Modelling the Impact of Chronic Cigarette Smoke Exposure in Obese Mice: Metabolic, Pulmonary, Intestinal, and Cardiac Issues
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective and Design of the Study
2.2. Experimental Animal Model
2.3. In Vivo Magnetic Resonance Imaging (MRI)
2.4. Intra-Peritoneal Glucose Tolerance Test (IP-GTT)
2.5. Evaluation of Lung Function
2.6. Assessment of Airway Inflammation
2.7. Cytokines and Insulin Quantification
2.8. Histological Analyses
2.9. RNA Extraction and Quantitative RT-PCR
2.10. Microbiota Analysis
2.11. Statistical Analysis
3. Results
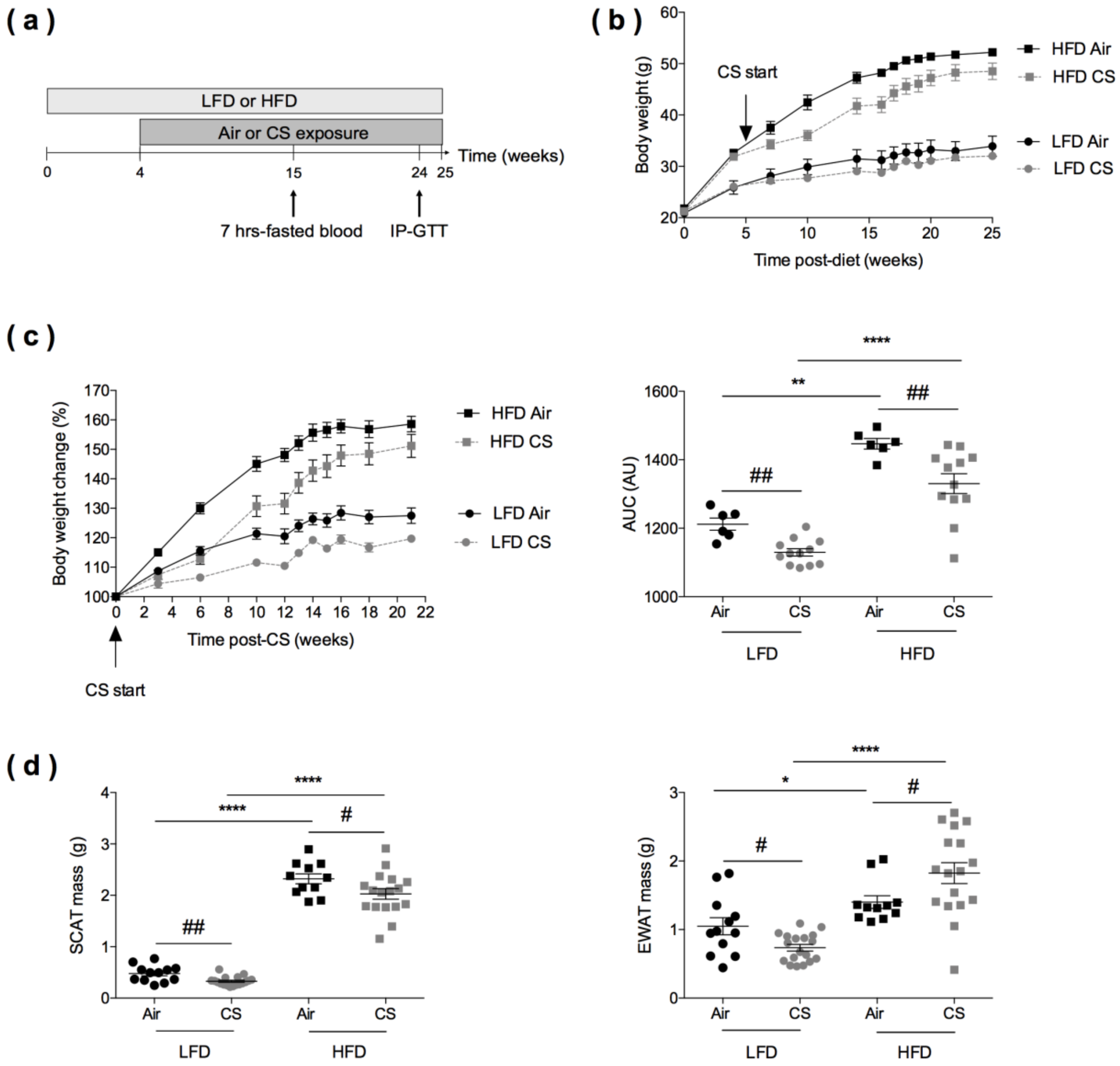
3.1. Experimental Design for Evaluating the Consequences of Chronic Cigarette Smoke Exposure in Lean and Obese Mice
3.2. Chronic Cigarette Smoke Exposure Induces White Adipose Tissue Redistribution in Obese Mice
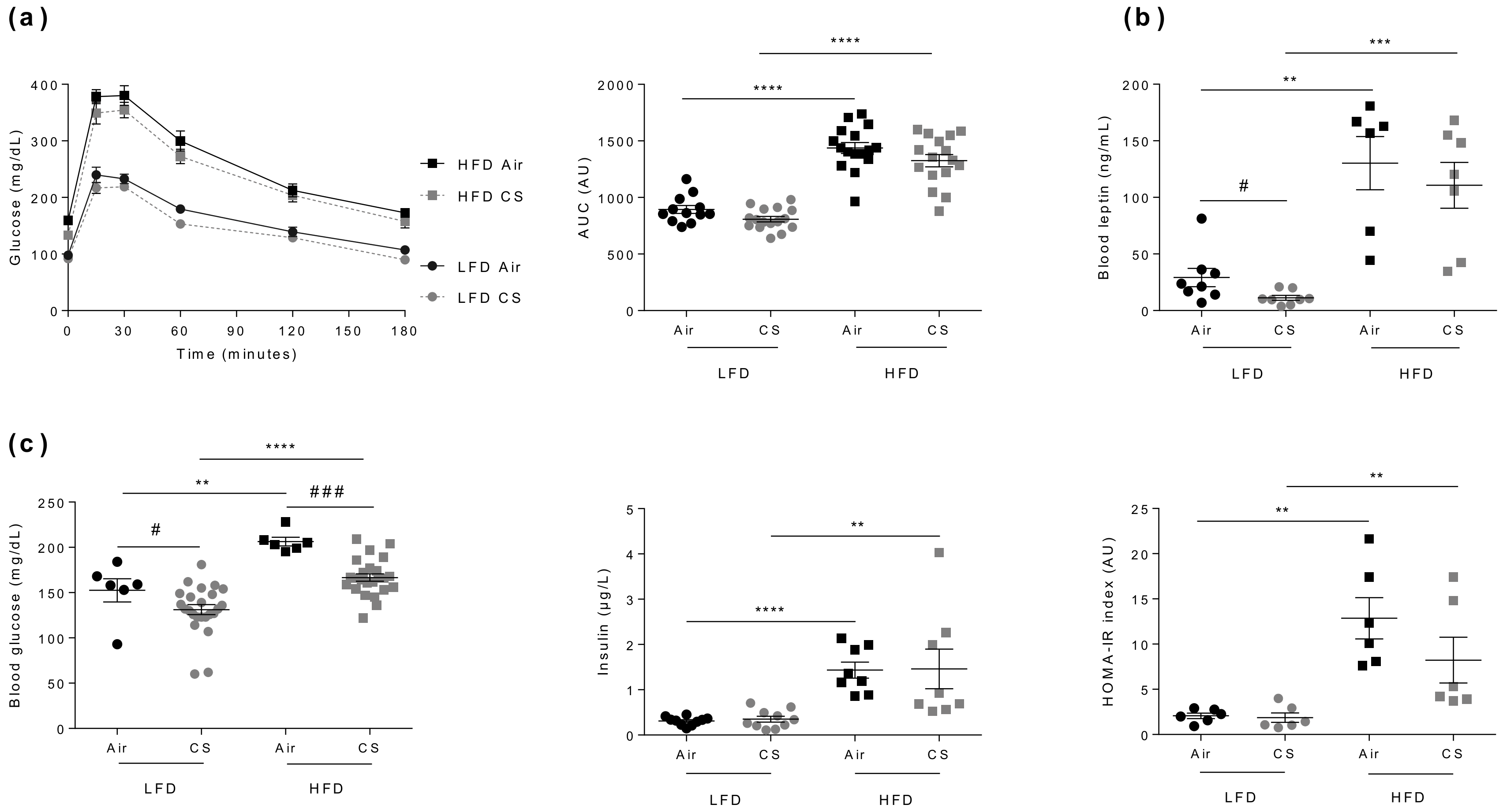
3.3. Chronic Cigarette Smoke Exposure Has No Impact on Glucose Homeostasis in Lean and Obese Mice
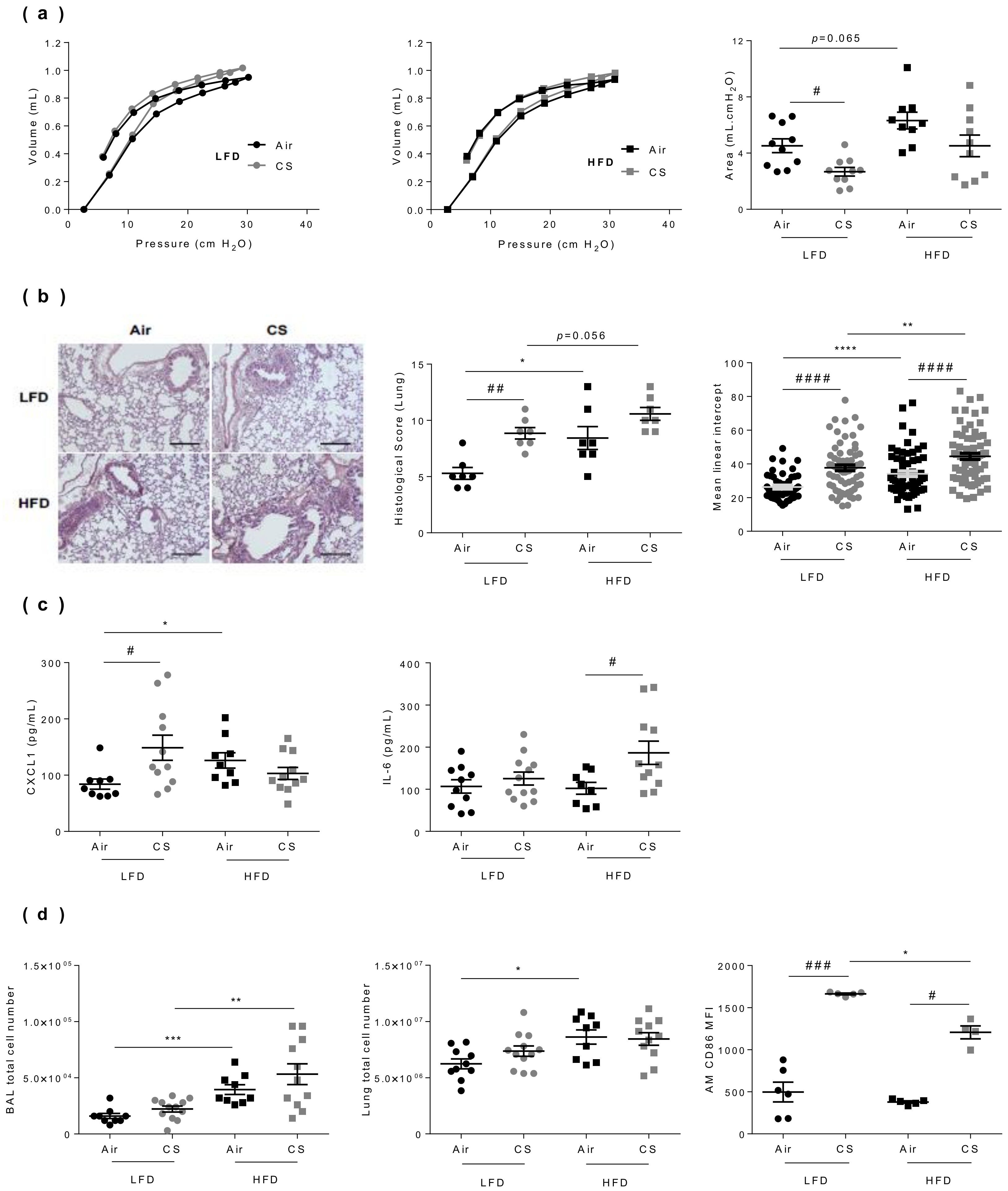
3.4. Lung Dysfunction and Inflammation Induced by Chronic Cigarette Smoke Exposure are Worsened in Obese Mice
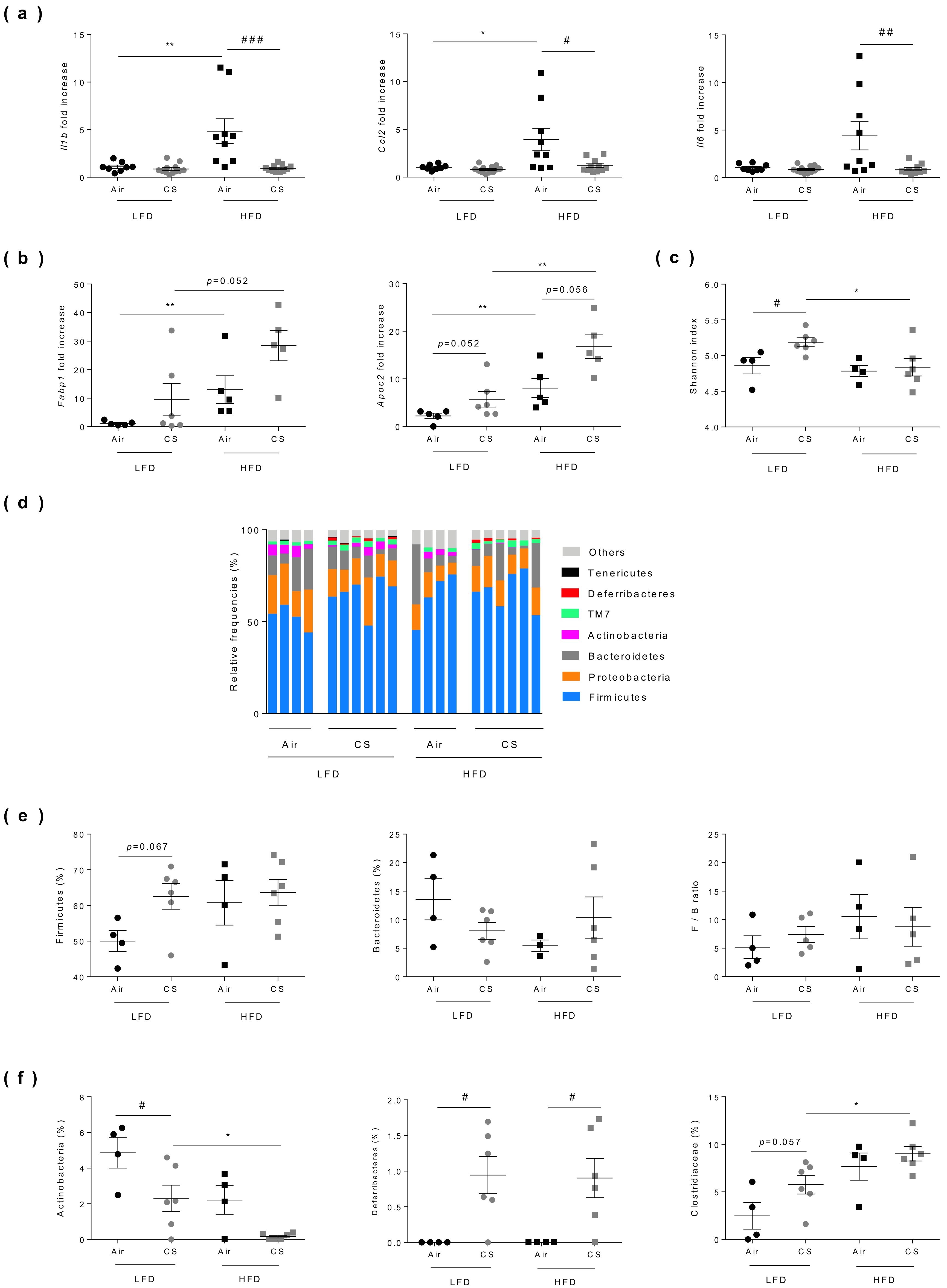
3.5. Chronic Cigarette Smoke Exposure Limits Obesity-Associated Gut Inflammation, Exacerbates Fatty Acid Metabolism and Modulates Caecal Microbiota
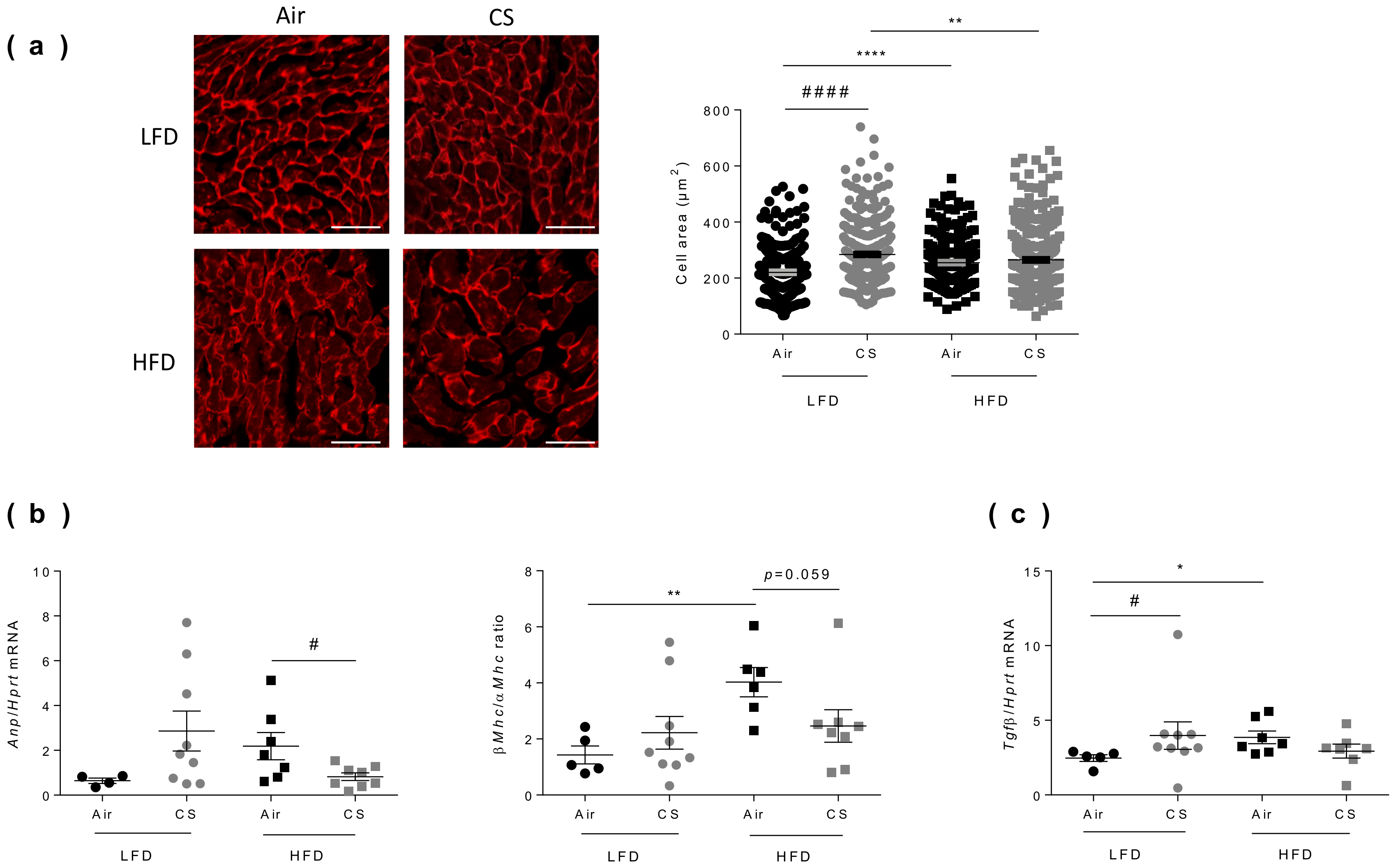
3.6. Chronic Cigarette Smoke Exposure Restricts Obesity-Induced Cardiac Hypertrophy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stenholm, S.; Head, J.; Kivimäki, M.; Kawachi, I.; Aalto, V.; Zins, M.; Goldberg, M.; Zaninotto, P.; Magnuson Hanson, L.; Westerlund, H.; et al. Smoking, physical inactivity and obesity as predictors of healthy and disease-free life expectancy between ages 50 and 75: A multicohort study. Int. J. Epidemiol. 