Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits
Abstract
1. Introduction
2. Polar Lipids
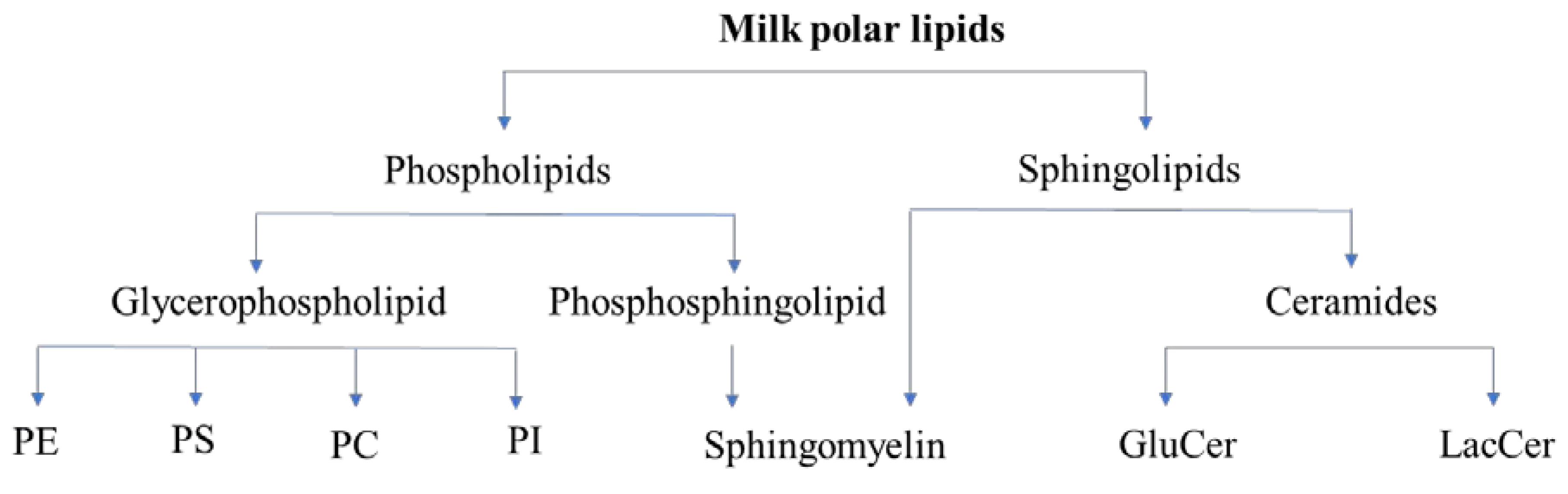
2.1. Classes of Polar Lipids
2.2. Biological Functions
2.3. Milk Polar Lipids: Classes and Quantity
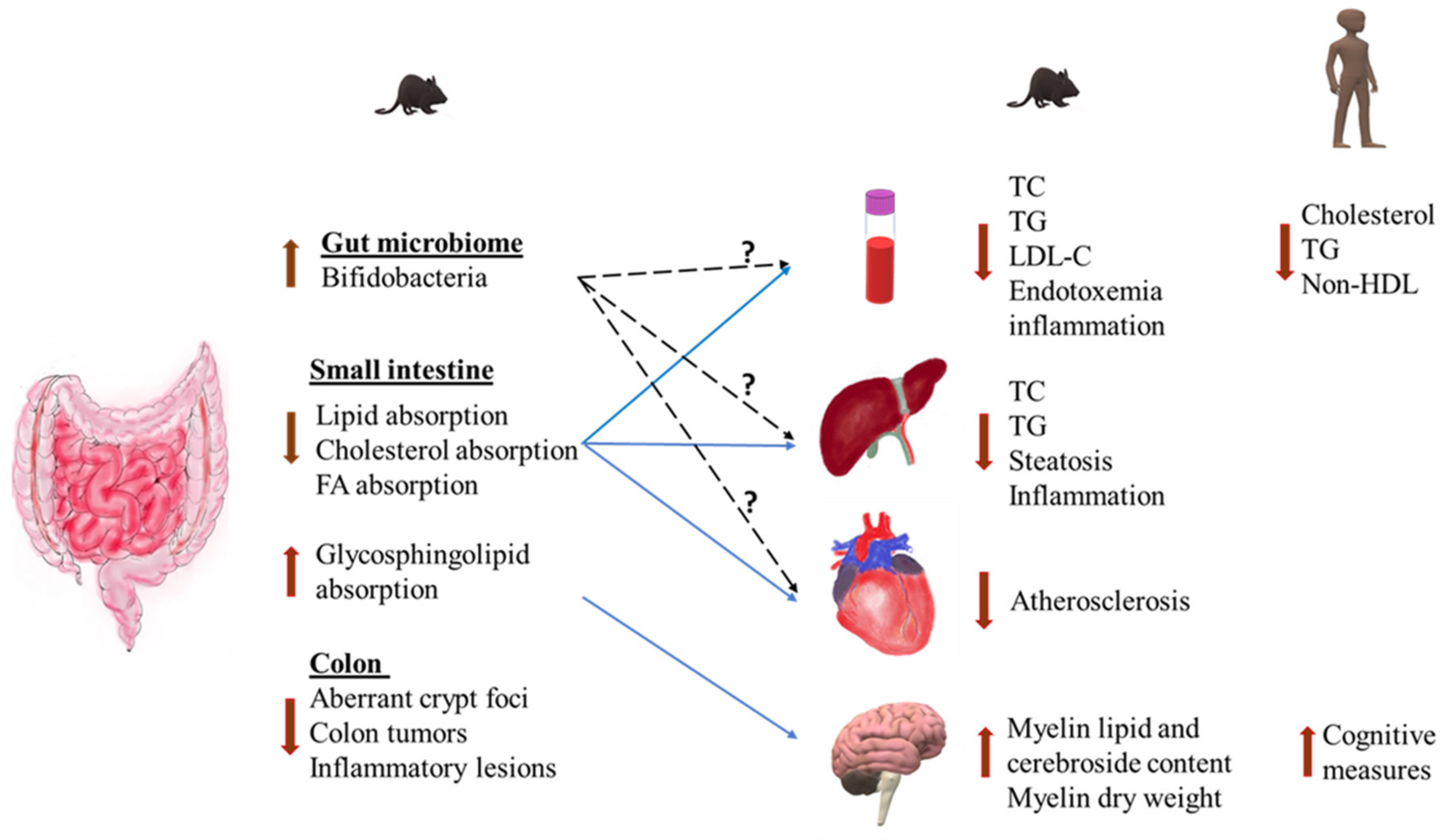
3. Health Effects of Milk Polar Lipids
3.1. Effects on Intestinal Lipid Absorption
3.2. Anti-Inflammatory Effects
3.3. Modulation of Gut Microbiota
3.4. Cardiovascular Disease
3.5. Non-Alcoholic Fatty Liver Disease (NAFLD)
3.6. Insulin Resistance and Type 2 Diabetes
3.7. Cognitive Function and Neurodevelopment
3.8. Colorectal Cancer and Colitis
4. Gaps in Scientific Literature and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Ogawa, T.; Ono, J.; Miyashita, R.; Aida, K.; Oda, Y.; Ohnishi, M. Analysis of sphingolipid classes and their contents in meals. Biosci. Biotechnol. Biochem. 2008, 72, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Michalski, M.C. Structure-function relationship of the milk fat globule. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Briard-Bion, V.; Ménard, O.; Beaucher, E.; Rousseau, F.; Fauquant, J.; Leconte, N.; Robert, B. Fat globules selected from whole milk according to their size: Different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chem. 2011, 125, 355–368. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Blesso, C.N. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731–2747. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef]
- Norris, G.H.; Porter, C.M.; Jiang, C.; Millar, C.L.; Blesso, C.N. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 40, 36–43. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef]
- Norris, G.; Porter, C.; Jiang, C.; Blesso, C.; Norris, G.H.; Porter, C.M.; Jiang, C.; Blesso, C.N. Dietary Milk Sphingomyelin Reduces Systemic Inflammation in Diet-Induced Obese Mice and Inhibits LPS Activity in Macrophages. Beverages 2017, 3, 37. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M.; Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef] [PubMed]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and lipid composition of bovine milk-fat-globule membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Beare-Rogers, J.; Dieffenbacher, A.; Holm, J.V. Lexicon of Lipid Nutrition (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 685–744. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary sphingolipids: Potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutr. Rev. 2017, 75, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, K.; Kolter, T. Biosynthesis and degradation of mammalian glycosphingolipids. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.D.; Morrison, W.R. Polar lipds in bovin milk. III. Isomeric cis and trans monoenoic and dienoic fatty acids, and alkyl and alkenyl ethers in phosphatidyl choline and phosphatidyl ethanolamin. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1971, 248, 71–79. [Google Scholar] [CrossRef]
- Murgia, S.; Mele, S.; Monduzzi, M. Quantitative characterization of phospholipids in milk fat via 31P NMR using a monophasic solvent mixture. Lipids 2003, 38, 585–591. [Google Scholar] [CrossRef]
- Gallier, S.; Gragson, D.; Cabral, C.; Jiménez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Mayor, S.; Rao, M. Rafts: Scale-dependent, active lipid organization at the cell surface. Traffic 2004, 5, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell - The Lipid Bilayer. In New York: Garland Science; Garland Science: New York, NY, USA, 2002; Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 13 August 2019).
