Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians
Abstract
1. Introduction
2. Materials and Methods
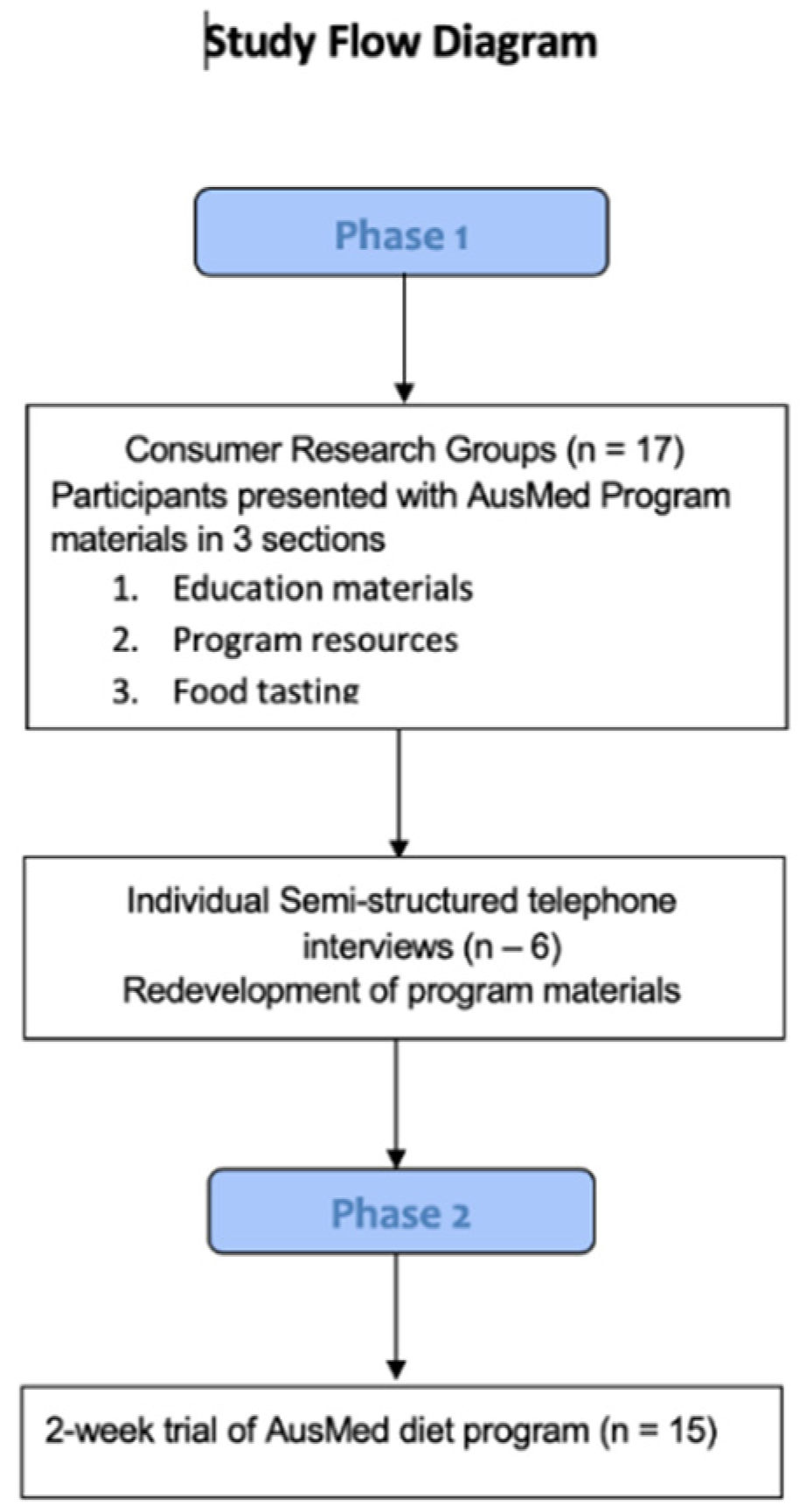
2.1. Phase 1 Process Evaluation
2.1.1. Phase 1 Participants
2.1.2. Phase 1 Study Design
- The AusMed education materials—background, diet–disease relationship, staple foods, plate ration and food pyramid.