2016, 45, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Methven, E.; McDowell, Z.C.; Hacking, B.; Alexander, D.; Stewart, L.; Kelnar, C.J.H. Health consequences of obesity. Arch. Dis. Child. 2003, 88, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Everaere, L.; Ait-Yahia, S.; Molendi-Coste, O.; Vorng, H.; Quemener, S.; LeVu, P.; Fleury, S.; Bouchaert, E.; Fan, Y.; Duez, C.; et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J. Allergy Clin. Immunol. 2016, 138, 1309–1318.e11. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.-J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef]
- Vineis, P. Smoking and impact on health. Eur. Respir. Rev. 2008, 17, 182–186. [Google Scholar] [CrossRef]
- Montbarbon, M.; Pichavant, M.; Langlois, A.; Erdual, E.; Maggiotto, F.; Neut, C.; Mallevaey, T.; Dharancy, S.; Dubuquoy, L.; Trottein, F.; et al. Colonic inflammation in mice is improved by cigarette smoke through iNKT cells recruitment. PLoS ONE 2013, 8, e62208. [Google Scholar] [CrossRef]
- Poulain, M.; Doucet, M.; Major, G.C.; Drapeau, V.; Sériès, F.; Boulet, L.-P.; Tremblay, A.; Maltais, F. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. Can. Med. Assoc. J. 2006, 174, 1293–1299. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Yadav, P.; Ellinghaus, D.; Rémy, G.; Freitag-Wolf, S.; Cesaro, A.; Degenhardt, F.; Boucher, G.; Delacre, M. International IBD Genetics Consortium, L.; Peyrin-Biroulet, L.; et al. Genetic Factors Interact With Tobacco Smoke to Modify Risk for Inflammatory Bowel Disease in Humans and Mice. Gastroenterology 2017, 153, 550–565. [Google Scholar] [CrossRef]