- Grecco, H.E.; Schmick, M.; Bastiaens, P.I.H. Signaling from the living plasma membrane. Cell 2011, 144, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Dowhan, W.; Bogdanov, M. Chapter 1 Functional roles of lipids in membranes. In Biochemistry of lipids, lipoproteins Membranes; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–37. [Google Scholar]
- Zimmerberg, J.; Gawrisch, K. The physical chemistry of biological membranes. Nat. Chem. Biol. 2006, 2, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, R.; Camp, J.V.; Dewettinck, K. Phospho- and sphingolipid distribution during processing of milk, butter and whey. Int. J. Food Sci. Technol. 2006, 41, 435–443. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
- Le, T.T.; Miocinovic, J.; Nguyen, T.M.; Rombaut, R.; van Camp, J.; Dewettinck, K. Improved Solvent Extraction Procedure and High-Performance Liquid Chromatography–Evaporative Light-Scattering Detector Method for Analysis of Polar Lipids from Dairy Materials. J. Agric. Food Chem. 2011, 59, 10407–10413. [Google Scholar] [CrossRef]
- Barry, K.M.; Dinan, T.G.; Murray, B.A.; Kelly, P.M. Comparison of dairy phospholipid preparative extraction protocols in combination with analysis by high performance liquid chromatography coupled to a charged aerosol detector. Int. Dairy J. 2016, 56, 179–185. [Google Scholar] [CrossRef]
- Ferreiro, T.; Gayoso, L.; Rodríguez-Otero, J.L. Milk phospholipids: Organic milk and milk rich in conjugated linoleic acid compared with conventional milk. J. Dairy Sci. 2015, 98, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, P.; Felipe, X.; Noriega, L.; Fontecha, J. Effect of processing of cow milk by high pressures under conditions up to 900 MPa on the composition of neutral, polar lipids and fatty acids. LWT - Food Sci. Technol. 2015, 62, 265–270. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Menard, O.; Rousseau, F.; Pradel, P.; Besle, J.-M. Phospholipid, Sphingolipid, and Fatty Acid Compositions of the Milk Fat Globule Membrane are Modified by Diet. J. Agric. Food Chem. 2008, 56, 5226–5236. [Google Scholar] [CrossRef] [PubMed]
- Puente, R.; García-Pardo, L.A.; Rueda, R.; Gil, A.; Hueso, P. Seasonal variations in the concentration of gangliosides and sialic acids in milk from different mammalian species. Int. Dairy J. 1996, 6, 315–322. [Google Scholar] [CrossRef]
- Castro-Gómez, M.P.; Rodriguez-Alcalá, L.M.; Calvo, M.V.; Romero, J.; Mendiola, J.A.; Ibañez, E.; Fontecha, J. Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L. Changes in Milk Fat Phospholipids During Lactation. J. Dairy Sci. 1990, 73, 1208–1216. [Google Scholar] [CrossRef]
- Fagan, P.; Wijesundera, C. Liquid chromatographic analysis of milk phospholipids with on-line pre-concentration. J. Chromatogr. A 2004, 1054, 241–249. [Google Scholar] [CrossRef]
- Donato, P.; Cacciola, F.; Cichello, F.; Russo, M.; Dugo, P.; Mondello, L. Determination of phospholipids in milk samples by means of hydrophilic interaction liquid chromatography coupled to evaporative light scattering and mass spectrometry detection. J. Chromatogr. A 2011, 1218, 6476–6482. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar] [CrossRef]
- MacKenzie, A.; Vyssotski, M.; Nekrasov, E. Quantitative Analysis of Dairy Phospholipids by 31P NMR. J. Am. Oil Chem. Soc. 2009, 86, 757–763. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Gori, A.; Losi, G.; Caboni, M.F. Bioactive lipids in the butter production chain from Parmigiano Reggiano cheese area. J. Sci. Food Agric. 2013, 93, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Avalli, A.; Contarini, G. Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J. Chromatogr. A 2005, 1071, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowicz, G.; Micek, P.; Wawrzeńczyk, C. A new liquid chromatography method with charge aerosol detector (CAD) for the determination of phospholipid classes. Application to milk phospholipids. Talanta 2013, 105, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- Britten, M.; Lamothe, S.; Robitaille, G. Effect of cream treatment on phospholipids and protein recovery in butter-making process. Int. J. Food Sci. Technol. 2008, 43, 651–657. [Google Scholar] [CrossRef]
- Rombaut, R.; Camp, J.V.; Dewettinck, K. Analysis of Phospho- and Sphingolipids in Dairy Products by a New HPLC Method. J. Dairy Sci. 2005, 88, 482–488. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K. Properties, analysis and purification of milk polar lipids. Int. Dairy J. 2006, 16, 1362–1373. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S. Dairy Lecithin from Cheese Whey Fat Globule Membrane: Its Extraction, Composition, Oxidative Stability, and Emulsifying Properties. J. Am. Oil Chem. Soc. 2013, 90, 217–224. [Google Scholar] [CrossRef]
- Kim, H.-H.Y.; Jimenez-Flores, R. Heat-Induced Interactions between the Proteins of Milk Fat Globule Membrane and Skim Milk. J. Dairy Sci. 1995, 78, 24–35. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Characterization of protein components of natural and heat-treated milk fat globule membranes. Int. Dairy J. 2002, 12, 393–402. [Google Scholar] [CrossRef]
- Cano-Ruiz, M.E.; Richter, R.L. Effect of Homogenization Pressure on the Milk Fat Globule Membrane Proteins. J. Dairy Sci. 1997, 80, 2732–2739. [Google Scholar] [CrossRef]
- Corredig, M.; Roesch, R.R.; Dalgleish, D.G. Production of a Novel Ingredient from Buttermilk. J. Dairy Sci. 2003, 86, 2744–2750. [Google Scholar] [CrossRef]
- Roesch, R.R.; Rincon, A.; Corredig, M. Emulsifying Properties of Fractions Prepared from Commercial Buttermilk by Microfiltration. J. Dairy Sci. 2004, 87, 4080–4087. [Google Scholar] [CrossRef]
- Rombaut, R.; Dejonckheere, V.; Dewettinck, K. Filtration of Milk Fat Globule Membrane Fragments from Acid Buttermilk Cheese Whey. J. Dairy Sci. 2007, 90, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Buchheim, W. Recovery of phospholipids from buttermilk using membrane processing. Kiel. Milchwirtsch. Forsch. 1997, 1, 47–68. [Google Scholar]
- Smith, L.M.; Jack, E.L. Isolation of Milk Phospholipids and Determination of Their Polyunsaturated Fatty Acids. J. Dairy Sci. 1959, 42, 767–779. [Google Scholar] [CrossRef]
- Garmy, N.; Taïeb, N.; Yahi, N.; Fantini, J. Interaction of cholesterol with sphingosine: Physicochemical characterization and impact on intestinal absorption. J. Lipid Res. 2005, 46, 36–45. [Google Scholar] [CrossRef]