- The AusMed diet program itself—menu plan, recipes, shopping lists, troubleshooting the menu plan.
- Foods typical to the Mediterranean diet—cooking demonstration and sample tasting of three recipes from the program.
2.1.3. Phase 1 Outcome Measures
2.2. Phase 2 Pilot Trial of AusMed Diet Program
2.2.1. Phase 2 Participants
2.2.2. Phase 2 Study Design
2.2.3. Phase 2 Outcome Measures
3. Results
3.1. Participant Characteristics
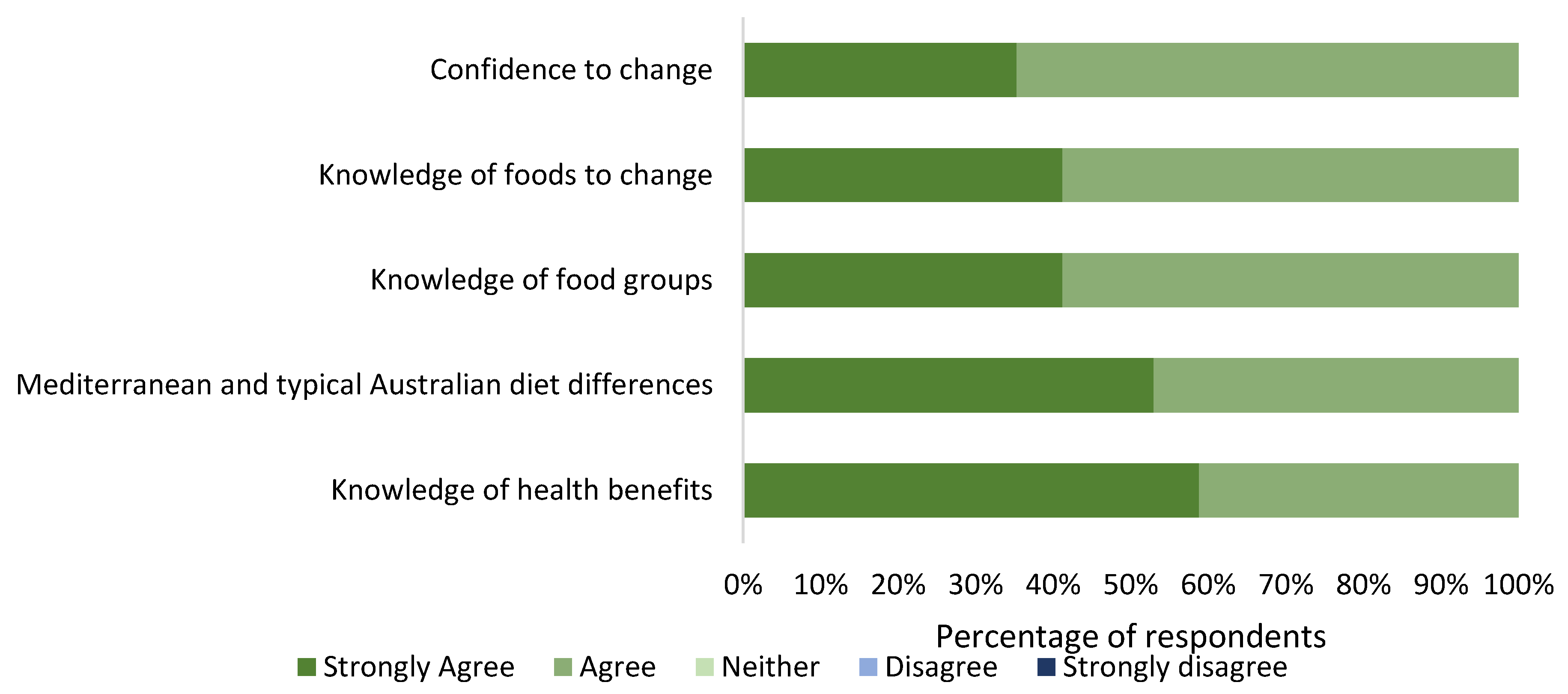
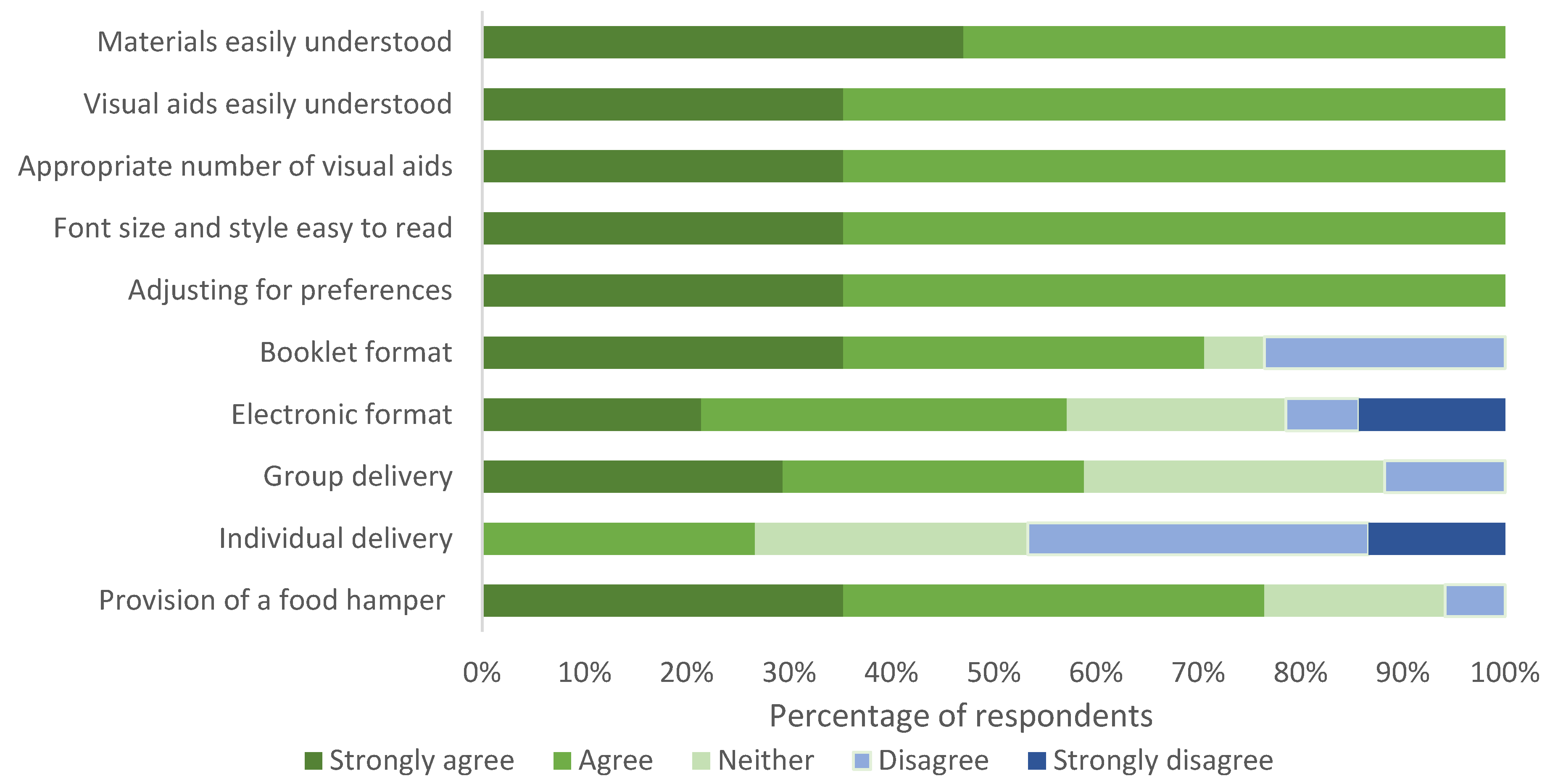
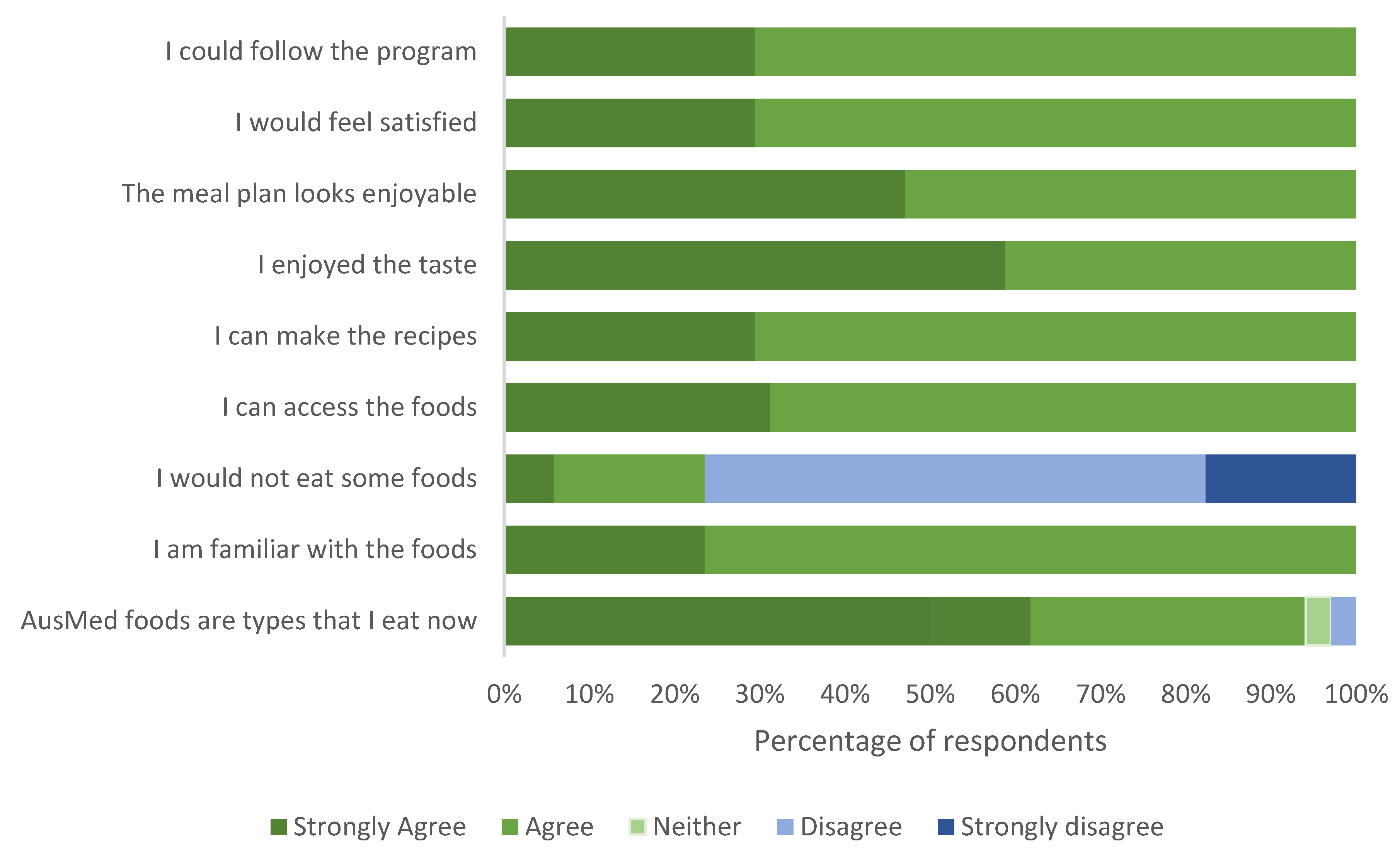
3.2. Phase 1 Quantitative Analysis
3.2.1. Phase 1 Nutrition Education Materials Survey
3.2.2. Phase 1 Program Materials Survey
3.2.3. AusMed Foods Survey Phase 1
3.3. Phase 1 Qualitative Analysis
“I guess you don’t really know for sure until you try to follow. It’s the doing of the thing isn’t it, that makes you realise what works and perhaps where the improvements are?” (Participant 6).
