Intravenous Arginine Administration Benefits CD4+ T-Cell Homeostasis and Attenuates Liver Inflammation in Mice with Polymicrobial Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
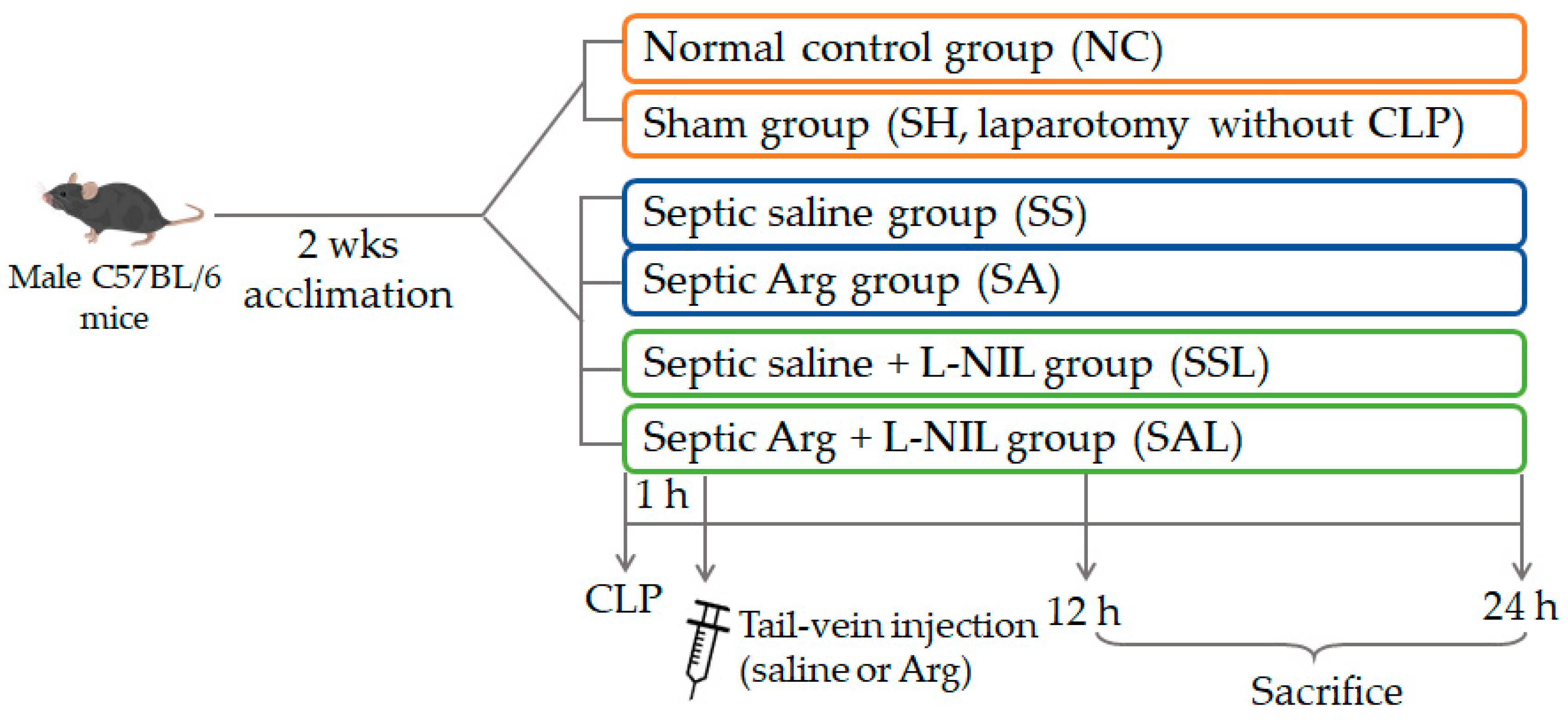
2.2. Study Protocol
2.3. Measurements of Plasma Biochemical Parameters
2.4. Evaluation of Plasma Amino Acid Concentrations and Arg Availability
2.5. Measurements of CD4+ T-Cell Subpopulations
2.6. Measurements of Thiobarbituric Acid-Reactive Substances (TBARSs) in Liver Tissues
2.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR) of Liver Tissues
2.8. Statistical Analysis
3. Results
3.1. Plasma Biochemical Markers and Liver Lipid Peroxide Concentrations
3.2. Plasma Amino Acid Concentrations and Arg Availability Assessment
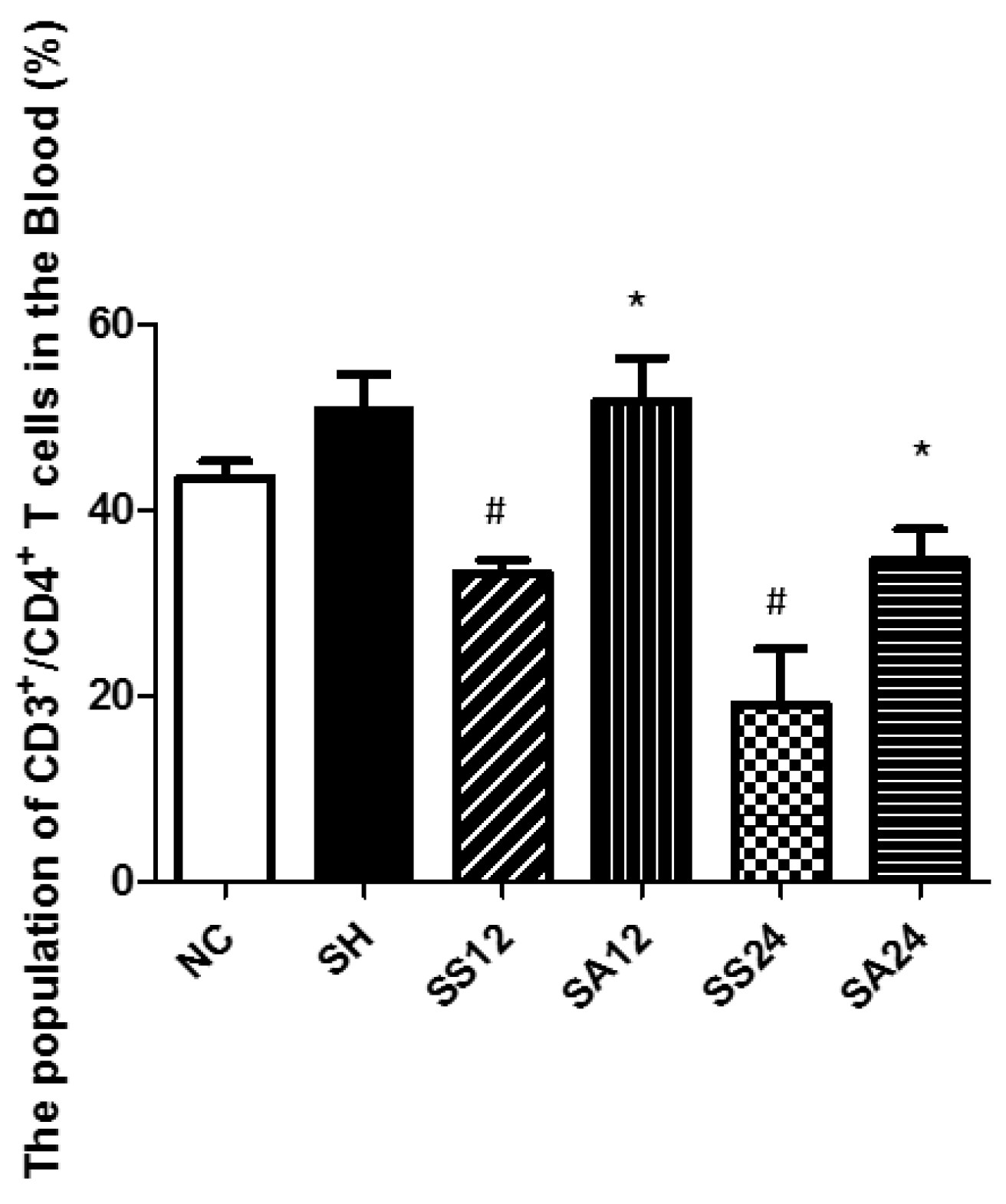
3.3. CD4+ T Lymphocyte Populations in Blood
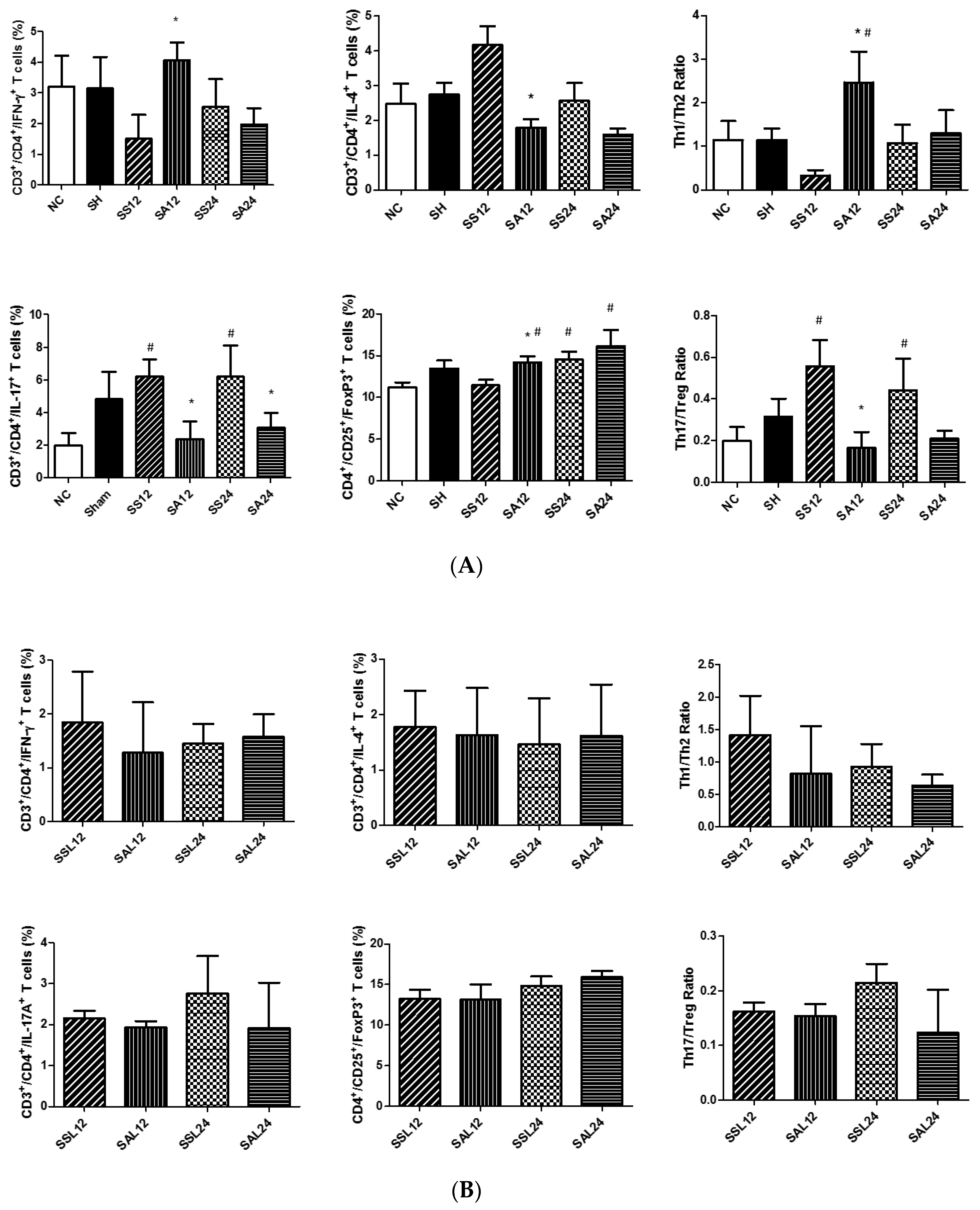
3.4. Distribution of T Lymphocyte Subpopulations in Para-Aortic Lymph Nodes
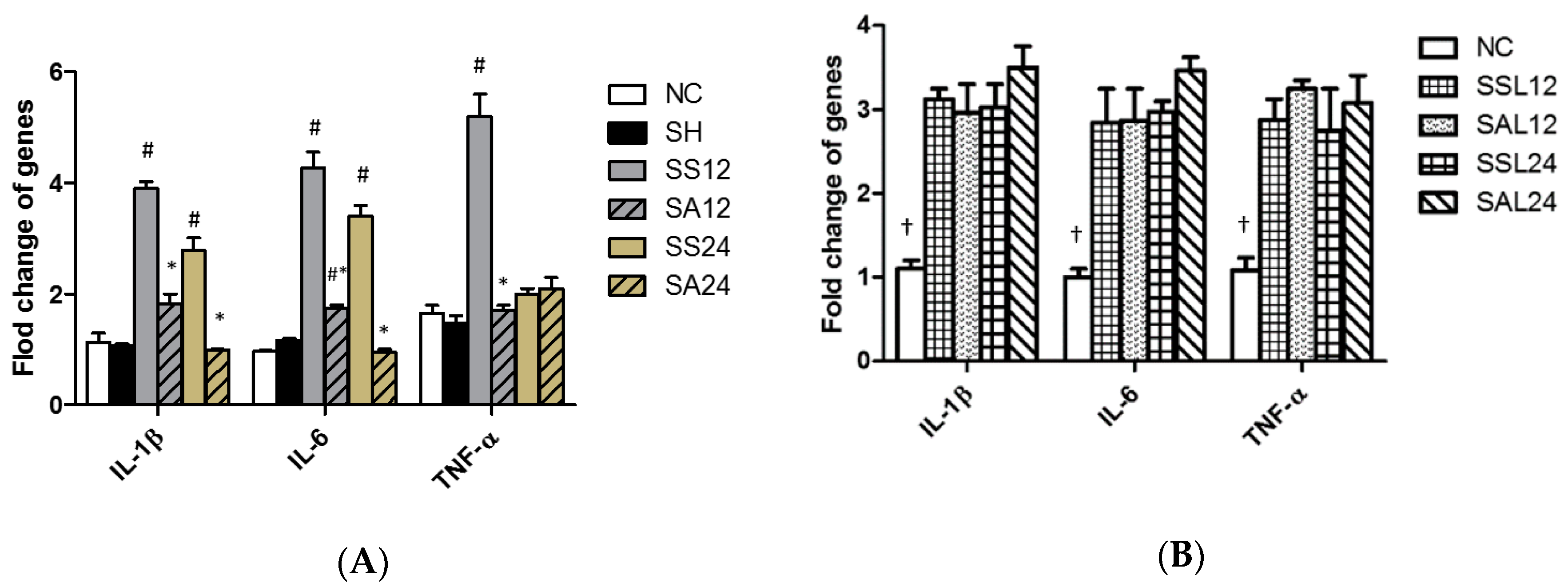
3.5. Gene Expression Levels in the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Perez, J.; Condotta, S.A.; Badovinac, V.P.; Griffith, T.S. Impact of sepsis on CD4 T cell immunity. J. Leukoc. Biol. 2014, 96, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Jenkins, M.K. Origins of CD4(+) Effector and central memory T Cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar] [CrossRef]
- Gupta, D.L.; Bhoi, S.; Mohan, T.; Galwnkar, S.; Rao, D.N. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine 2016, 88, 214–221. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J. Immunol. 2005, 175, 5–14. [Google Scholar]
- Allen, J.E.; Maizels, R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Wu, W.; Rozo, C.; Bowdridge, S.; Millman, A.; Van Rooijen, N.; Urban, J.F., Jr.; Wynn, T.A.; Gause, W.C. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 2012, 18, 260–266. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Peng, X.; Zhu, J.; Chen, X.; Liu, H.; Tang, C.; Dong, Z.; Liu, F.; Peng, Y. Mangiferin attenuate sepsis-induced acute kidney injury via antioxidant and anti-inflammatory effects. Am. J. Nephrol. 2014, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.B.; Strange, J.; Kearney, P.; Gellin, G.; Endean, E.; Fitzpatrick, E. Effects of L-arginine on the proliferation of T lymphocyte subpopulations. J. Parenter. Enteral Nutr. 2001, 25, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Miller, K.R.; Rosenthal, C.; Rosenthal, M.D. When is it appropriate to use arginine in critical illness? Nutr. Clin. Pract. 2016, 31, 438–444. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Castermans, T.M.; Hommen, M.P.; Meesters, D.M.; Poeze, M. Arginine and citrulline and the immune response in sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef]
- Shi, H.P.; Most, D.; Efron, D.T.; Witte, M.B.; Barbul, A. Supplemental L-arginine enhances wound healing in diabetic rats. Wound Repair Regen. 2003, 11, 198–203. [Google Scholar] [CrossRef]
- Everett, J.; Turner, K.; Cai, Q.; Gordon, V.; Whiteley, M.; Rumbaugh, K. Arginine is a critical substrate for the pathogenesis of pseudomonas aeruginosa in burn wound infections. mBio 2017, 8, e02160-16. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Yeh, C.L.; Pai, M.H.; Shih, Y.M.; Shih, J.M.; Yeh, S.L. Intravenous arginine administration promotes proangiogenic cells mobilization and attenuates lung injury in mice with polymicrobial sepsis. Nutrients 2017, 9, 507. [Google Scholar] [CrossRef]
- Kieft, H.; Roos, A.N.; van Drunen, J.D.; Bindels, A.J.; Bindels, J.G.; Hofman, Z. Clinical outcome of immunonutrition in a heterogeneous intensive care population. Intensive Care Med. 2005, 31, 524–532. [Google Scholar] [CrossRef]
- Tsuei, B.J.; Bernard, A.C.; Barksdale, A.R.; Rockich, A.K.; Meier, C.F.; Kearney, P.A. Supplemental enteral arginine is metabolized to ornithine in injured patients. J. Surg. Res. 2005, 123, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Iapichino, G.; Radrizzani, D.; Facchini, R.; Simini, B.; Bruzzone, P.; Zanforlin, G.; Tognoni, G. Early enteral immunonutrition in patients with severe sepsis: Results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003, 29, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Poeze, M.; Deutz, N.E. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin. Sci. (Lond.) 2015, 128, 57–67. [Google Scholar] [CrossRef] [PubMed]
- De Waele, E.; Malbrain, M.L.N.G.; Spapen, H. Nutrition in sepsis: A bench-to-bedside review. Nutrients 2020, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S. Bench to bedside review: Therapeutic modulation of nitric oxide in sepsis-an update. Intensive Care. Med. Exp. 2019, 7, 64. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Poeze, M.; Dejong, C.H.; Ramsay, G.; Deutz, N.E. Sepsis: An arginine deficiency state? Crit. Care Med. 2004, 32, 2135–2145. [Google Scholar] [CrossRef]
- Hubbard, W.J.; Choudhry, M.; Schwacha, M.G.; Kerby, J.D.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. Cecal ligation and puncture. Shock 2005, 24 (Suppl. 1), 52–57. [Google Scholar] [CrossRef]
- Tiwari, M.M.; Brock, R.W.; Megyesi, J.K.; Kaushal, G.P.; Mayeux, P.R. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: Role of nitric oxide and caspases. Am. J. Physiol. Renal Physiol. 2005, 289, F1324–F1332. [Google Scholar] [CrossRef]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. L-arginine availability and metabolism is altered in ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Zhou, M.; Martindale, R.G. Arginine in the critical care setting. J. Nutr. 2007, 137 (Suppl. 2), 1687S–1692S. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Novak, F.; Drover, J.W.; Jain, M.; Su, X.; Suchner, U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001, 286, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Poeze, M.; Deutz, N.E. A randomized-controlled trial of arginine infusion in severe sepsis on microcirculation and metabolism. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lortie, M.J.; Satriano, J.; Gabbai, F.B.; Thareau, S.; Khang, S.; Deng, A.; Pizzo, D.P.; Thomson, S.C.; Blantz, R.C.; Munger, K.A. Production of arginine by the kidney is impaired in a model of sepsis: Early events following LPS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1434–R1440. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A Unified Theory of Sepsis-Induced Acute Kidney Injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Gao, H.; Hoesel, L.M.; Nadeau, B.A.; Day, D.E.; Zetoune, F.S.; Sarma, J.V.; Huber-Lang, M.S.; Ferrara, J.L.; et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008, 22, 2198–2205. [Google Scholar] [CrossRef]
- Daly, J.M.; Reynolds, J.; Thom, A.; Kinsley, L.; Dietrick-Gallagher, M.; Shou, J.; Ruggieri, B. Immune and metabolic effects of arginine in the surgical patient. Ann. Surg. 1988, 208, 512–523. [Google Scholar] [CrossRef]
- Heemskerk, S.; Masereeuw, R.; Russel, F.G.; Pickkers, P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat. Rev. Nephrol. 2009, 5, 629. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xu, G.; Liang, X.; Wei, J.; Luo, J.; Chen, G.N.; Yan, X.D.; Wen, X.P.; Zhong, M.; Lv, X. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. Am. J. Transl. Res. 2016, 8, 5685–5695. [Google Scholar]
- Gudowska, M.; Gruszewska, E.; Cylwik, B.; Panasiuk, A.; Rogalska, M.; Flisiak, R.; Szmitkowski, M.; Chrostek, L. Galectin-3 concentration in liver diseases. Ann. Clin. Lab. Sci. 2015, 45, 669–673. [Google Scholar]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.; DiSomma, S. Emergency department use of galectin-3. Crit. Pathw. Cardiol. 2014, 13, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Dieplinger, B.; Egger, M.; Leitner, I.; Firlinger, F.; Poelz, W.; Lenz, K.; Haltmayer, M.; Mueller, T. Interleukin 6, galectin 3, growth differentiation factor 15, and soluble st2 for mortality prediction in critically ill patients. J. Crit. Care 2016, 34, 38–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Hu, W.; Zhu, M.L.; Yin, T.; Su, J.; Wang, J.R. Serum galectin-3 levels and delirium among postpartum intensive care unit women. Brain Behav. 2017, 7, e00773. [Google Scholar] [CrossRef]
- Hammerich, L.; Heymann, F.; Tacke, F. Role of IL-17 and Th17 cells in liver diseases. Clin. Dev. Immunol. 2011, 2011, 345803. [Google Scholar] [CrossRef]
- de Roquetaillade, C.; Kandara, K.; Gossez, M.; Peronnet, E.; Monard, C.; Cour, M.; Rimmele, T.; Argaud, L.; Monneret, G.; Venet, F. Intracellular calcium signaling and phospho-antigen measurements reveal functional proximal TCR activation in lymphocytes from septic shock patients. Intensive Care. Med. Exp. 2019, 7, 74. [Google Scholar] [CrossRef]
- Harbrecht, B.G. Therapeutic use of nitric oxide scavengers in shock and sepsis. Curr. Pharm. Des. 2006, 12, 3543–3549. [Google Scholar] [CrossRef]
- Cauwels, A. Nitric oxide in shock. Kidney Int. 2007, 72, 557–565. [Google Scholar] [CrossRef]
- Antonucci, E.; Fiaccadori, E.; Donadello, K.; Taccone, F.S.; Franchi, F.; Scolletta, S. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J. Crit. Care 2014, 29, 500–511. [Google Scholar] [CrossRef]
- Levy, B.; Collin, S.; Sennoun, N.; Ducrocq, N.; Kimmoun, A.; Asfar, P.; Perez, P.; Meziani, F. Vascular hyporesponsiveness to vasopressors in septic shock: From bench to bedside. Intensive Care Med. 2010, 36, 2019–2029. [Google Scholar] [CrossRef]
- Dyson, A.; Bryan, N.S.; Fernandez, B.O.; Garcia-Saura, M.F.; Saijo, F.; Mongardon, N.; Rodriguez, J.; Singer, M.; Feelisch, M. An integrated approach to assessing nitroso-redox balance in systemic inflammation. Free Radic. Biol. Med. 2011, 51, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Madden, H.P.; Breslin, R.J.; Wasserkrug, H.L.; Efron, G.; Barbul, A. Stimulation of T cell immunity by arginine enhances survival in peritonitis. J. Surg. Res. 1988, 44, 658–663. [Google Scholar] [CrossRef]
- Niedbala, W.; Cai, B.; Liew, F.Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), 37–40. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Wei, X.Q.; Piedrafita, D.; Xu, D.; Liew, F.Y. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 1999, 29, 2498–2505. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E. Exogenous Arginine in Sepsis. Crit. Care Med. 2007, 35, S557–S563. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Baudry, N.; Duranteau, J.; Vicaut, E. Effects of vasopressin, norepinephrine, and L-arginine on intestinal microcirculation in endotoxemia. Crit. Care Med. 2006, 34, 1752–1757. [Google Scholar] [CrossRef]
- Boselli, M.; Aquilani, R.; Baiardi, P.; Dioguardi, F.S.; Guarnaschelli, C.; Achilli, M.P.; Arrigoni, N.; Iadarola, P.; Verri, M.; Viglio, S.; et al. Supplementation of essential amino acids may reduce the occurrence of infections in rehabilitation patients with brain injury. Nutr. Clin. Pract. 2012, 27, 99–114. [Google Scholar] [CrossRef]



| Gene | Primer Sequence |
|---|---|
| IL-1β | F: 5′-TGCCACCTTTTGACAGTGATG-3′ R: 5′-ATGTGCTGCTGCGAGATTTG-3′ |
| IL-6 | F: 5′-TCCTACCCCAACTTCCAATGCTC-3′ R: 5′-TTGGATGGTCTTGGTCCTTAGCC-3′ |
| TNF-α | F: 5′-ATGGCCTCCCTCTCATCAGT-3′ R: 5′-TTTGCTACGACGTGGGCTAC-3′ |
| β-actin | F: 5′-GTCAGAAGGACTCCTATGTG-3′ R: 5′-ACGCAGCTCATTGTAGAAG-3′ |
| Parameters | NC | SS12 | SA12 | SS24 | SA24 |
| Plasma | |||||
| AST (U/L) | 58.2 ± 2.4 | 449.1 ± 35.3 # | 130.9 ± 7.7 #,* | 224.4 ± 16.6 # | 87.87 ± 1.93 #,* |
| ALT (U/L) | 37.3 ± 5.5 | 611.7 ± 71.9 # | 251.9 ± 6.1#,* | 387.6 ± 39.4 # | 217.4 ± 25.38 #,* |
| Gal-3 (ng/mL) | 6.3 ± 0.9 | 25.9 ± 3.2 # | 11.1 ± 1.4 #,* | 20.2 ± 3.1# | 9.2 ± 1.2 * |
