Kim, C.; Kim, J.; Hwang, J.-K. 5,7-Dimethoxyflavone attenuates obesity by inhibiting adipogenesis in 3T3-L1 adipocytes and high-fat diet-induced obese C57BL/6J mice. J. Med. Food 2016, 19, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-G.; Choi, E.-J.; Choi, Y.; Hwang, J.-K. 5,7-Dimethoxyflavone induces melanogenesis in B16F10 melanoma cells through cAMP-dependent signalling. Exp. Dermatol. 2011, 20, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Takeda, S.; Hitoe, S.; Nakamura, S.; Matsuda, H.; Shimoda, H. Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J. Nat. Med. 2016, 70, 163–172. [Google Scholar] [CrossRef]
- Horigome, S.; Yoshida, I.; Tsuda, A.; Harada, T.; Yamaguchi, A.; Yamazaki, K.; Inohana, S.; Isagawa, S.; Kibune, N.; Satoyama, T. Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora. Biosci. Biotechnol. Biochem. 2014, 78, 851–860. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.R.; Campos, G.R. Skeletal muscle fiber types in C57BL6J mice. Braz. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Van Dijk, M.; Nagel, J.; Dijk, F.J.; Salles, J.; Verlaan, S.; Walrand, S.; Van Norren, K.; Luiking, Y. Sarcopenia in older mice is characterized by a decreased anabolic response to a protein meal. Arch. Gerontol. Geriatr. 2017, 69, 134–143. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xie, F.; Muzik, O. Alteration of copper fluxes in brain aging: A longitudinal study in rodent using 64CuCl2-PET/CT. Aging Dis. 2018, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.-B.; Hwang, J.-K. Standardized Kaempferia parviflora wall. Ex baker (Zingiberaceae) extract inhibits fat accumulation and muscle atrophy in ob/ob mice. Evid. Based Complement. Alternat. Med. 2018, 2018, 8161042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-K.; Jie, L.; Jin, L.; Baosheng, G.; Leung, A.; Zhang, G.; Zhang, B.-T. Icaritin requires phosphatidylinositol 3 kinase (PI3k)/Akt signaling to counteract skeletal muscle atrophy following mechanical unloading. Sci. Rep. 2016, 6, 20300. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Kim, M.; Park, T. α-Cedrene, a newly identified ligand of MOR23, increases skeletal muscle mass and strength. Mol. Nutr. Food Res. 2018, 62, 1800173. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Yoshida, N.; Maekawa, D.; Kitakaze, T.; Kobayashi, Y.; Kitano, T.; Fujita, T.; Okuwa-Hayashi, H.; Harada, N.; Nakano, Y. 5-Hydroxy-7-methoxyflavone derivatives from Kaempferia parviflora induce skeletal muscle hypertrophy. Food Sci. Nutr. 2019, 7, 312–321. [Google Scholar] [CrossRef]
- Bei, D.; An, G. Pharmacokinetics and tissue distribution of 5,7-dimethoxyflavone in mice following single dose oral administration. J. Pharm. Biomed. Anal. 2016, 119, 65–70. [Google Scholar] [CrossRef]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Kim, M.; Kim, N.; Han, J. Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem. 2014, 62, 12377–12383. [Google Scholar] [CrossRef]



| Gene | Direction | Sequence (5’-3’) |
|---|---|---|
| MuRF1 | Forward | TCTGGACTTAGAACACATAGCAGAG |
| Reverse | TCTCCTTCTTCATTGGTGTTCTTCT | |
| Atrogin-1 | Forward | CAGTGATCCATTCTGTTCATCCTTG |
| Reverse | TTATTTCCAGCCAAATGGAGAGAGA | |
| Beclin1 | Forward | CTCCTTAGGGGATGTTTGCCTT |
| Reverse | TCAGGAAAGAGGGAAAGGATGC | |
| LC3 | Forward | GGGAGGTCCTGGCTCCTAAA |
| Reverse | CAGACAGGCAAGGGCCTAAC | |
| Atg4 | Forward | GAGCCTTCCTCCATGTTCTTTCTC |
| Reverse | CTCATATCTAGGGGGAGGAAAGGT | |
| Atg7 | Forward | TAGAGAGGACCTTGGCCTAACA |
| Reverse | CAAGCCATCTATGTGTGTGCTG | |
| PGC-1α | Forward | GAGTGTTCTGGTACCCAAGGC |
| Reverse | CTGGGCCGTTTAGTCTTCCTT | |
| NRF-1 | Forward | AGATTCGTGGGTGGTAGGGT |
| Reverse | TCTAGCAGAGGTCTAGGCGG | |
| Tfam | Forward | AGACTACACTGGGAAACCACAG |
| Reverse | GGCTTATAGGGACCCAGTGATG | |
| IL-6 | Forward | AGACAAAGCGAGAGTCCTTCAG |
| Reverse | GTCCTTAGCCACTCCTTCTGTG | |
| TNF-α | Forward | GAGTCATTGCTCTGTGAAGGGA |
| Reverse | ATTCTGAGACAGAGGCAACCTG | |
| Genomic DNA | Forward | GCCAGCCTCTCCTGATTTTAGTGT |
| Reverse | GGGAACACAAAAGACCTCTTCTGG | |
| Mitochondria DNA | Forward | CCGCAAGGGAAAGATGAAAGAC |
| Reverse | TCGTTTGGTTTCGGGGTTTC | |
| β-Actin | Forward | GAAGGAGATTACTGCTCTGGCTC |
| Reverse | CTCAGTAACAGTCCGCCTAGAA |
| Parameter | Young | Aged | Aged+DMF25 | Aged+DMF50 |
|---|---|---|---|---|
| Body weight | ||||
| Initial weight (g) | 21.92 ± 0.69 | 33.43 ± 0.86 ## | 32.53 ± 0.78 | 32.90 ± 4.55 |
| Final weight (g) | 22.65 ± 0.78 | 35.12 ± 2.74 ## | 35.26 ± 1.26 | 32.98 ± 5.57 |
| Muscle weight /BW (mg·g−1) | ||||
| Gastrocnemius | 10.56 ± 0.38 | 6.81 ± 0.77 ## | 7.71 ± 0.45 * | 8.01 ± 0.46 ** |
| Tibialis anterior | 4.30 ± 0.17 | 2.83 ± 0.13 ## | 2.94 ± 0.23 | 3.12 ± 0.07 * |
| Extensor digitorum longus | 1.87 ± 0.07 | 1.11 ± 0.08 ## | 1.38 ± 0.13 ** | 1.48 ± 0.13 ** |
| Soleus | 0.74 ± 0.04 | 0.53 ± 0.04 ## | 0.69 ± 0.07 ** | 0.71 ± 0.05 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Hwang, J.-K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. https://doi.org/10.3390/nu12041079
Kim C, Hwang J-K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients. 2020; 12(4):1079. https://doi.org/10.3390/nu12041079
Chicago/Turabian StyleKim, Changhee, and Jae-Kwan Hwang. 2020. "The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways" Nutrients 12, no. 4: 1079. https://doi.org/10.3390/nu12041079
APA StyleKim, C., & Hwang, J.-K. (2020). The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients, 12(4), 1079. https://doi.org/10.3390/nu12041079