3.4. Phase 2 Quantitative Analysis
3.4.1. The 14-Point Mediterranean Diet Score
3.4.2. Phase 2 AusMed Foods Survey
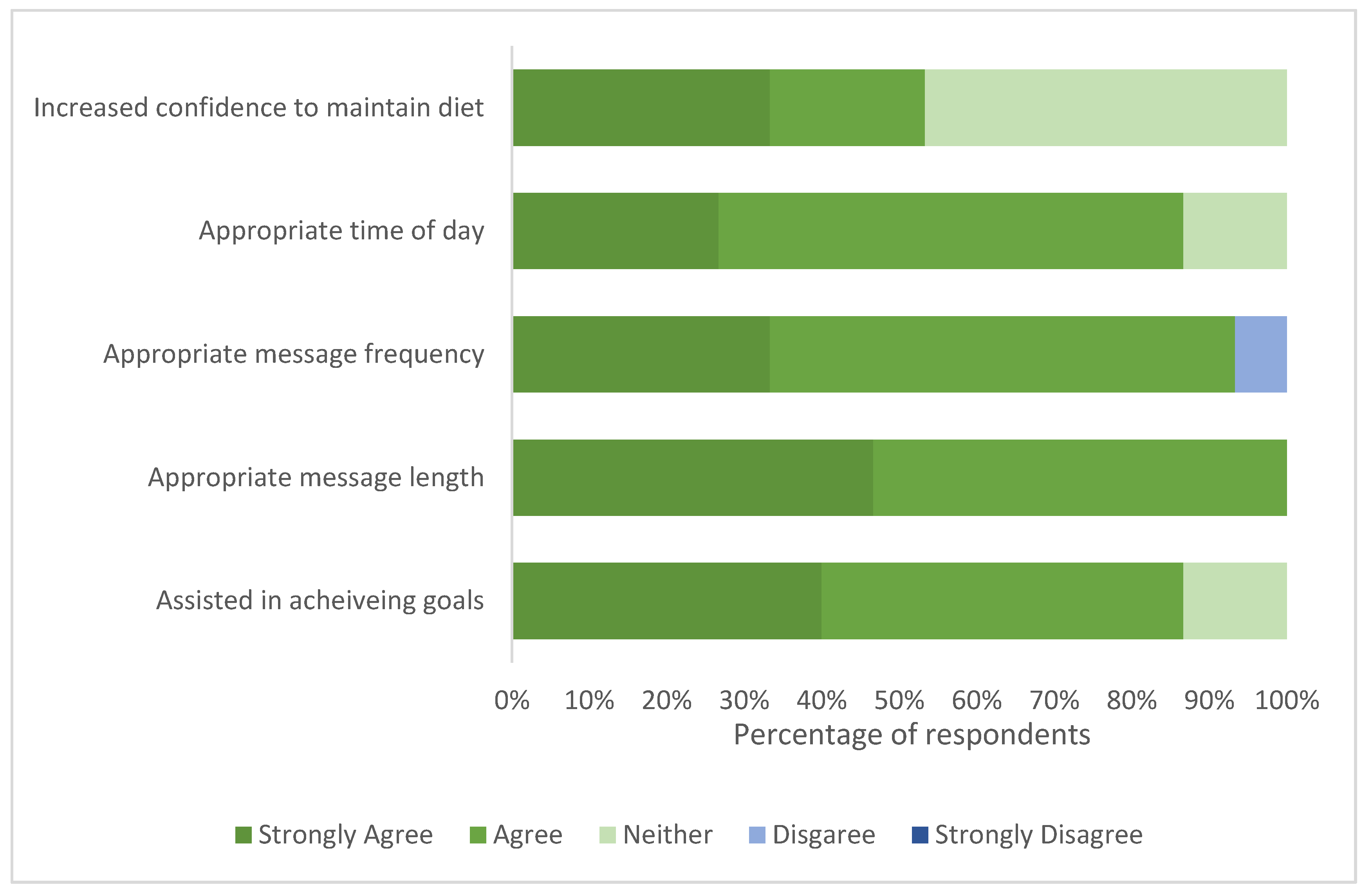
3.4.3. Phase 2 Text Message Support Evaluation Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Australian Bureau of Statistics. 4430.0 Disability, Ageing and Carers, Australia: Summary of Findings, 2015; Australian Bureau of Statistics: Canberra, Australia, 2017.