- Savin, Z.; Kivity, S.; Yonath, H.; Yehuda, S. Smoking and the intestinal microbiome. Arch. Microbiol. 2018, 200, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J. Cardiol. 2017, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette Smoking and Inflammation. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef]
- Wang, Z.; Nakayama, T. Inflammation, a Link between Obesity and Cardiovascular Disease. Mediators Inflamm. 2010, 2010, 1–17. [Google Scholar] [CrossRef]
- Blauw, L.L.; Boon, M.R.; Rosendaal, F.R.; de Mutsert, R.; Gast, K.B.; van Dijk, K.W.; Rensen, P.C.N.; Dekkers, O.M. NEO study group Smoking is associated with increased resting energy expenditure in the general population: The NEO study. Metabolism 2015, 64, 1548–1555. [Google Scholar] [CrossRef]
- Carreras-Torres, R.; Johansson, M.; Haycock, P.C.; Relton, C.L.; Davey Smith, G.; Brennan, P.; Martin, R.M. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ 2018, 361, k1767. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, M.; Rémy, G.; Bekaert, S.; Le Rouzic, O.; Kervoaze, G.; Vilain, E.; Just, N.; Tillie-Leblond, I.; Trottein, F.; Cataldo, D.; et al. Oxidative stress-mediated iNKT-cell activation is involved in COPD pathogenesis. Mucosal Immunol. 2014, 7, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.; Duriez, P.; Martin-Nizard, F.; Durieux, N.; Bordet, R.; Pétrault, O. Long-term risperidone treatment induces visceral adiposity associated with hepatic steatosis in mice: A magnetic resonance approach. Schizophr. Res. Treat. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Taront, S.; Molendi-Coste, O.; Bouchaert, E.; Neve, B.; Eury, E.; Lobbens, S.; Labalette, M.; Duez, H.; Staels, B.; et al. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS ONE 2014, 9, e92684. [Google Scholar] [CrossRef]