- Feng, D.; Ohlsson, L.; Ling, W.; Nilsson, Å.; Duan, R.-D. Generating Ceramide from Sphingomyelin by Alkaline Sphingomyelinase in the Gut Enhances Sphingomyelin-Induced Inhibition of Cholesterol Uptake in Caco-2 Cells. Dig. Dis. Sci. 2010, 55, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Naganuma, T.; Sase, Y.; Kihara, A. Long-chain bases of sphingolipids are transported into cells via the acyl-CoA synthetases. Sci. Rep. 2016, 6, 25469. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Milk Sphingomyelin Is More Effective than Egg Sphingomyelin in Inhibiting Intestinal Absorption of Cholesterol and Fat in Rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef]
- Norris, G.H.; Milard, M.; Michalski, M.-C.; Blesso, C.N. Protective Properties of Milk Sphingomyelin against Dysfunctional Lipid Metabolism, Gut Dysbiosis, and Inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, E.R.M.; Wang, D.Q.-H.; Donovan, J.M.; Carey, M.C. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 2002, 122, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, J.H.; Nejrup, R.G.; Frøkiær, H.; Nilsson, Å.; Ohlsson, L.; Hellgren, L.I. Emulsifying triglycerides with dairy phospholipids instead of soy lecithin modulates gut lipase activity. Eur. J. Lipid Sci. Technol. 2015, 117, 1522–1539. [Google Scholar] [CrossRef]
- Patton, J.S.; Carey, M.C. Inhibition of human pancreatic lipase-colipase activity by mixed bile salt-phospholipid micelles. Am. J. Physiol. - Gastrointest. Liver Physiol. 1981, 4, 328–336. [Google Scholar] [CrossRef]
- Homan, R.; Hamelehle, K.L. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J. Lipid Res. 1998, 39, 1197–1209. [Google Scholar]
- Rodgers, J.B.; O’Connor, P.J. Effect of phosphatidylcholine on fatty acid and cholesterol absorption from mixed micellar solutions. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1975, 409, 192–200. [Google Scholar] [CrossRef]
- Rampone, A.J.; Long, L.R. The effect of phosphatidylcholine and lysophosphatidylcholine on the absorption and mucosal metabolism of oleic acid and cholesterol in vitro. Biochim. Biophys. Acta - Lipids Lipid Metab. 1977, 486, 500–510. [Google Scholar] [CrossRef]
- Beil, F.U.; Grundy, S.M. Studies on plasma lipoproteins during absorption of exogenous lecithin in man. J. Lipid Res. 1980, 21, 525–536. [Google Scholar]
- Young, S.C.; Hui, D.Y. Pancreatic Lipase/Colipase-Mediated Triacylglycerol Hydrolysis Is Required for Cholesterol Transport from Lipid Emulsions to Intestinal Cells. Biochem. J. 1999, 339, 615–620. [Google Scholar] [CrossRef]
- Hollander, D.; Morgan, D. Effect of plant sterols, fatty acids and lecithin on cholesterol absorption in vivo in the rat. Lipids 1980, 15, 395–400. [Google Scholar] [CrossRef]
- Imaizumi, K.; Mawatari, K.; Murata, M.; Ikeda, I.; Sugano, M. The Contrasting Effect of Dietary Phosphatidylethanolamine and Phosphatidylcholine on Serum Lipoproteins and Liver Lipids in Rats. J. Nutr. 1983, 113, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Sekihara, K.; Sugano, M. Hypocholesterolemic action of dietary phosphatidylethanolamine in rats sensitive to exogenous cholesterol. J. Nutr. Biochem. 1991, 2, 251–254. [Google Scholar] [CrossRef]
- Grzybek, M.; Kubiak, J.; Łach, A.; Przybyło, M.; Sikorski, A.F. A raft-associated species of phosphatidylethanolamine interacts with cholesterol comparably to sphingomyelin. A Langmuir-Blodgett monolayer study. PLoS ONE 2009, 4, e5053. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Brzustowicz, M.R.; Gustafson, N.; Stillwell, W.; Wassall, S.R. Monounsaturated PE does not phase-separate from the lipid raft molecules sphingomyelin and cholesterol: Role for polyunsaturation? Biochemistry 2002, 41, 10593–10602. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor γ expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef]
- Józefowski, S.; Czerkies, M.; Łukasik, A.; Bielawska, A.; Bielawski, J.; Kwiatkowska, K.; Sobota, A. Ceramide and Ceramide 1-Phosphate Are Negative Regulators of TNF-α Production Induced by Lipopolysaccharide. J. Immunol. 2010, 185, 6960–6973. [Google Scholar] [CrossRef]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.Q.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef]
- Milard, M.; Penhoat, A.; Durand, A.; Buisson, C.; Loizon, E.; Meugnier, E.; Bertrand, K.; Joffre, F.; Cheillan, D.; Garnier, L.; et al. Acute effects of milk polar lipids on intestinal tight junction expression: Towards an impact of sphingomyelin through the regulation of IL-8 secretion? J. Nutr. Biochem. 2019, 65, 128–138. [Google Scholar] [CrossRef]
- Snow, D.R.; Ward, R.E.; Olsen, A.; Jimenez-Flores, R.; Hintze, K.J. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J. Dairy Sci. 2011, 94, 2201–2212. [Google Scholar] [CrossRef]
- Ten Bruggencate, S.J.; Frederiksen, P.D.; Pedersen, S.M.; Floris-Vollenbroek, E.G.; Lucas-van de Bos, E.; van Hoffen, E.; Wejse, P.L. Dietary Milk-Fat-Globule Membrane Affects Resistance to Diarrheagenic Escherichia coli in Healthy Adults in a Randomized, Placebo-Controlled, Double-Blind Study. J. Nutr. 2016, 146, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Mao, X. Milk fat globule membrane supplementation modulates the gut microbiota and attenuates metabolic endotoxemia in high-fat diet-fed mice. J. Funct. Foods 2018, 47, 56–65. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bawden, E. Gut Microbiome. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 2010, 156, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Wu, X.; Ma, C.; Han, L.; Nawaz, M.; Gao, F.; Zhang, X.; Yu, P.; Zhao, C.; Li, L.; Zhou, A.; et al. Molecular Characterisation of the Faecal Microbiota in Patients with Type II Diabetes. Curr. Microbiol. 2010, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, G.; Huys, G.; Bekaert, M.; Boulanger, L.; De Boeck, K.; Vandamme, P. Dysbiosis of bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J. Cyst. Fibros. 2013, 12, 206–215. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Carmen Collado, M.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. Obesity in school-aged children and its correlation with Gut E.coli and Bifidobacteria: A case–control study. BMC Pediatr. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Sprong, R.C.; Hulstein, M.F.E.; Van Der Meer, R. Bactericidal Activities of Milk Lipids. Antimicrob. Agents Chemother. 2001, 45, 1298–1301. [Google Scholar] [CrossRef]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid bases are taken up by Escherichia coli and Staphylococcus aureus and induce ultrastructural damage. Skin Pharmacol. Physiol. 2013, 26, 36–44. [Google Scholar] [CrossRef]