- World Health Organisation. World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Australian Institute of Health and Welfare. Evidence for Chronis Disease Risk Factors; Australian Government: Canberra, Australia, 2018.
- World Health Organisation. Global Strategy on Diet, Physical Activity and Health: Diet, Nutrition and the Prevention of Chronic Disease; Report of the Joint WHO/FAO Espert Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A modified Mediterranean dietary intervention for adults with major depression: Dietary protocol and feasibility data from the SMILES trial. Nutr. Neurosci. 2018, 21, 487–501. [Google Scholar] [CrossRef]
- O’Neil, A.; Berk, M.; Itsiopoulos, C.; Castle, D.; Opie, R.; Pizzinga, J.; Brazionis, L.; Hodge, A.; Mihalopoulous, C.; Chatterton, M.; et al. A randomised, controlled trial of a dietary intervention for adults with major depression (the ‘SMILES’ trial): Study protocol. BMC Psychiatry 2013, 13, 114. [Google Scholar] [CrossRef]
- Davis, C.; Hodgson, J.; Bryan, J.; Garg, M.; Woodman, R.; Murphy, K. Older Australians Can Achieve High Adherence to the Mediterranean Diet during a 6 Month Randomised Intervention; Results from the Medley Study. Nutrients 2017, 9, 534. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocke, M.; Peeters, P.; van der Schouw, Y.; Boeing, H.; Hoffman, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Murphy, K.; Dyer, K.; Hyde, B.; Davis, C.; Hodgson, J.; Woodman, R. Australians Can Adopt Mediterranean Diet (MedDiet) Principles up to 1-Year Following Completion of the Medley Trail (OR22-01-19). Curr. Dev. Nutr. 2019, 1. [Google Scholar] [CrossRef]
- NHMRC. The Australian Guide to Healthy Eating; Australian Government: Canberra, Australia, 2016.
- Thorne, S. Data analysis in qualitative research. EBN 2000, 3, 68–70. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Atkins, L.S.; West, R. The Behaviour Change Wheel: A Guide to Designing Interventions; Silverback Publishing: Sutton, UK, 2014. [Google Scholar]
- Rinaldi de Alvarenga, J.F.; Tran, C.; Hurtado-Barroso, S.; Martinez-Huelamo, M.; Illan, M.; Lamuela-Raventos, R.M. Home cooking and ingredient synergism improve lycopene isomer production in Sofrito. Food Res. Int. 2017, 99, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Andajani-Sutjaho, S.; Ball, K.; Warren, N.; Inglis, V.; Crawford, D. Perceived personal, social and environmental barriers to weight maintenance among young women: A community survey. Int. J. Behav. Nutr. Phys. Act. 2004, 1, 15. [Google Scholar] [CrossRef]
- Government, A. Age Pension: Payment Rates; Commonwealth of Australia: Canberra, Australia, 2018. [Google Scholar]
- Anderson, R.T.; Ory, M.; Cohen, S.; McBride, J.S. Issues of Aging and Adherence to Health Interventions. Control. Clin. Trials 2000, 21, S171–S183. [Google Scholar] [CrossRef]
- Culos-Reed, S.N.; Rejeski, W.J.; McAuley, E.; Ockene, J.K.; Roter, D.L. Predictors of Adherence to Behavior Change Interventions in the Elderly. Control. Clin. Trials 2000, 21, S200–S205. [Google Scholar] [CrossRef]
- World Health Organisation. mHealth New Horizons for Health through Mobile Technologies: Based on the Findings of the Second Survey on eHealth; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Sheeran, P.; Montanaro, E.; Bryan, A.; Miles, E.; Maki, A.; Avishai-Yitshak, A.; Klein, W.M.P.; Rothman, A.J. The Impact of Changing Attitudes, Norms, and Self-efficacy on Health-Related Intentions and Behavior: A Meta-Analysis. Health Psychol. 2016, 35, 1178–1188. [Google Scholar] [CrossRef]