- Robichaud, A.; Fereydoonzad, L.; Limjunyawong, N.; Rabold, R.; Allard, B.; Benedetti, A.; Martin, J.G.; Mitzner, W. Automated full-range pressure-volume curves in mice and rats. J. Appl. Physiol. 2017, 123, 746–756. [Google Scholar] [CrossRef]
- Van Dijk, T.H.; Laskewitz, A.J.; Grefhorst, A.; Boer, T.S.; Bloks, V.W.; Kuipers, F.; Groen, A.K.; Reijngoud, D.J. A novel approach to monitor glucose metabolism using stable isotopically labelled glucose in longitudinal studies in mice. Lab. Anim. 2013, 47, 79–88. [Google Scholar] [CrossRef]
- Madouri, F.; Barada, O.; Kervoaze, G.; Trottein, F.; Pichavant, M.; Gosset, P. Production of Interleukin-20 cytokines limits bacterial clearance and lung inflammation during infection by Streptococcus pneumoniae. EBioMedicine 2018, 37, 417–427. [Google Scholar] [CrossRef]
- Belge, C.; Hammond, J.; Dubois-Deruy, E.; Manoury, B.; Hamelet, J.; Beauloye, C.; Markl, A.; Pouleur, A.-C.; Bertrand, L.; Esfahani, H.; et al. Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 2014, 129, 451–462. [Google Scholar] [CrossRef]
- Gallou-Kabani, C.; Vigé, A.; Gross, M.-S.; Rabès, J.-P.; Boileau, C.; Larue-Achagiotis, C.; Tomé, D.; Jais, J.-P.; Junien, C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity 2007, 15, 1996–2005. [Google Scholar] [CrossRef]
- Facchini, F.S.; Hollenbeck, C.B.; Jeppesen, J.; Ida Chen, Y.D.; Reaven, G.M. Insulin resistance and cigarette smoking. Lancet 1992, 339, 1128–1130. [Google Scholar] [CrossRef]