- Nejrup, R.G.; Bahl, M.I.; Vigsnæs, L.K.; Heerup, C.; Licht, T.R.; Hellgren, L.I. Lipid hydrolysis products affect the composition of infant gut microbial communities in vitro. Br. J. Nutr. 2015, 114, 63–74. [Google Scholar] [CrossRef]
- Nilsson, Å.; Duan, R.-D. Pancreatic and mucosal enzymes in choline phospholipid digestion. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G425–G445. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Hansen, G.H.; Niels-Christiansen, L.L.; Koentgen, F.; Ohlsson, L.; Nilsson, Å.; Duan, R.D. Crucial role of alkaline sphingomyelinase in sphingomyelin digestion: A study on enzyme knockout mice. J. Lipid Res. 2011, 52, 771–781. [Google Scholar] [CrossRef]
- Millar, C.L.; Jiang, C.; Norris, G.H.; Garcia, C.; Seibel, S.; Anto, L.; Lee, J.-Y.; Blesso, C.N. Cow’s milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a Western-type diet. J. Nutr. Biochem. 2020, 79, 108351. [Google Scholar] [CrossRef]
- Milard, M.; Laugerette, F.; Durand, A.; Buisson, C.; Meugnier, E.; Loizon, E.; Louche-Pelissier, C.; Sauvinet, V.; Garnier, L.; Viel, S.; et al. Milk Polar Lipids in a High-Fat Diet Can Prevent Body Weight Gain: Modulated Abundance of Gut Bacteria in Relation with Fecal Loss of Specific Fatty Acids. Mol. Nutr. Food Res. 2019, 63, 1801078. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Reis, M.G.; Roy, N.C.; Bermingham, E.N.; Ryan, L.; Bibiloni, R.; Young, W.; Krause, L.; Berger, B.; North, M.; Stelwagen, K.; et al. Impact of Dietary Dairy Polar Lipids on Lipid Metabolism of Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2013, 61, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, X.; Wang, K.-X.; Zou, L.-J.; Ji, P.; Yin, Y.-L. Ethanolamine enhances intestinal functions by altering gut microbiome and mucosal anti-stress capacity in weaned rats. Br. J. Nutr. 2018, 120, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2019, 69, 487–501. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Gérard, P.; Lepercq, P.; Leclerc, M.; Gavini, F.; Raibaud, P.; Juste, C. Bacteroides sp. Strain D8, the First Cholesterol-Reducing Bacterium Isolated from Human Feces. Appl. Environ. Microbiol. 2007, 73, 5742–5749. [Google Scholar] [CrossRef]
- Gérard, P. Metabolism of Cholesterol and Bile Acids by the Gut Microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef]
- Sekimoto, H.; Shimada, O.; Makanishi, M.; Nakano, T.; Katayama, O. Interrelationship between serum and fecal sterols. Jpn. J. Med. 1983, 22, 14–20. [Google Scholar] [CrossRef]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents - physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Grill, J.P.; Schneider, F.; Ballongue, J. Bifidobacteria and probiotic effects: Action of Bifidobacterium species on conjugated bile salts. Curr. Microbiol. 1995, 31, 23–27. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Radhakrishnan Nair, U. Pellissery and Uma (2013). Lactic Acid Bacteria as Mucosal Delivery Vaccine Review Article ARTICLE HISTORY ABSTRACT. Adv. Anim. Vet. Sci. 2013, 1, 183–187. [Google Scholar]
- Di Gioia, D.; Aloisio, I.; Mazzola, G.; Biavati, B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biotechnol. 2014, 98, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.Y.D.; et al. Plasma choline metabolites and colorectal cancer risk in the women’s health initiative observational study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61, 1700363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lorenzen, J.; Astrup, A.; Larsen, L.; Yde, C.; Clausen, M.; Bertram, H. Metabolic Effects of a 24-Week Energy-Restricted Intervention Combined with Low or High Dairy Intake in Overweight Women: An NMR-Based Metabolomics Investigation. Nutrients 2016, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yde, C.C.; Clausen, M.R.; Kristensen, M.; Lorenzen, J.; Astrup, A.; Bertram, H.C. Metabolomics investigation to shed light on cheese as a possible piece in the French paradox puzzle. J. Agric. Food Chem. 2015, 63, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine- N -oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Daviglus, M.L.; Stamler, J.; Orencia, A.J.; Dyer, A.R.; Liu, K.; Greenland, P.; Walsh, M.K.; Morris, D.; Shekelle, R.B. Fish Consumption and the 30-Year Risk of Fatal Myocardial Infarction. N. Engl. J. Med. 1997, 336, 1046–1053. [Google Scholar] [CrossRef]
- Dewailly, É.; Blanchet, C.; Gingras, S.; Lemieux, S.; Holub, B.J. Fish consumption and blood lipids in three ethnic groups of Québec (Canada). Lipids 2003, 38, 359–365. [Google Scholar] [CrossRef]
- Kromhout, D.; Bosschieter, E.B.; Cor de Lezenne, C. The Inverse Relation between Fish Consumption and 20-Year Mortality from Coronary Heart Disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Shekelle, R.B.; Missell, L.; Paul, O.; Shryock, A.M.; Stamler, J.; Vollset, S.E.; Heuch, I.; Bjelke, E.; Curb, J.D.; Reed, D.M.; et al. Fish Consumption and Mortality from Coronary Heart Disease. N. Engl. J. Med. 1985, 313, 820–824. [Google Scholar]
- Gao, X.; Xu, J.; Jiang, C.; Zhang, Y.; Xue, Y.; Li, Z.; Wang, J.; Xue, C.; Wang, Y. Fish oil ameliorates trimethylamine N-oxide-exacerbated glucose intolerance in high-fat diet-fed mice. Food Funct. 2015, 6, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of causal direction between gut microbiota- dependent metabolites and cardiometabolic health: A bidirectional mendelian randomization analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Santulli, S. Santulli Gaetano Epidemiology of Cardiovascular Disease in the 21st Century: Updated Numbers and Updated Facts. J. Cardiovasc. Dis. 2013, 1, 1–2. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Watanabe, S.; Takahashi, T.; Tanaka, L.; Haruta, Y.; Shiota, M.; Hosokawa, M.; Miyashita, K. The effect of milk polar lipids separated from butter serum on the lipid levels in the liver and the plasma of obese-model mouse (KK-Ay). J. Funct. Foods 2011, 3, 313–320. [Google Scholar] [CrossRef]
- Wat, E.; Tandy, S.; Kapera, E.; Kamili, A.; Chung, R.W.S.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar] [CrossRef]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study1. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef]
- Conway, V.; Couture, P.; Richard, C.; Gauthier, S.F.; Pouliot, Y.; Lamarche, B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1255–1262. [Google Scholar] [CrossRef]