| Liver | |||||
| MDA (nmol/mg protein) | 3.26 ± 0.6 | 6.5 ± 0.3 # | 4.8 ± 0.4 * | 5.7 ± 0.3 # | 3.8 ± 0.2 * |
| Parameters | SH | SSL12 | SAL12 | SSL24 | SAL24 |
| Plasma | |||||
| AST (U/L) | 60.5 ± 3.1 | 368.8 ± 40.5 # | 346.5 ± 38.2 # | 222.5 ± 46.3 # | 216.5 ± 35.6 # |
| ALT (U/L) | 40.5 ± 5.6 | 564.3 ± 50.6 # | 462.3 ± 41.3 # | 326.5 ± 35.6 # | 200.4 ± 43.5 # |
| Gal-3 (ng/mL) | 7.1 ± 0.6 | 24.5 ± 1.3 # | 22.6 ± 1.1 # | 23.6 ± 1.3 # | 20.5 ± 1.2 # |
| Liver | |||||
| MDA (nmol/mg protein) | 4.1 ± 0.5 | 7.5 ± 0.2 # | 6.6 ± 0.3 # | 7.3 ± 0.5 # | 7.1 ± 0.2 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Tanuseputero, S.A.; Wu, J.-M.; Tseng, Y.-R.; Yang, P.-J.; Lee, P.-C.; Yeh, S.-L.; Lin, M.-T. Intravenous Arginine Administration Benefits CD4+ T-Cell Homeostasis and Attenuates Liver Inflammation in Mice with Polymicrobial Sepsis. Nutrients 2020, 12, 1047. https://doi.org/10.3390/nu12041047
Yeh C-L, Tanuseputero SA, Wu J-M, Tseng Y-R, Yang P-J, Lee P-C, Yeh S-L, Lin M-T. Intravenous Arginine Administration Benefits CD4+ T-Cell Homeostasis and Attenuates Liver Inflammation in Mice with Polymicrobial Sepsis. Nutrients. 2020; 12(4):1047. https://doi.org/10.3390/nu12041047
Chicago/Turabian StyleYeh, Chiu-Li, Sharon Angela Tanuseputero, Jin-Ming Wu, Yi-Ru Tseng, Po-Jen Yang, Po-Chu Lee, Sung-Ling Yeh, and Ming-Tsan Lin. 2020. "Intravenous Arginine Administration Benefits CD4+ T-Cell Homeostasis and Attenuates Liver Inflammation in Mice with Polymicrobial Sepsis" Nutrients 12, no. 4: 1047. https://doi.org/10.3390/nu12041047
APA StyleYeh, C.-L., Tanuseputero, S. A., Wu, J.-M., Tseng, Y.-R., Yang, P.-J., Lee, P.-C., Yeh, S.-L., & Lin, M.-T. (2020). Intravenous Arginine Administration Benefits CD4+ T-Cell Homeostasis and Attenuates Liver Inflammation in Mice with Polymicrobial Sepsis. Nutrients, 12(4), 1047. https://doi.org/10.3390/nu12041047