- Wareham, N.J.; Ness, E.M.; Byrne, C.D.; Cox, B.D.; Day, N.E.; Hales, C.N. Cigarette smoking is not associated with hyperinsulinemia: Evidence against a causal relationship between smoking and insulin resistance. Metabolism 1996, 45, 1551–1556. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased e coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Massot, N.; Carbonnel, F.; Cattan, S.; Gendre, J.-P.; Cosnes, J. Impact of cessation of smoking on the course of ulcerative colitis. Am. J. Gastroenterol. 2001, 96, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Capasso, J.M. Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. J. Mol. Cell. Cardiol. 1995, 27, 849–856. [Google Scholar] [CrossRef]
- Swynghedauw, B. Molecular Mechanisms of Myocardial Remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef]
- Lompre, A.-M.; Schwartz, K.; d’Albis, A.; Lacombe, G.; Van Thiem, N.; Swynghedauw, B. Myosin isoenzyme redistribution in chronic heart overload. Nature 1979, 282, 105–107. [Google Scholar] [CrossRef]
- Gardner, D.G.; Chen, S.; Glenn, D.J.; Grigsby, C.L. Molecular biology of the natriuretic peptide system: Implications for physiology and hypertension. Hypertension 2007, 49, 419–426. [Google Scholar] [CrossRef]
- Richards, A.M. Natriuretic peptides: Update on Peptide release, bioactivity, and clinical use. Hypertension 2007, 50, 25–30. [Google Scholar] [CrossRef]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Williams, J.; Townsend, N.; Mikkelsen, B.; Roberts, N.; Foster, C.; Wickramasinghe, K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: A systematic review. Lancet Glob. Health 2017, 5, e277–e289. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Luppi, F.; Beghe, B.; Rabe, K.F. Complex chronic comorbidities of COPD. Eur. Respir. J. 2008, 31, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.; Lehrter, V.; Rhimi, M.; Mangin, I.; Peucelle, V.; Abraham, A.-L.; Mariadassou, M.; Maguin, E.; Waligora-Dupriet, A.-J.; Pot, B.; et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ. Microbiol. 2016, 18, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Audrain-McGovern, J.; Benowitz, N.L. Cigarette Smoking, Nicotine, and Body Weight. Clin. Pharmacol. Ther. 2011, 90, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Filozof, C.; Fernández Pinilla, M.C.; Fernández-Cruz, A. Smoking cessation and weight gain. Obes. Rev. 2004, 5, 95–103. [Google Scholar] [CrossRef]
- Bergman, R.N.; Kim, S.P.; Hsu, I.R.; Catalano, K.J.; Chiu, J.D.; Kabir, M.; Richey, J.M.; Ader, M. Abdominal Obesity: Role in the Pathophysiology of Metabolic Disease and Cardiovascular Risk. Am. J. Med. 2007, 120, S3–S8. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Sposato, B.; Scalese, M. Can overweight/obesity and smoking have combined effects on bronchial hyperresponsiveness? Eur. Respir. J. 2014, 43, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Obesity, Inflammation, and Lung Injury (OILI): The Good. Mediators Inflamm. 2014, 2014, 978463. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kawamura, Y.; Sakakibara, T.; Nogaki, H.; Takahashi, H.; Murohara, T. Long-term clinical outcome after surgical or percutaneous coronary revascularization in hemodialysis patients. Circ. J. 2014, 78, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- O’Carroll, C.; Fagan, A.; Shanahan, F.; Carmody, R.J. Identification of a Unique Hybrid Macrophage-Polarization State following Recovery from Lipopolysaccharide Tolerance. J. Immunol. 2014, 192, 427–436. [Google Scholar] [CrossRef]