- Millar, C.L.; Norris, G.H.; Vitols, A.; Garcia, C.; Seibel, S.; Anto, L.; Blesso, C.N. Dietary egg sphingomyelin prevents aortic root plaque accumulation in apolipoprotein-E knockout mice. Nutrients 2019, 11, 1124. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Wang, Z.; Bursill, C.A.; Wu, B.J.; Barter, P.J.; Rye, K.-A. Effect of long-term dietary sphingomyelin supplementation on atherosclerosis in mice. PLoS ONE 2017, 12, e0189523. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006, 87, 1–16. [Google Scholar] [CrossRef]
- Weiland, A.; Bub, A.; Barth, S.W.; Schrezenmeir, J.; Pfeuffer, M. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men – Two double-blind, randomised, controlled, clinical trials. J. Nutr. Sci. 2016, 5, e21. [Google Scholar] [CrossRef]
- Nyberg, L.; Duan, R.D.; Nilsson, Å. A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J. Nutr. Biochem. 2000, 11, 244–249. [Google Scholar] [CrossRef]
- Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr. Metab. (Lond). 2010, 7, 90. [Google Scholar] [CrossRef]
- Zhou, A.L.; Hintze, K.J.; Jimenez-Flores, R.; Ward, R.E. Dietary fat composition influences tissue lipid profile and gene expression in fischer-344 rats. Lipids 2012, 47, 1119–1130. [Google Scholar] [CrossRef]
- Lecomte, M.; Bourlieu, C.; Meugnier, E.; Penhoat, A.; Cheillan, D.; Pineau, G.; Loizon, E.; Trauchessec, M.; Claude, M.; Ménard, O.; et al. Milk Polar Lipids Affect In Vitro Digestive Lipolysis and Postprandial Lipid Metabolism in Mice. J. Nutr. 2015, 145, 1770–1777. [Google Scholar] [CrossRef]
- Lecomte, M.; Couëdelo, L.; Meugnier, E.; Plaisancié, P.; Létisse, M.; Benoit, B.; Gabert, L.; Penhoat, A.; Durand, A.; Pineau, G.; et al. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Mol. Nutr. Food Res. 2016, 60, 609–620. [Google Scholar] [CrossRef]
- Yamauchi, I.; Uemura, M.; Hosokawa, M.; Iwashima-Suzuki, A.; Shiota, M.; Miyashita, K. The dietary effect of milk sphingomyelin on the lipid metabolism of obese/diabetic KK-Ay mice and wild-type C57BL/6J mice. Food Funct. 2016, 7, 3854–3867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Ward, R.E. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J. Dairy Sci. 2019, 102, 4816–4831. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Burling, H.; Nilsson, A. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis. 2009, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Hertervig, E.; Jönsson, B.A.; Duan, R.-D.; Nyberg, L.; Svernlöv, R.; Nilsson, Å. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am. J. Clin. Nutr. 2010, 91, 672–678. [Google Scholar] [CrossRef]
- Ohlsson, L.; Burling, H.; Duan, R.-D.; Nilsson, Å. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur. J. Clin. Nutr. 2010, 64, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Malarski, A.; Reuther, C.; Kertscher, R.; Kiehntopf, M.; Jahreis, G. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Kelly, E.R.; van der Made, S.; Berendschot, T.T.J.M.; Husche, C.; Lütjohann, D.; Plat, J. The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition 2013, 29, 1237–1244. [Google Scholar] [CrossRef]
- Severins, N.; Mensink, R.P.; Plat, J. Effects of lutein-enriched egg yolk in buttermilk or skimmed milk on serum lipids & lipoproteins of mildly hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 210–217. [Google Scholar]
- Grip, T.; Dyrlund, T.S.; Ahonen, L.; Domellöf, M.; Hernell, O.; Hyötyläinen, T.; Knip, M.; Lönnerdal, B.; Orešič, M.; Timby, N. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatr. Res. 2018, 84, 726–732. [Google Scholar] [CrossRef]
- Lebovitz, H. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2012, 35, S11. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Nakamichi, H.; Hama, Y.; Higashiyama, S.; Igarashi, Y.; Mitsutake, S. Phytosphingosine is a novel activator of GPR120. J. Biochem. 2018, 164, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Leppimäki, P.; Axberg, M.; Slotte, J.P. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem. 1999, 266, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.L.; Nelson, C.A. Brain Development and the Role of Experience in the Early Years. Zero Three 2009, 30, 9–13. [Google Scholar] [PubMed]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Kuchta-Noctor, A.M.; Murray, B.A.; Stanton, C.; Devery, R.; Kelly, P.M. Anticancer Activity of Buttermilk Against SW480 Colon Cancer Cells is Associated with Caspase-Independent Cell Death and Attenuation of Wnt, Akt, and ERK Signaling. Nutr. Cancer 2016, 68, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.M.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H. Colonic Cell Proliferation and Aberrant Crypt Foci Formation Are Inhibited by Dairy Glycosphingolipids in 1,2-Dimethylhydrazine-Treated CF1 Mice. J. Nutr. 2000, 130, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, D.L.; Webb, S.K.; Schmelz, E.-M.; Merrill, A.H. Dietary Sphingomyelin Inhibits 1,2-Dimethylhydrazine-Induced Colon Cancer in CF1 Mice. J. Nutr. 1994, 124, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.M.; Dillehay, D.L.; Webb, S.K.; Reiter, A.; Adams, J.; Merrill, A.H. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: Implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996, 56, 4936–4941. [Google Scholar] [PubMed]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary Milk Fat Globule Membrane Reduces the Incidence of Aberrant Crypt Foci in Fischer-344 Rats. J. Agric. Food Chem 2010, 58, 2157. [Google Scholar] [CrossRef] [PubMed]
| Product | PL (g/100 g DM) | PL (g/100 g Fat) | PE (% of Total PL) | PI (% of Total PL) | PS (% of Total PL) | PC (% of Total PL) | SM (% of Total PL) | Reference |
|---|---|---|---|---|---|---|---|---|
| Whole milk | 0.2–0.3 | 0.7–2.3 | 23.2–72.2 | 1.4–7.5 | 3.4–24.5 | 8.0–46.4 | 4.0–29.5 | [20,30,31,32,33,34,39,40,41,42,45] |
| Skim milk | 0.1 | 10.7–11.1 | 26.7–38.2 | 5.5–8.4 | 8.4–9.9 | 19.6–35.2 | 16.7–21.2 | [30,32] |
| Cream | 0.2–0.4 | 0.3–5.6 | 17.7–45.6 | 6.8–15.4 | 6.7–14.8 | 14.6–33.7 | 11.9–28.6 | [19,30,32,42,43,44,46] |
| Butter | 0.3 | 0.2–5.31 | 17.7–43.3 | 4.3–15.8 | 7.0–15.3 | 19.9–35.6 | 16.6–21.8 | [30,31,32,43,44,46] |
| Buttermilk | 1.1- 2.0 | 4.5–35.3 | 17.0–44.8 | 2.4–17.3 | 8.0–18.0 | 17.3–46.0 | 12.1–21.5 | [29,30,37,42,44,47,48,49] |
| Butter serum | 11.5 | 46.7–48.4 | 26.7–31.4 | 9.0–11.2 | 6.9–10.1 | 24.9–27.2 | 23.8–28.9 | [30,32,47] |
| Cheese whey | 0.3–1.8 | 5.3–23.7 | 27.4–41.1 | 2.8–3.7 | 3.9–9.3 | 19.0–32.2 | 9.9–16.4 | [29,30,48,50] |
| Yogurt (skimmed) | 0.2 | 5.5 | 31.1 | 6.3 | 7.9 | 19.9 | 24.9 | [30] |
| Ricotta cheese | 1.16 | 2.7 | 45.4 | 4.4 | 5.8 | 15.8 | 14.2 | [30] |
| Mozzarella cheese | 0.28 | 0.5 | 42.5 | 5.7 | 5.6 | 19.4 | 14.6 | |
| Cheddar cheese | 0.25 | 0.5 | 38.0 | 7.7 | 8.5 | 20.3 | 16.3 |
| Authors | Model | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Reis et al. (2013) | C57BL/6J mice | HFD (n = 13) | HFD followed by supplementation of total polar lipids (TPL), phospholipids (PL), or sphingolipids (SPL) through HFD (n = 13) | 5 weeks on HFD followed by 5 weeks on TPL/PL/SM | Little effect of the polar lipid dietary supplementation on the composition of cecal microbiota was observed (p > 0.05). | [107] |
| Nejrup et al. (2015) | Fecal samples from nine healthy infants (aged 2–5 months) | Medium chained and long chain NEFA with and without 10 mol% sphingosine | 24 h in vitro | LC-NEFA with sphingosine: increased bifidobacteria | [99] | |
| Zhou et al. (2018) | 21-d-old Sprague–Dawley rats | 0 µM Ethanolamine in drinking water | 250, 500 and 1000 μM Ethanolamine from milk in drinking water for 2 weeks | 2 weeks | Increased: Bacteroidetes (500 and 1000 μM) Decreased: Proteobacteria, Elusimicrobia and Tenericutes (500 and 1000 μM) Spirochetes (500 μM) | [109] |
| Norris et al. (2016) | Male C57BL/6 mice | HFD (21% added milk fat by weight) (n = 3) | 0.25% (w/w) milk SM in HFD (n = 10) | 4 weeks | Increased: Firmicutes, bifidobacteria, Actinobacteria and Gram-positives Decreased: Bacteroidetes, Tenericutes and Gram-negatives | [9] |
| Norris et al. (2017) | Male C57BL/6 mice | HFD (31% lard; 0.15% cholesterol by weight) (n = 14) | 0.1% (w/w) milk SM in HFD. (n = 14) | 10 weeks | Increased Acetatifactor | [11] |
| Bhinder et al. (2017) | 5 to 15 days old Rats (Used pup in a cup model) | Fed with mothers’ milk (MM) | Formula with MFGM comprising part of the fat component or Formula with fat derived entirely from vegetable source | 15 days | MFGM formula: microbial richness and evenness similar to MM. Similar abundances of Firmicutes and Proteobacteria compared to MM MFGM formula: Increased Lactobacilli, Enterococcus, Clostridiales, Streptococcus, and Morganella vs. vegetable fat formula. | [108] |
| Li et al. (2018) | 5 weeks old C57BL/6J mice | Chow diet (n = 10) | HFD (n = 10) or HFD + MFGM (Lacprodan® MFGM-10) at 200 mg/kg BW (n = 10) | MFGM diet increased the relative abundance of Porphyromonadaceae, S24–7, norank_f_Bacteroidates_S24- 7_group, unclassified_f_Lachnospiraceae, and Odoribacter compared with the HFD group Increased ACE index compared with HFD. MFGM supplementation recovered 13 key genera found enriched in control group Simpson’s index showed no difference among three group | [84] | |
| Milard et al. (2019) | Male C57BL/6J mice | HFD (21% w/w palm oil in chow) | 8 weeks on HFD with 1.1% (w/w) milk PL or 1.6% (w/w) of milk PL | 8 weeks | Increased: Bifidobacterium, in particular Bifidobacterium animalis in 1.1% of milk PL group Decreased: Lactobacillus in 1.6% of milk PL group Positive correlation between Bifidobacterium animalis and Akkermansia muciniphila | [103] |
| Vors et al. (2019) | Double-blind, parallel clinical trial in 58 Overweight postmenopausal women | No milk PL via butter serum (n = 19) | 3 mg (n = 19) or 5 mg (n = 20) of milk PL via butter serum | 4 weeks | No change in major phylogenetic groups and bacterial species of gut microbiota Increased fecal coprostanol/cholesterol ratio | [110] |
| Millar et al. (2020) | LDLr−/− mice | HFD (45%) for (n = 15) | HFD (45%) with 1% or 2% milk PL (MPL) (n = 15) | 14 weeks | 2% MPL: Increased Actinobacteria, Bacteroidetes, Bifidobacterium, Bacteriodales_unclassified. Reduced Firmicutes/Bacteroidetes ratio 1% MPL: Increased Shannon diversity | [102] |
| Authors | Animal Model | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Nyberg et al. (2000) | Male Sprague-Dawley rats (n = 5–8) | Cholesterol mixed in soybean oil (without PL) | 2.6:1, 1:1 or 0.5:1 molar ratio of cholesterol:SM | 3 days | Decreased intestinal cholesterol absorption (lowest in cholesterol:SM ratio 1:1) | [148] |
| Eckhardt et al. (2002) | Male C57BL/6 mice (n = 6) | Chow | Chow diet enriched in PL (containing 0.1%, 0.5% or 5% of milk SM by weight) | 4 days | Decreased intestinal cholesterol absorption | [64] |
| Wat et al. (2009) | Male C57BL/6 mice (n = 10) | LFD or HFD without milk PL | LFD or Western-type diet with 1.2% (w/w) PL from phospholipid-rich dairy milk extract (PLRDME) | 8 weeks | Serum lipids: PLRDME with western-type diet group: Decreased TG (−20%), phospholipids (−21%) and HDL-C (−19%) PLRDME with LFD group: No change in TC, TG, phospholipids and HDL-C Hepatic Lipids: PLRDME with western-type diet group: Decreased total lipid (−33%), TG (−44%), TC (−48%) and phospholipids (−16%) PLRDME with LFD group: No change in total lipid, TG, TC and phospholipids | [139] |
| Kamili et al. (2010) | Male C57BL/6 mice (n = 10) | Western-type diet without milk PL | Western-type diet (21% AMF; 0.15% cholesterol by weight) with 1.2% (w/w) PL from PLRDME or milk phospholipid concentrate (PC-700) | 3, 5 or 8 weeks | Plasma lipids: PLRDME after 8 weeks: Decreased plasma TC (−23%) Hepatic lipids: PLRDME after 5 weeks: Decreased total lipid (−41%), TG (−47%) and TC (−39%) PLRDME after 8 weeks: Decreased total lipid (−18%) and TG (−28%) PC−700 after 5 weeks: Decreased total lipid (−45%), TG (−63%) and TC (−57%) | [149] |
| Watanabe et al. (2011) | Female KK-Ay mice (n = 7) | AIN-93G diet | AIN-93G diet with 1.7% (w/w) of lipid-concentrated butter serum (LC-BS) or 0.5% (w/w) of ceramide-rich fraction (Cer-fr) or 0.5% (w/w) of SM-rich fraction (SM-fr) | 4 weeks | Plasma lipids: SM-fr: no change LC-BS: Decreased TC (−18%) and LDL-C (−45%) Cer-fr: Change only in TC (−25%) Hepatic lipids: SM-fr: No change LC-BS: Decreased TG (−27%) Cer-fr: Decreased TG (−38%) and TC (−47%) | [138] |
| Zhou et al. (2012) | Fischer-344 rats (n = 3–4) | AIN-76A diet with corn oil or AMF (0.5% w/w) | 2.5% (w/w) MFGM, 2.5% (w/w) AMF in AIN-76A diets | 12 weeks | Decreased esterified cholesterol and increased TG in liver | [150] |
| Reis et al. (2013) | Male C57BL/6 (n = 13) | HFD | HFD (~20% lard by weight) with 1.7% (w/w) total polar lipids extracts or 1.4% (w/w) phospholipids-rich extract or 0.4% (w/w) SM-rich extract | 5 weeks | Decreased FA synthesis in liver by total PL extract and PL-rich extract Decreased 16:1n-7/16:0 in liver by SM-rich extract | [107] |
| Lecomte et al. (2015) | Female Swiss mice (n = 7) | Emulsion with soybean PL (gavaged) | Emulsion with 5.7 mg milk PL (gavaged) | 1, 2 or 4 h | After 1 h: Increased plasma NEFA and a trend to increase TG After 4 h: Decreased plasma TG and NEFA associated with a decreased duodenal gene expression of APOB 48 and Sar1b | [151] |
| Norris et al. (2016a) | Male C57BL/6 mice (n = 10) | HFD (21% AMF by weight) | 0.25% (w/w) milk SM in HFD (21% AMF by weight) | 4 weeks | Decreased serum TC and hepatic TG No change in serum TG and hepatic TC | [10] |
| Norris et al. (2017) | Male C57BL/6 mice (n = 14) | HFD (31% lard; 0.15% cholesterol by weight) | 0.1% (w/w) milk SM in HFD | 10 weeks | No change in serum lipids Decreased hepatic TC (−23%) and TG (−30%) | [9] |
| Lecomte et al. (2016) | Male C57BL/6J mice (n = 10–12) | HFD (17% w/w palm oil) + soybean PL | 1.2 % (w/w) milk PL or SPL in HFD (17% w/w palm oil) | 8 weeks | No change in plasma and hepatic lipids Increased fecal VLCFA such as C22:0, C24:0 and C22:4(n-6) | [152] |
| Yamauchi et al. (2016) | Obese/diabetic KK-Ay (n = 7) and male C57BL/6 mice (n = 6) | HFD (lard, soybean, linseed or fish) | 1% (w/w) milk SM in HFD (lard, soybean, linseed or fish) | 4 weeks | No effect on wild type mice In KK-Ay mice: Soybean + SM: decreased serum LDL-C and non-HDL-C. Increased hepatic total lipids, cholesterol, bile acid. Linseed + SM: Decreased serum LDL-C. Decreased hepatic total FA and increased fecal total lipid and cholesterol. Lard + SM: Increased fecal total lipids, cholesterol, and decreased hepatic total FA. | [153] |
| Zhou et al. (2019) | Male ob/ob mice (n = 11–18) | Moderately high-fat AIN-93G diet (34% kcal as fat) without milk PL or gangliosides | (0.2% (w/w) milk gangliosides (GG) or 1% (w/w) milk PL (PL) in moderately high-fat AIN-93G diet (34% kcal as fat) | 2 weeks | No change in plasma and hepatic lipids by milk GG. PL increased plasma NEFA, PL, SM and DAG and decreased hepatic CE. | [154] |
| Millar et al. (2020) | LDLr−/− mice | HFD (45%) for (n = 15) | HFD (45%) with 1% or 2% milk PL (MPL) (n = 15) | 14 weeks | 2% MPL: Decreased serum cholesterol (−51%), with dose-dependent reduction in VLDL-C and LDL-C. Decreased hepatic TC (−55%) 1% MPL: Decreased hepatic TC (−53%) | [102] |
| Authors | Population and Study Design | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Ohlsson et al. (2009) | Parallel group study with 33 healthy men and 15 healthy women | 119 mg of total SL (isocaloric) | 2 drinks/day totaling 975 mg SL containing 700 mg SM, 180 mg GC and 95 mg GS | 4 weeks | No change in plasma lipids. Trend for decreasing LDL-C (only in women) | [155] |
| Ohlsson et al. (2010) | Human ileostomy contents from 6 men and 6 women | 1. Milk SM (250 mg) mixed in skimmed milk 2. Milk SM (50,100 or 200 mg) mixed in milk-like oat drink | Collected after 8 h | Increased the out-put of VLCFA specific of milk SM (22:0, 23:0, 24:0) | [156] | |
| Ohlsson et al. (2010) | Crossover study in 18 healthy adult males | High-fat (40 g) standard breakfast together with a milk-like formulation lacking polar milk lipids | High-fat (40 g) standard breakfast together with a milk-like formulation containing 975 mg of milk SL | 1 to 7 h | No change in plasma lipids after 1 h Trend for decreasing cholesterol in large TG- rich lipoproteins. | [157] |
| Keller et al. (2013) | Parallel study in 14 healthy women | Baseline | 2 supplementation cycle–3 g milk PL/day followed by 6 g milk PL/day | 10 days each | 3 g milk PL: Decreased plasma TC, HDL-C After 6 g milk PL supplementation: Increased plasma TC and LDL-C | [158] |
| Conway et al. (2013) | Double-blinded crossover study in 34 healthy adults | 45 g/day of a macro/micronutrient matched placebo | 45 g buttermilk powder/day | 4 weeks | Decreased serum cholesterol (−3.1%), TG (−10.7%) and trend for decreasing LDL-C (p = 0.057) Decreased LDL-C (−5.6%) in participants with highest (top 50%) baseline LDL-C | [141] |
| Baumgartner et al. (2013) | Single-blind parallel study in 97 healthy adults | One or two eggs a week (n = 20) | 1. One egg/day (n = 57) 2. 100 mL/day of buttermilk drink containing one egg yolk (n = 20) | 12 weeks | No difference in serum lipids, liver inflammatory markers, Apo-A1, Apo-B100, campesterol, or lathosterol between the two treatment groups | [159] |
| Rosqvist et al. (2015) | Single-blind, parallel study in 57 overweight adults | Butter oil (1.3 mg total PL), matched for calories, macronutrients, and calcium | 40 g milkfat/day as whipping cream (198 mg total PL) | 8 weeks | Decreased plasma cholesterol, LDL-C, non-HDL-C, and apoB:apoA1 ratio | [140] |
| Severins et al. (2015) | Single-blind, parallel study in 92 mildly hypercholesterolemic adults | 80 mL of skim-milk powder (n = 25) | 1. 80 mL skim-milk with lutein enriched egg yolk (28 g from 1.5 eggs providing 323 mg cholesterol) 2. Buttermilk (72 mg PL) 3. Buttermilk with lutein enriched egg yolk (28 g from 1.5 eggs providing 323 mg cholesterol) | 12 weeks | Buttermilk addition could not change the increased serum lipids levels due to of egg yolk Buttermilk group showed a trend for decreasing TC (p = 0.077), but not for LDL-C | [160] |
| Weiland et al. (2016) | Double-blind parallel-group intervention trials in overweight or obese males. | Milk enriched with 2 g milk fat (n = 31) | Milk enriched with 2 g milk PL (n = 31) | 8 weeks | Decreased GGT and waist circumference No change in plasma lipids (total, HDL- and LDL-cholesterol, total cholesterol:HDL-cholesterol ratio, TAG, PL), ALT, AST, apoB, apoA1, glucose, insulin, insulin sensitivity index, C-reactive protein, IL-6, soluble intracellular adhesion molecule and total homocysteine (tHcy). | [147] |
| Milk enriched with 2.8 g soy PL (n = 57) | Milk enriched with 3 g milk PL (n = 57) | 7 weeks | Decreased only GGT No change in plasma lipids (TC, HDL-C, LDL-C, TG, phospholipids, TC:HDL-C ratio), apoA1, apoB, glucose, insulin, HOMA-IR, hs-CRP, IL-6, sICAM, ALT, AST | |||
| Grip et al. (2018) | Double blinded study in formula fed infants. | Breast fed infants (n = 80) | Formula without MFGM (n = 160) | 4, 6 and 12 months | Decreased plasma PC and SM | [161] |
| Vors et al. (2019) | Double blinded parallel study in 58 postmenopausal women | No milk PL via butter serum (n = 19) | 3 g (n = 19) or 5 g (n = 20) of milk PL via butter serum | 4 weeks | Decreased fasting total cholesterol, LDL-C, TC/HDL-C ratio, ApoB/ApoA1 ratio, post-prandial total cholesterol, chylomicron lipids. | [110] |
