Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Subgroup Analysis & Investigation of Heterogeneity
2.1.1. Neonatal Probiotic Supplementation
2.1.2. Neonatal Vitamin A Supplementation
2.1.3. Oral Dextrose Gel Supplementation
2.2. Sensitivity Analysis
3. Results
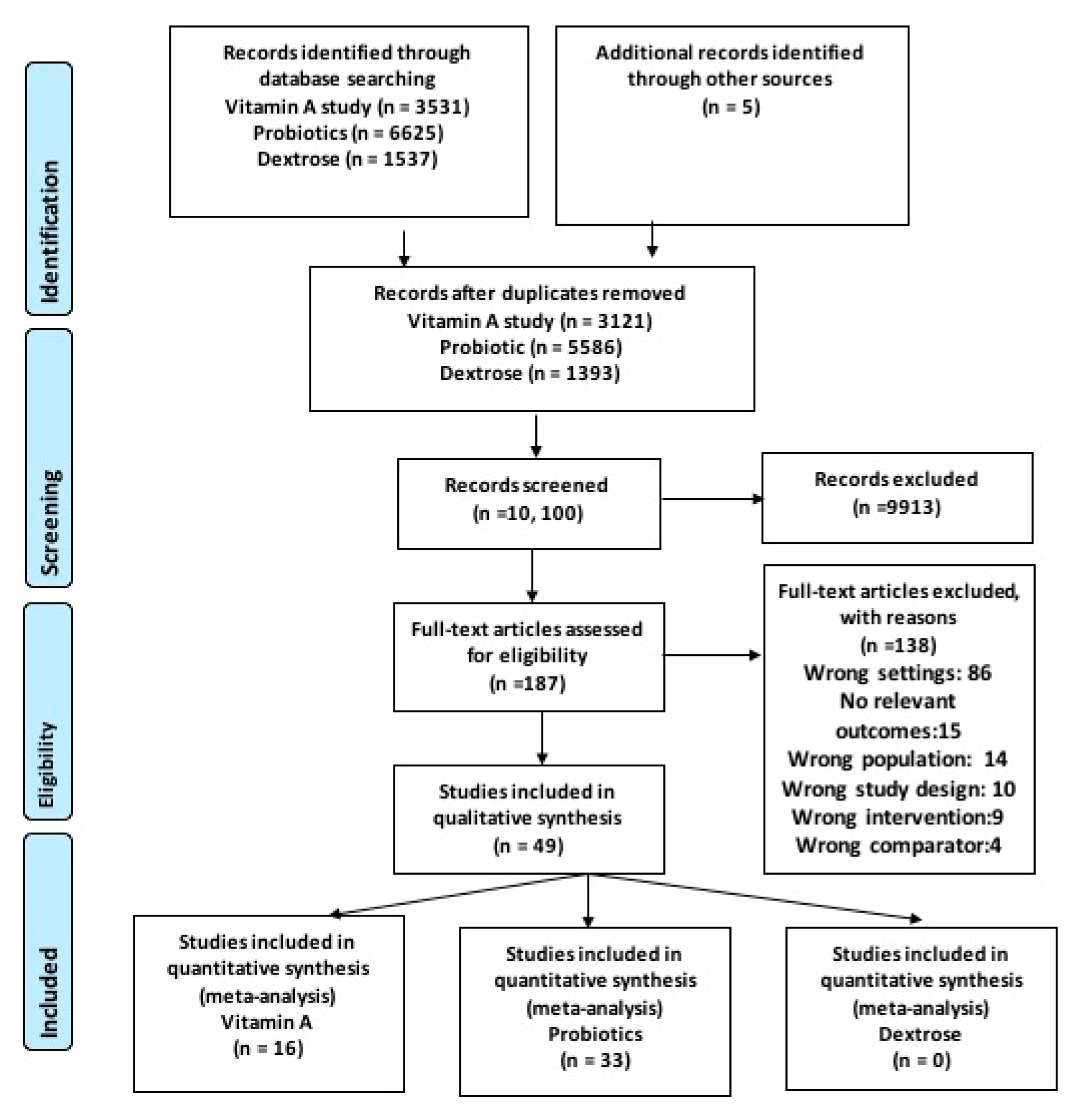
3.1. Literature Search
3.2. Characteristics of Included Studies
3.2.1. Vitamin A Supplementation during the Neonatal Period
3.2.2. Probiotic Supplementation during the Neonatal Period
3.2.3. Risk of Bias
3.2.4. