Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.1.1. Materials
2.1.2. Animals
2.2. Preparation, Characterization, and Stabilization of SeNPs
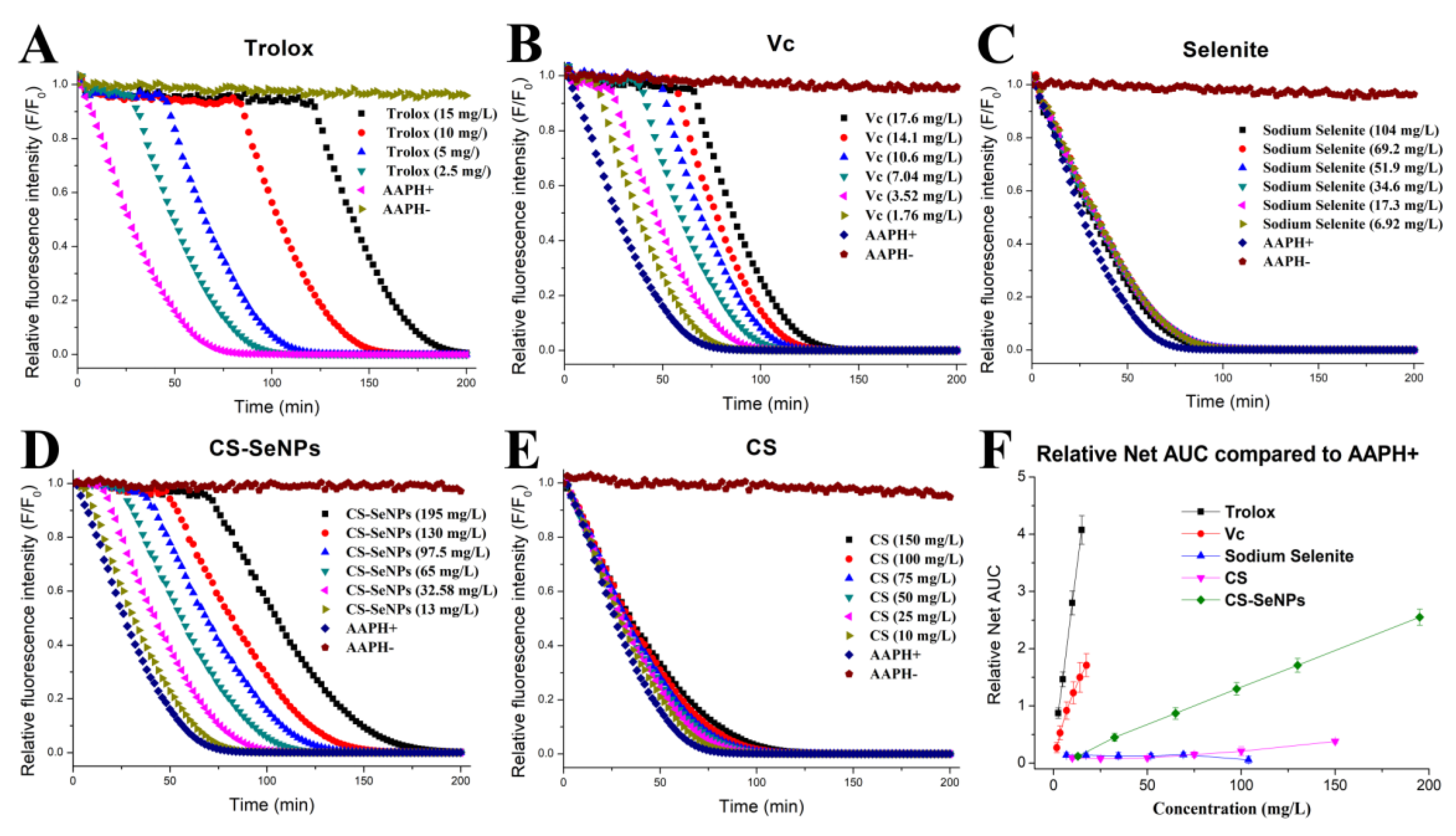
2.3. Oxygen Radical Absorbance Capacity (ORAC) Test
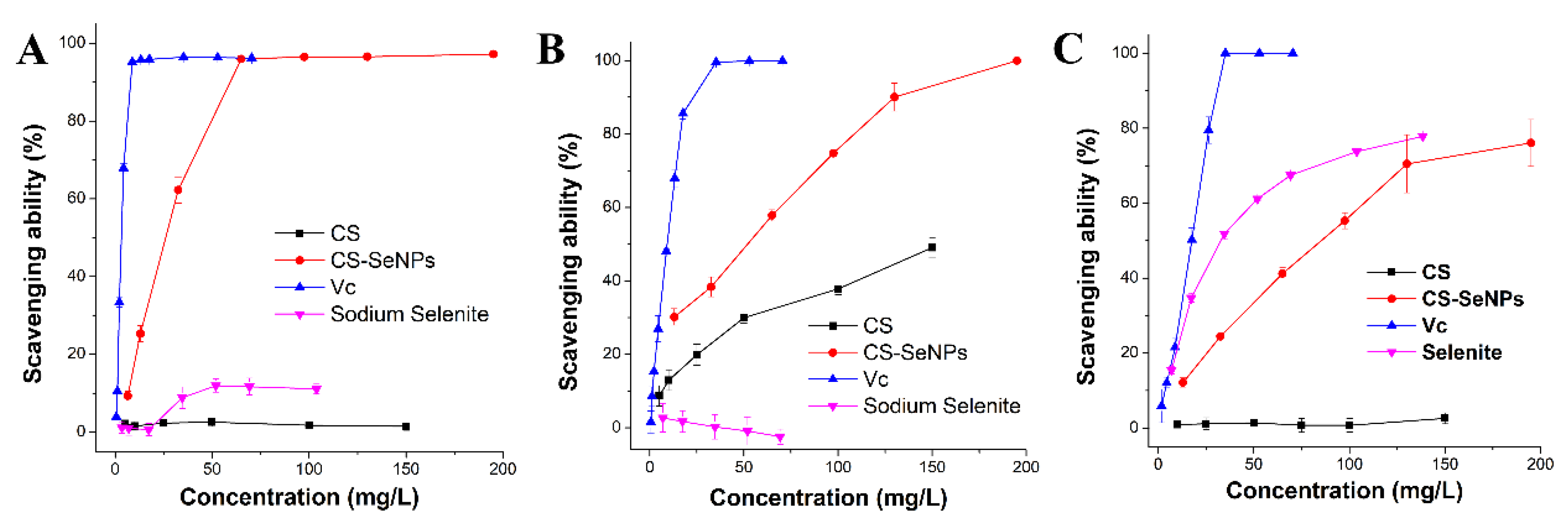
2.4. Free-Radical Scavenging Tests
2.5. Animal Experiments
2.5.1. Experiment Design and Animal Grouping
2.5.2. Liver Histopathology Assessment
2.5.3. Determination of Se Deposition
2.5.4. Biochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. UF-assisted Preparation of CS-SeNPs
3.2. Antioxidant Capacity of CS-SeNPs Determined by the ORAC Methodology
3.3. Free-Radical Scavenging Ability of CS-SeNPs
3.4. Hepatoprotection by Dried CS-SeNPs against Con A
3.4.1. Dried CS-SeNPs Powder Used as a SeNPs Sample
3.4.2. Growth and Viscera Status of KM Mice
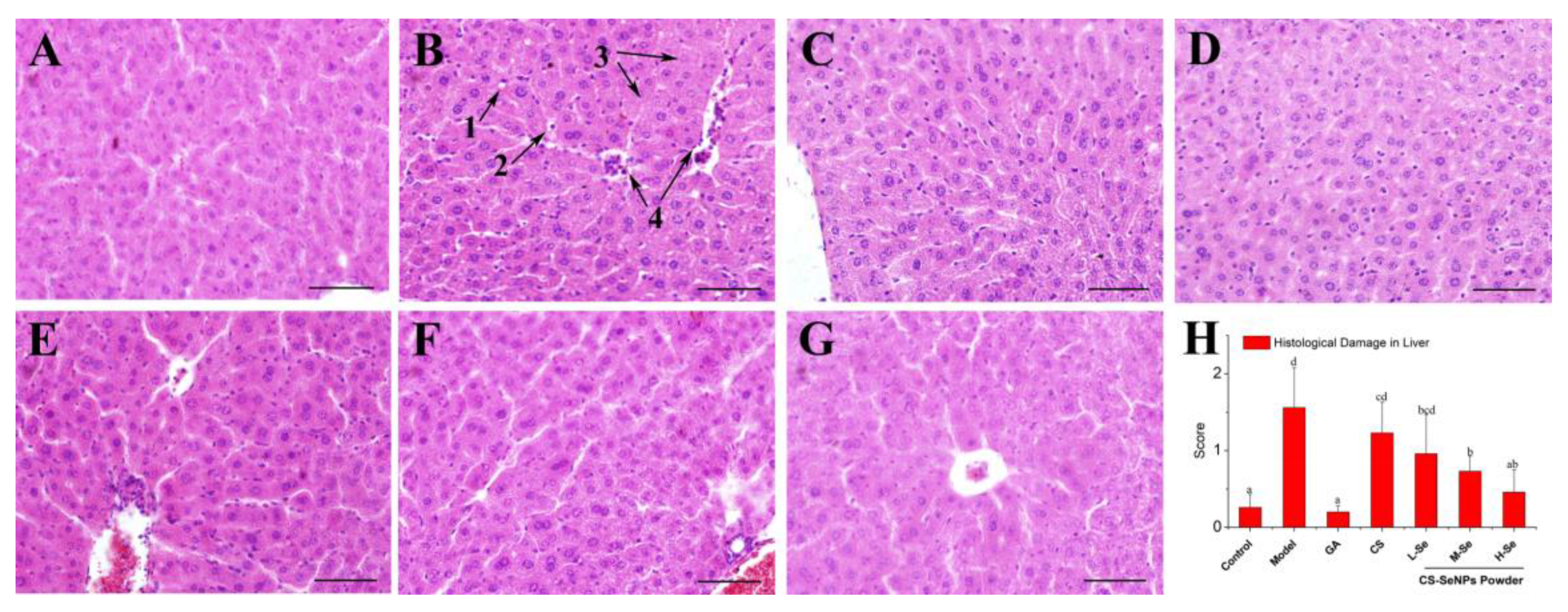
3.4.3. Histological Assessment of Hepatic Damage
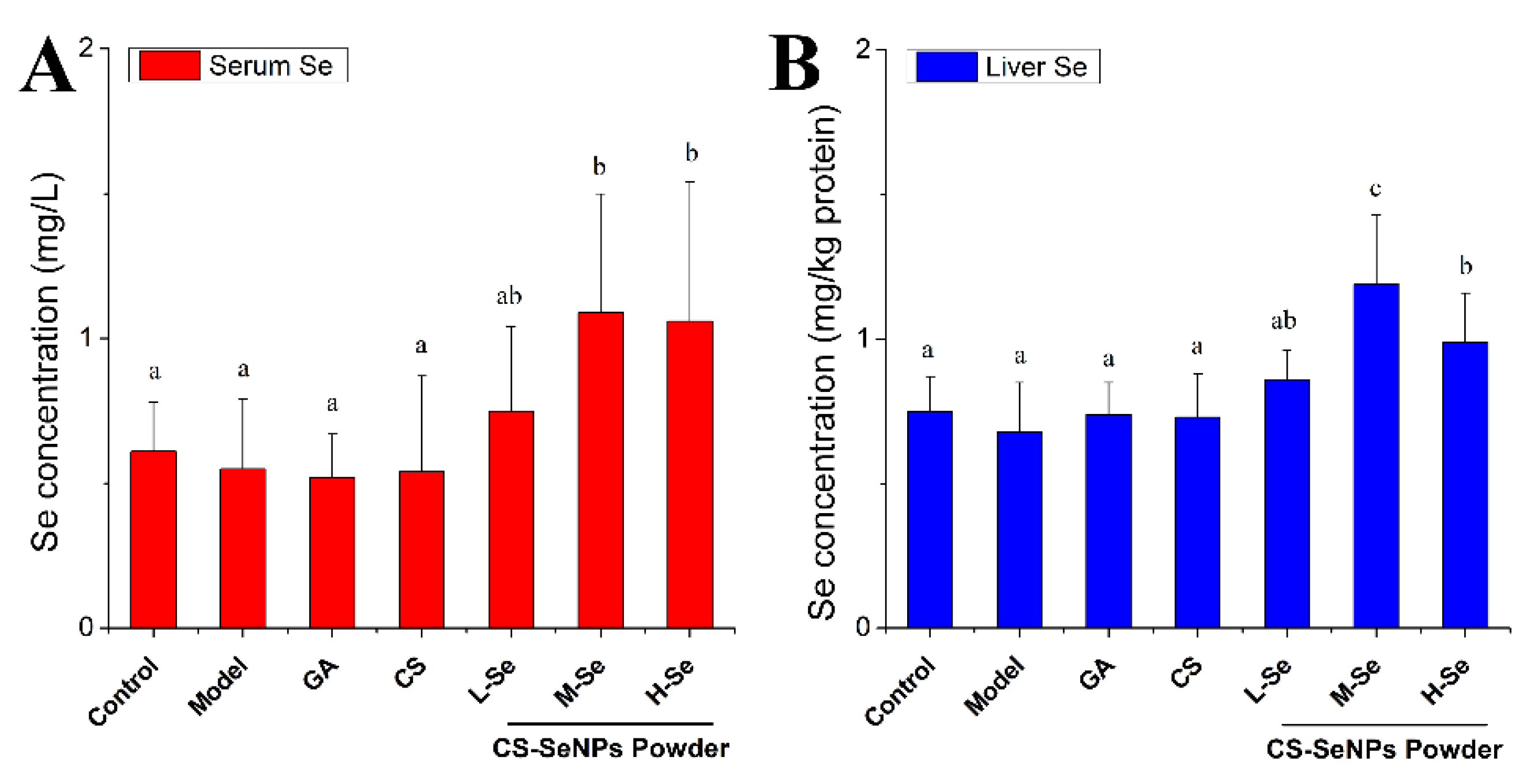
3.4.4. Se Retention
3.4.5. Serum Levels of Liver Enzymes and GSH
3.4.6. Antioxidant Activity in the Liver
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2’-Azobis-(2-amidinopropane)-dihydrochloride |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| AUC | area under the curve |
| bw | body weight |
| Con A | concanavalin A |
| CS | chitosan |
| CS-SeNPs | chitosan-stabilized selenium nanopartilcels |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| •DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| EC50 | concentration for 50% of maximal effect |
| EDS | energy-dispersive X-ray spectroscopy |
| GA | glycyrrhizic acid |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| ICP-MS | inductively coupled plasma mass spectrometry |
| i.p, | intraperitoneal |
| i.v, | intravenously |
| LDH | lactic dehydrogenase |
| KM | Kunming |
| KMnO4 | potassium permanganate |
| LD50 | median lethal dose |
| MW | molecular weight |
| MWCO | molecular weight cut-off |
| •O2− | superoxide anion radical |
| •OH− | hydroxyl radical |
| ORAC | oxygen radical absorbance capacity |
| RH | relative humidity |
| ROS | radical oxygen species |
| SA | salicylic acid |
| Se | selenium |
| SeNPs | selenium nanoparticles |
| SOD | superoxide dismutase |
| SPF | specifc-pathogen-free |
| TBARS | thiobarbituric acid-reactive substances |
| TEM | transmission electron microscopy |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| UF | ultra-filtration |
| Vc | ascorbic acid |
References
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Zhang, J.; Spallholz, J.E. Toxicity of selenium compounds and nano-selenium particles. In General, Applied and Systems Toxicology, 2nd ed.; Casciano, D.A., Sahu, S.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 787–802. [Google Scholar]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; Surai, P.F. Selenium in human and animal nutrition: Resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914–1978) on the occasion of the thirtieth anniversary of his death. Crit. Rev. Biotechnol. 2009, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Wang, J.H.; Kim, H.G.; Park, H.J.; Bok, H.S.; Son, C.G. CGX, a traditional Korean medicine ameliorates concanavalin A-induced acute liver injury. Food Chem. Toxicol. 2010, 48, 3308–3315. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, J.; Zhu, P.; Fujino, M.; Takahara, T.; Toyama, S.; Tomita, A.; Zhao, L.; Yang, Z.; Hei, M.; et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int. Immunopharmacol. 2015, 28, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Li, Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur. J. Pharmacol. 2008, 586, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; Feki, A.E. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef]
- Taskin, E.; Dursun, N. Recovery of adriamycin induced mitochondrial dysfunction in liver by selenium. Cytotechnology 2015, 67, 977–986. [Google Scholar] [CrossRef]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.; Lin, H.; Chang, Y.; Xing, J. Role of Hydrogen Sulfide on Autophagy in Liver Injuries Induced by Selenium Deficiency in Chickens. Biol. Trace Elem. Res. 2017, 175, 194–203. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Z.; Hu, F.; Zhang, Y.; Li, C.; Yan, P.; Feng, L.; Shen, J.; Huang, B. The protective effects of selenium-enriched Spirulina platensis on chronic alcohol-induced liver injury in mice. Food Funct. 2018, 9, 3155–3165. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Bishayee, A. Selenium in the prevention and treatment of hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 2010, 10, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Chen, Y.; Zhang, Y.Q.; Zhou, M.Z.; Song, X.M.; Zhang, B.Z.; Luo, L.; Xu, P.M.; Zhao, Y.N.; Zhao, Y.B.; et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol. Trace Elem. Res. 1997, 56, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; Hong, Z.; Sun, J.; Wang, C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in D-galactose-induced aging mice. J. Nanobiotechnol. 