- Nova-Lamperti, E.; Fanelli, G.; Becker, P.D.; Chana, P.; Elgueta, R.; Dodd, P.C.; Lord, G.M.; Lombardi, G. Hernandez-Fuentes, M.P. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+ T-cell responses. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- De Maia, L.A.; Cruz, F.F.; De Oliveira, M.V.; Samary, C.S.; De Fernandes, M.V.S.; De Trivelin, S.A.A.; De Rocha, N.N.; De Abreu, M.G.; Pelosi, P.; Silva, P.L.; et al. Effects of obesity on pulmonary inflammation and remodeling in experimental moderate acute lung injury. Front. Immunol. 2019, 10, 1215. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.X.; Hu, N.; Ren, J.; Du, M.; Zhu, M.J. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J. Gastroenterol. 2012, 18, 2180–2187. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Derikx, J.P.M.; Vreugdenhil, A.C.E.; Van den Neucker, A.M.; Grootjans, J.; van Bijnen, A.A.; Damoiseaux, J.G.M.C.; van Heurn, L.W.E.; Heineman, E.; Buurman, W.A. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J. Clin. Gastroenterol. 2009, 43, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef] [PubMed]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota. mSystems 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Al Hariri, M.; Zibara, K.; Farhat, W.; Hashem, Y.; Soudani, N.; Al Ibrahim, F.; Hamade, E.; Zeidan, A.; Husari, A.; Kobeissy, F. Cigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Front. Pharmacol. 2016, 7, e397. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhang, L.; Zhu, M.; Ga, L.; Gao, L. A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J. Cell. Biochem. 2018, 119, 8750–8762. [Google Scholar] [CrossRef]