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and REal-World Settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef]
- Buenza, J.J.; Toledo, E.; Hu, F.; Bes-Rastrollo, M.; Serrano-Martinez, M.; Sanchez-Vellegas, A.; Martinez, J.A.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: The Seguimiento Universidad de Navarra (SUN) cohort. Am. J. Clin. Nutr. 2010, 93, 1484–1493. [Google Scholar] [CrossRef]
- McKay, K.; Cheng, C.; Wright, A.; Shill, J.; Stephens, H. Evaluating mobile phone applications for health behaviour change: A systematic review. J. Telemed. Telecare 2018, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Giakoumidakis, K.; Khan, E.; Patelarou, E. Mobile phone text messaging for improving secondary prevention in cardiovascular diseases: A systematic review. Heart Lung 2018, 47, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Courtney, K.L.; Naylor, P.J.; E Rhodes, R. Tailored mobile text messaging interventions targeting type 2 diabetes self-management: A systematic review and a meta-analysis. Digit. Health 2019, 5, 2055207619845279. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED. trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019. [Google Scholar] [CrossRef]
- Goran, M.I.; Walker, R.; Allayee, H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 392–396. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Wang, S.; Cao, G.; Jin, C.; Yu, J.; Li, X.; Yan, J.; Wang, F.; Yu, W.; et al. Carbohydrate Intake, Glycemic Index, Glycemic Load, and Stroke: A Meta-analysis of Prospective Cohort Studies. Asia Pac. J. Public Health 2015, 27, 486–496. [Google Scholar] [CrossRef]

| Variables | All Subjects (n = 17) | Female (n = 12) | Male (n = 5) |
|---|---|---|---|
| Age (years) | 71.2 ± 4.2 | 70.7 ± 4.2 | 72.8 ± 4.2 |
| Marital status | |||
| Married | 13 (76%) | 8 (67%) | 5 (100%) |
| Divorced | 2 (12%) | 2 (17%) | - |
| Single | 1 (6%) | 1 (8%) | - |
| Widowed | 1 (6%) | 1 (8%) | - |
| Education | |||
| School certificate/HSC | 7 (41%) | 6 (50%) | 1 (20%) |
| Certificate/diploma | 6 (35%) | 4 (33%) | 2 (40%) |
| University degree | 4 (24%) | 2 (17%) | 2 (40%) |
| Household income | |||
| Rather not say/unknown | 3 (17%) | 3 (25%) | - |
| $25,000 to $49,999 | 9 (53%) | 7 (58%) | 2 (40%) |
| $50,000 to $99,999 | 3 (17%) | 2 (17%) | 1 (20%) |
| $100,000 to $199,000 | 2 (13%) | - | 2 (40%) |
| Theme | Sub-Theme | Verbatim Evidence |
|---|---|---|
| Barriers to adherence |
| “I think it’s overcoming the fat issue, you know, the oils and the…even though I use the olive oil and sprays, it’s the amount” Participant 5 “The meal plan is very big. I know you said in the presentation that it is flexible but there is a lot of it. I tend to have the same thing for breakfast most days and sometimes a fairly similar type thing for lunch so the, just the sheer number of recipes is a little overwhelming for me” Participant 2 “But it’s when you first look at it, I thought, Oh goodness me! And then I thought, no, come on, you’ve probably got just about everything that’s on there already in the house. That’s just my first impression of the list” Participant 5 “What I’d like to do is eat more fish, I went fishing and we caught some lovely whiting, so it will be easier, but it is expensive. I love sardines and mackerel and all those sorts. We do get a bit of canned stuff, that works for us” Participant 4 |
| Additional support |
| “We can form a Mediterranean club here…like some people play cards, some go bowling, some go fishing and so on…. not just ourselves, but encourage other people to come into the group as well” Participant 1 “No, no, I wouldn’t go. No, no, no, not a group. No, I don’t think it’s, well for me, it’s not the environment that I would have.” Participant 2 “I’ll be more than happy with an app or something like that…. if you’re away from home for quite a period of time and still wanting to follow the guidelines then, to have an app to refer to…” Participant 5 “Well yes, absolutely yes! If you did it, that text message then that would be fun. Absolutely yes. A text message would be my preferred method to get information” Participant 2 |
| Simplification and individualisation of materials |
| “Well, I was even thinking of some of those recipes I’d cheat with, the chicken and leek pot pies, we’d buy a chicken already done…a few little cheats or hacks for other people” Participant 2 “I mean, you know, we know salt in moderation, but instead of, maybe some tips on how to flavour and season your food” Participant 5 “…a sort of template thing…where you pick and choose your ingredients...where you tick boxes, perhaps a meal template or a plan template to make our own plan” Participant 6 “I’m on my own too, so I think there might be just too much. I tend to keep things pretty simple” Participant 5 |
| MD Score Individual Questions | Baseline (n = 15) Mean ± SD | Post Intervention (n = 15) Mean ± SD | p |
|---|---|---|---|
| Total MD Score | 5.4 ± 2.38 | 9.6 ± 2.03 | <0.001 |
| 1. Use EVOO as main culinary fat | 73% | 100% | * 0.099 |
| 2. Olive oil tbsp/day | 1.15 ± 0.86 | 2.53 ± 1.19 | 0.001 |
| 3. Vegetable serves/day | 1.20 ± 0.47 | 1.71 ± 0.59 | <0.001 |
| 4. Fruit serve/day | 1.83 ± 0.59 | 2.20 ± 0.94 | 0.135 |
| 5. Red meat serves/day | 1.13 ± 0.71 | 0.65 ± 0.63 | 0.015 |
| 6. Butter/cream/margarine serves/day | 0.82 ± 1.05 | 0.50 ± 0.49 | 0.237 |
| 7. Soft drink serves/day | 0.08 ± 0.26 | 0.07 ± 0.26 | 0.237 |
| 8. Wine glasses/week | 10.27 ± 7.04 | 10.67 ± 6.15 | 0.620 |
| 9. Legumes serves/week | 0.47 ± 0.64 | 1.8 ± 0.94 | <0.001 |
| 10. Fish or shellfish serves/week | 3.20 ± 2.57 | 4.33 ± 2.32 | 0.009 |
| 11. Commercial sweets/pastries serves/week | 1.60 ± 1.72 | 0.67 ± 0.82 | 0.021 |
| 12. Nuts serves/week | 3.53 ± 3.83 | 3.53 ± 1.96 | 1.000 |
| 13. White meat preferentially | 80% | 87% | * 1.000 |
| 14. Sofrito serves/week | 0.93 ± 0.59 | 2.07 ± 1.62 | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacharia, K.; Patterson, A.J.; English, C.; MacDonald-Wicks, L. Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians. Nutrients 2020, 12, 1044. https://doi.org/10.3390/nu12041044
Zacharia K, Patterson AJ, English C, MacDonald-Wicks L. Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians. Nutrients. 2020; 12(4):1044. https://doi.org/10.3390/nu12041044
Chicago/Turabian StyleZacharia, Karly, Amanda J. Patterson, Coralie English, and Lesley MacDonald-Wicks. 2020. "Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians" Nutrients 12, no. 4: 1044. https://doi.org/10.3390/nu12041044
APA StyleZacharia, K., Patterson, A. J., English, C., & MacDonald-Wicks, L. (2020). Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians. Nutrients, 12(4), 1044. https://doi.org/10.3390/nu12041044