2017, 15, 92–105. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Jing, W.; Fan, J.; Dahms, H.U.; Lee, S.C.; Wang, L. Biogenic selenium and its hepatoprotective activity. Sci. Rep. 2017, 7, 15627. [Google Scholar] [CrossRef]
- Amin, K.A.; Hashem, K.S.; Alshehri, F.S.; Awad, S.T.; Hassan, M.S. Antioxidant and Hepatoprotective Efficiency of Selenium Nanoparticles Against Acetaminophen-Induced Hepatic Damage. Biol. Trace Elem. Res. 2017, 175, 136–145. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Motaan, S.E.; Malik, N. Protective and Antioxidant Role of Selenium Nanoparticles and Vitamin C Against Acrylamide Induced Hepatotoxicity in Male Mice. Int. J. Pharmacol. 2019, 15, 664–674. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Bauomy, A.A.; Diab, M.S.M.; Al-Quraishy, S. Protective role of selenium nanoparticles against Schistosoma mansoni-induced hepatic injury in mice. Biomed. Res. 2016, 27, 214–219. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dufailly, V.; Noël, L.; Guérin, T. Determination of chromium, iron and selenium in foodstuffs of animal origin by collision cell technology, inductively coupled plasma mass spectrometry (ICP-MS), after closed vessel microwave digestion. Anal. Chim. Acta 2006, 565, 214–221. [Google Scholar] [CrossRef]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Atala, E.; Vásquez, L.; Speisky, H.; Lissi, E.; López-Alarcón, C. Ascorbic acid contribution to ORAC values in berry extracts: An evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2009, 113, 331–335. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics 2020, 12, 43. [Google Scholar] [CrossRef]
- Peralta, E.; Roab, G.; Hernandez-Servin, J.A.; Romero, R.; Balderas, P.; Natividad, R. Hydroxyl Radicals quantification by UV spectrophotometry. Electrochim. Acta 2014, 129, 137–141. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.; Wang, H.; Li, Q.; Cui, Z.; Zhang, Y. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int. Immunopharmacol. 2016, 31, 140–148. [Google Scholar] [CrossRef]
- Anraku, M.; Hiraga, A.; Iohara, E.; Uekama, K.; Tomida, H.; Otagiri, M.; Hirayama, F. Preparation and antioxidant activity of PEGylated chitosans with different particle sizes. Int. J. Biol. Macromol. 2014, 70, 64–69. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Yang, J.; Lin, F.; Sun, Y.; Li, T.; Wu, C. Hepatoprotective effect of apple polyphenols against concanavalin A-induced immunological liver injury in mice. Chem. Biol. Interact 2016, 258, 159–165. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Chen, H.; Shin, D.W.; Nam, J.G.; Kwon, K.W.; Yoo, J.B. Selenium nanowires and nanotubes synthesized via a facile template-free solution method. Mater. Res. Bull. 2010, 45, 699–704. [Google Scholar] [CrossRef]
- Zheng, J.S.; Zheng, S.Y.; Zhang, Y.B.; Zheng, W.; Yang, F.; Chen, T. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloid Surf. B 2011, 83, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, O.; Mace, C.R.; Martinez, R.V.; Kumar, A.A.; Nie, Z.; Patton, M.R.; Whitesides, G.M. Separation of nanoparticles in aqueous multiphase systems through centrifugation. Nano Lett. 2012, 12, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.H.; Sampath, K.S.; Arunkumar, P.; Suresh, K.M.; Sujatha, V.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4–15. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Hu, C.H.; Li, Y.L.; Xiong, L.; Zhang, H.M.; Song, J.; Xia, M.S. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Dai, H.C.; Long, L.Q.; Zhang, X.W.; Zhang, W.M.; Wu, X.X. Cloning and expression of the duck leptin gene and the effect of leptin on food intake and fatty deposition in mice. J. Anim. Sci. 2007, 20, 850–855. [Google Scholar] [CrossRef]
- Barnes, K.M.; Evenson, J.K.; Raines, A.M.; Sunde, R.A. Transcript Analysis of the Selenoproteome Indicates That Dietary Selenium Requirements of Rats Based on Selenium-Regulated Selenoprotein mRNA Levels Are Uniformly Less Than Those Based on Glutathione Peroxidase Activity. J. Nutr. 2009, 139, 199–206. [Google Scholar] [CrossRef]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or supernutritional selenium status vastly alters the transcriptome in rodents. BMC Genom. 2011, 12, 26–40. [Google Scholar] [CrossRef]
- El-Demerdash, F.; Yousef, M.; El-Naga, N. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem. Toxicol. 2005, 43, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kodai, S.; Takemura, S.; Minamiyama, Y.; Hai, S.; Yamamoto, S.; Kubo, S.; Yoshida, Y.; Niki, E.; Okada, S.; Hirohashi, K.; et al. S-allyl cysteine prevents CCl4-induced acute liver injury in rats. Free Radic. Res. 2007, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, M.J.; Wang, C.; Nie, H.; Huang, W.J.; Yuan, T.D.; Sun, T.; Shu, K.G.; Wang, C.F.; Gong, Q.; et al. Protective effects of hesperidin on concanavalin A-induced hepatic injury in mice. Int. Immunopharmacol. 2014, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kinoshita, M.; Nakashima, M.; Habu, Y.; Shono, S.; Uchida, T.; Shinomiya, N.; Seki, S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology 2008, 48, 1979–1988. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.D.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef]

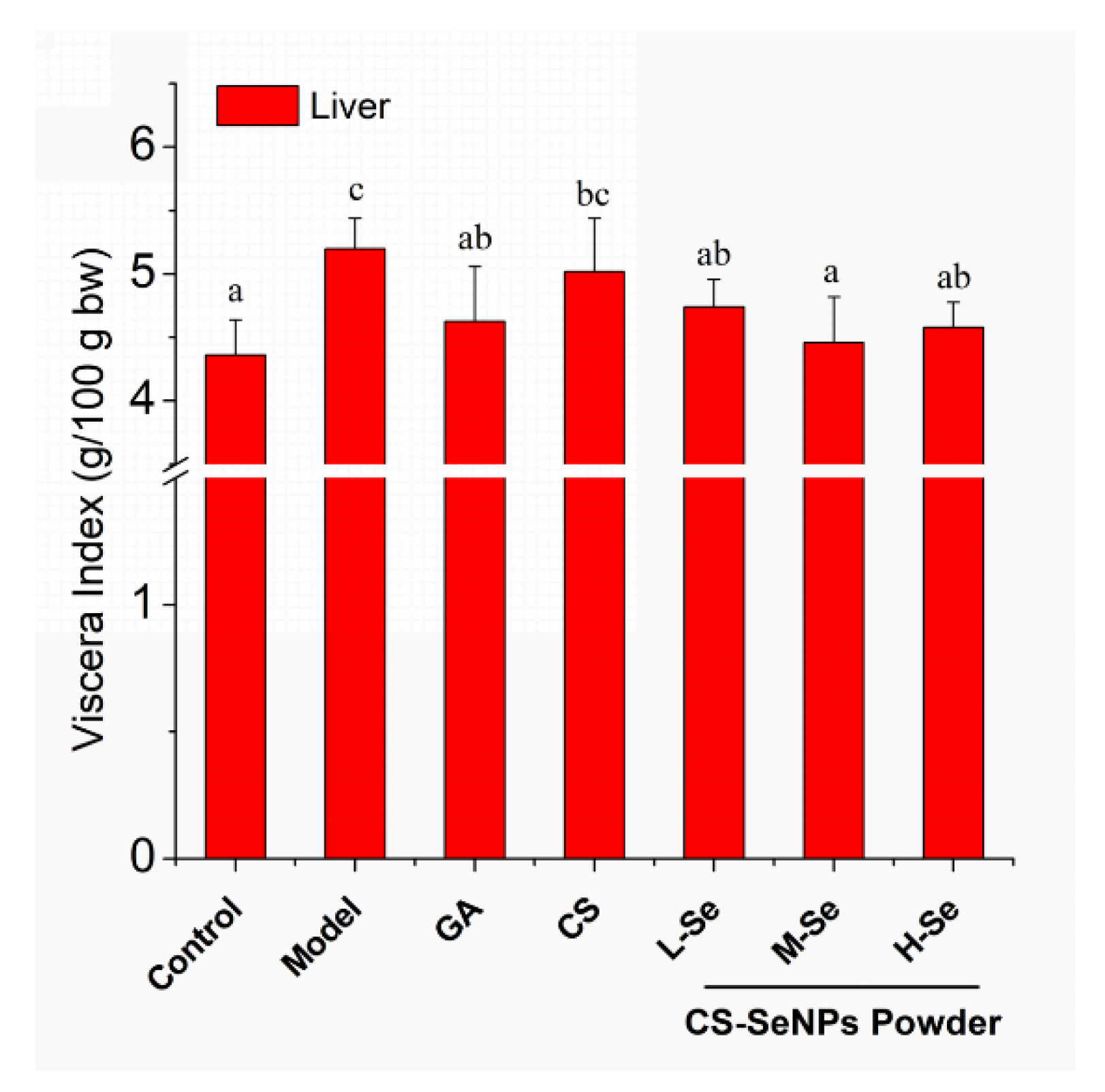
| Group | Pre-treatment (once daily, ig, Day 1–35) | Treatment (once, iv, Day 35) |
|---|---|---|
| Control | Normal saline | Normal saline |
| Model | Normal saline | Con A (10 mg/kg bw) |
| GA | Glycyrrhizic acid (10 mg/kg bw) | Con A (10 mg/kg bw) |
| CS | Chitosan (1.2 mg kg−1 bw) | Con A (10 mg/kg bw) |
| L-Se | Dried CS-SeNPs power (0.6 mg kg−1 bw) | Con A (10 mg/kg bw) |
| M-Se | Dried CS-SeNPs power (1.2 mg kg−1 bw) | Con A (10 mg/kg bw) |
| H-Se | Dried CS-SeNPs power (2.4 mg kg−1 bw) | Con A (10 mg/kg bw) |
| Sample | ORAC 1 (mg Trolox/100 mg) | Relative ORAC 2 (%) |
|---|---|---|
| Trolox | 100 | 100 |
| VC | 35.16 ± 3.54 | 35.16 |
| Selenite | <0.001 | <0.001 |
| CS-SeNPs | 5.10 ± 0.22 | 5.10 |
| CS | 0.75 ± 0.11 | 0.75 |
| Free Radical | VC | Sodium selenite | CS | CS-SeNPs |
|---|---|---|---|---|
| •DPPH | 2.90 ± 0.04 | >>150 | >>150 | 20.2 ± 0.6 |
| •O2- | 5.79 ± 1.0 | >>150 | >150 | 17.5 ± 0.6 |
| •OH- | 15.0 ± 1.2 | 34.8 ± 1.3 | >>150 | 75.1 ± 6.1 |
| Group | ALT(U/L) | AST(U/L) | LDH (U/L) | GSH (μmol/L) |
|---|---|---|---|---|
| Control | 10.3 ± 2.1 ab | 13.2 ± 6.0 a | 2929 ± 396 a | 17.4 ± 7.8 a |
| Model | 32.1 ± 9.2 c | 62.3 ± 22.5 b | 3655 ± 331 c | 5.9 ± 4.9 b |
| GA | 15.7 ± 5.3 ab | 10.7 ± 6.0 a | 3151 ± 233 ab | 7.2 ± 5.4 b |
| CS | 20.3 ± 8.0 b | 54.2 ± 24.6 b | 3411 ± 185 bc | 7.1 ± 2.9 b |
| L-Se | 16.4 ± 6.1 ab | 26.1 ± 5.2 a | 2920 ± 349 a | 8.2 ± 3.3 b |
| M-Se | 7.1 ± 4.8 a | 13.9 ± 6.5 a | 2983 ± 277 a | 17.4 ± 8.4 a |
| H-Se | 20.2 ± 13.1 b | 60.6 ± 30.2 b | 3342 ± 517 abc | 21.7 ± 5.4 a |
| Group | GSH (μmol/g prot) | TBARS (μmol/g prot) | SOD (U/g prot) | CAT (U/g prot) | GSH-Px (U/g prot) |
|---|---|---|---|---|---|
| Control | 7.99 ± 2.81 ab | 1.13 ± 0.35 a | 243 ± 39 a | 41.4 ± 7.5 ab | 473 ± 146 ab |
| Model | 2.08 ± 1,75 c | 4.68 ± 1.79 c | 154 ± 22 c | 28.9 ± 6.2 a | 183 ± 103 c |
| GA | 3.26 ± 2.01 bc | 1.28 ± 0.43 a | 151 ± 21 c | 56.6 ± 26.1 b | 399 ± 58 abc |
| CS | 4.69 ± 3.03 abc | 3.22 ± 1.06 bc | 184 ± 20 bc | 30.3 ± 10.9 a | 278 ± 133 bc |
| L-SeM | 2.37 ± 1.82 bc | 1.44 ± 0.50 ab | nt | 34.6 ± 13.2 ab | 450 ± 162 ab |
| M-SeM | 7.91 ± 3.81 ab | 0.96 ± 0.50 a | 240 ± 34 a | 50.4 ± 13.9 ab | 519 ± 97 a |
| H-SeM | 9.10 ± 3.56 a | 1.44 ± 1.06 ab | 204 ± 27 ab | 34.9 ± 7.7 ab | 461 ± 114 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, K.; Hong, B.; He, J.; Huang, W. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients 2020, 12, 857. https://doi.org/10.3390/nu12030857
Bai K, Hong B, He J, Huang W. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients. 2020; 12(3):857. https://doi.org/10.3390/nu12030857
Chicago/Turabian StyleBai, Kaikai, Bihong Hong, Jianlin He, and Wenwen Huang. 2020. "Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice" Nutrients 12, no. 3: 857. https://doi.org/10.3390/nu12030857
APA StyleBai, K., Hong, B., He, J., & Huang, W. (2020). Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients, 12(3), 857. https://doi.org/10.3390/nu12030857