- Tilton, S.C.; Karin, N.J.; Webb-Robertson, B.-J.M.; Waters, K.M.; Mikheev, V.; Lee, K.M.; Corley, R.A.; Pounds, J.G.; Bigelow, D.J. Impaired transcriptional response of the murine heart to cigarette smoke in the setting of high fat diet and obesity. Chem. Res. Toxicol. 2013, 26, 1034–1042. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Li, L.; Zhao, H.; Peng, S.; Zuo, Z.; Hospital, S.Y.M. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism 2015, 64, 917–925. [Google Scholar] [CrossRef]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rémy, G.; Dubois-Deruy, E.; Alard, J.; Kervoaze, G.; Chwastyniak, M.; Baron, M.; Beury, D.; Siegwald, L.; Caboche, S.; Hot, D.; et al. Modelling the Impact of Chronic Cigarette Smoke Exposure in Obese Mice: Metabolic, Pulmonary, Intestinal, and Cardiac Issues. Nutrients 2020, 12, 827. https://doi.org/10.3390/nu12030827
Rémy G, Dubois-Deruy E, Alard J, Kervoaze G, Chwastyniak M, Baron M, Beury D, Siegwald L, Caboche S, Hot D, et al. Modelling the Impact of Chronic Cigarette Smoke Exposure in Obese Mice: Metabolic, Pulmonary, Intestinal, and Cardiac Issues. Nutrients. 2020; 12(3):827. https://doi.org/10.3390/nu12030827
Chicago/Turabian StyleRémy, Gaëlle, Emilie Dubois-Deruy, Jeanne Alard, Gwenola Kervoaze, Maggy Chwastyniak, Morgane Baron, Delphine Beury, Léa Siegwald, Ségolène Caboche, David Hot, and et al. 2020. "Modelling the Impact of Chronic Cigarette Smoke Exposure in Obese Mice: Metabolic, Pulmonary, Intestinal, and Cardiac Issues" Nutrients 12, no. 3: 827. https://doi.org/10.3390/nu12030827
APA StyleRémy, G., Dubois-Deruy, E., Alard, J., Kervoaze, G., Chwastyniak, M., Baron, M., Beury, D., Siegwald, L., Caboche, S., Hot, D., Gosset, P., Grangette, C., Pinet, F., Wolowczuk, I., & Pichavant, M. (2020). Modelling the Impact of Chronic Cigarette Smoke Exposure in Obese Mice: Metabolic, Pulmonary, Intestinal, and Cardiac Issues. Nutrients, 12(3), 827. https://doi.org/10.3390/nu12030827