| Double blind cross-over study in 4 ileostomized subjects | No milk PL via butter serum (n = 19) with 2H-cholesterol tracer | 3 g (n = 19) or 5 g (n = 20) of milk PL via butter serum with 2H-cholesterol tracer | Acute post-prandial | Decreased 2H-cholesterol tracer in plasma and chylomicrons. Increased ileal output of total cholesterol and of milk SM |
| Author | Model | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Nagasawa et al. (2018) | 293 T cells | Dihydrosphingosine or phytosphingosine or sphingosine | 24 h | Significant upregulation of GPR120 (a receptor for long chain fatty acids) by dihydrosphingosine and phytosphingosine. Sphingosine - no effect. | [164] | |
| Yamauchi et al. (2016) | obese/diabetic KK-Ay mice (n = 7) | Lard or soybean oil or linseed oil | Lard + 1% SM or soybean oil +1% SM or linseed oil + 1% SM | 4 weeks | No difference in blood glucose level | [153] |
| wild-type C57BL/6J mice (n = 7) | Linseed oil or fish oil or lard + soybean oil | Linseed oil + 1% SM or fish oil + 1% SM or lard + soybean oil + 1% SM | 4 weeks | No difference in blood glucose level | ||
| Weiland et al. (2016) | Double-blind parallel-group intervention trials in overweight or obese males. | Milk enriched with 2 g milk fat (n = 31) | Milk enriched with 2 g milk PL (n = 31) | 8 weeks | No difference in blood glucose, insulin and HOMA-IR between groups | [147] |
| Milk enriched with 2.8 g soy PL (n = 57) | Milk enriched with 3 g milk PL (n = 57) | 7 weeks | No difference in blood glucose, insulin and HOMA-IR between groups | |||
| Norris et al. (2017) | Male C57BL/6 mice | HFD (31% lard; 0.15% cholesterol by weight) (n = 14) | 0.1% (w/w) milk SM (n = 14) in HFD | 10 weeks | No difference in fasting serum insulin, glucose concentrations and HOMA-IR between groups | [9] |
| Authors | Model/Population and Study Design | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Oshida et al. (2003) | Male Wister rat pups (n = 30) | No l-Cycloserine (LCS) treatment or dietary SM (non-LCS) | Daily s/c injection of 100 mg/kg of LCS from 8 days old + diet without (LCS group) or with 810 mg/100 g of SM (SM-LCS group) from 17 days old | Until 28 days old | Significantly high myelin dry weight, myelin total lipid content, and cerebroside content in the SM-LCS group than in the LCS group. Axon diameter, nerve fiber diameter, myelin thickness, and g value of optic nerve were similar in SM-LCS and non-LCS groups. | [167] |
| Tanaka et al. (2013) | Randomized, double-blind controlled trial in 28 premature infants with birth weight less than 1500 g | Milk (13 g SM/100 g PL) (n = 14) | Sphingomyelin fortified milk (20 g SM/100 g PL) (n = 14) | 18 months | Significantly better Behavior Rating Scale of the BSID-II, Fagan test scores, latency of VEP, and sustained attention test scores | [172] |
| Gurnida et al. (2012) | Double-blind, parallel study in infants 2 to 8 weeks of age | Standard infant formula (0.22% milk PL and 0.006% gangliosides) (n = 30) | Complex lipid-supplemented formula (0.235% milk PL and 0.009% gangliosides) (n = 29) | From 2–8 weeks of age to until 24 weeks of age | Increased Hand and Eye coordination IQ score (p < 0.006), Performance IQ score (p < 0.001) and General IQ score (p = 0.041). | [168] |
| Authors | Model | Control | Treatment | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Kutchta-Noctor et al. (2016) | SW480 colon cancer cells and FHC cells (normal human colon cells) | Controls contained only media. Sodium butyrate (5 mM), a potent apoptotic fatty acid, served as a positive control. | Buttermilk between 0 and 0.94 mg/mL of media | 3 days at 37 ℃ in CO2 incubator | Inhibited growth of SW480 colon cancer cells in dose-dependent manner with selective antiproliferative activity toward cancer cells Downregulated growth signaling pathways mediated by Akt, ERK1/2, and c-myc. | [173] |
| Schmelz et al. (2000) | 5 weeks old female CF1 mice | i/p injection of 1,2-DMH (DMH)@ 30 mg/kg body weight for 6 weeks + sphingolipid free AIN 76A diet | i/p injection of 1,2-dimethylhydrazine (DMH) at 30 mg/kg body weight for 6 weeks + AIN 76A diet with 0.025 or 0.1 g/100 g of milk GluCer, LacCer or ganglioside GD3 after 1 week | 4 weeks | Glycosphingolipid groups: >40% reduction (p < 0.001) in appearance of aberrant crypt foci, reduced proliferation (up to 80%; p < 0.001) in colonic crypts. | [174] |
| Dillehay et al. (1994) | CF1 mice | Injection of 1,2-DMH + diet without SM | Injection of DMH + diets with 0.025 to 0.1 g/100 g of SM for 28 weeks followed by diet without SM | 52 weeks | SM fed groups: 20% incidence of colon tumors (vs 47% in controls) | [175] |
| Schmelz et al. (1996) | 5 weeks old female CF1 mice | i/p injection of 0.5 mL/kg of DMH once weekly for 6 weeks followed by diet without SM | i/p injection of 0.5 mL/kg of DMH once weekly for 6 weeks followed by diet supplemented with 0 to 0.1% (w/w) buttermilk or powdered milk SM | 34 weeks | 0.1% SM: Reduced appearance of aberrant colonic crypt foci (p < 0.001) and significantly fewer aberrant crypts per colonic focus | [176] |
| Snow et al. (2010) | Fischer-344 rats | i/p injection of 1,2-DMH (25 mg/kg BW) once weekly for 2 weeks followed by AIN-76A diet corn oil | i/p injection of 1,2-dimethylhydrazine (25 mg/kg BW) once weekly for 2 weeks followed by AIN-76A diet with AMF or with 50% MFGM, 50% AMF | 9 weeks | MFGM group had significantly fewer aberrant crypt foci | [177] |
| Mazzei et al. (2011) | PPARγ+/+ and PPARγ−/− mice | Semi-purified sphingolipid-free AIN76A diet for 7 weeks followed by single injection of azoxymethane (10 mg/kg BW). | 0.1% SM (w/w) supplemented diet for 7 weeks followed by single injection of azoxymethane (10 mg/kg BW). | 9 weeks | SM group of both genotypes: Decreased disease activity and colonic inflammatory lesions (more efficiently in PPARγ+/+ mice). | [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients 2020, 12, 1001. https://doi.org/10.3390/nu12041001
Anto L, Warykas SW, Torres-Gonzalez M, Blesso CN. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients. 2020; 12(4):1001. https://doi.org/10.3390/nu12041001
Chicago/Turabian StyleAnto, Liya, Sarah Wen Warykas, Moises Torres-Gonzalez, and Christopher N. Blesso. 2020. "Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits" Nutrients 12, no. 4: 1001. https://doi.org/10.3390/nu12041001
APA StyleAnto, L., Warykas, S. W., Torres-Gonzalez, M., & Blesso, C. N. (2020). Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients, 12(4), 1001. https://doi.org/10.3390/nu12041001






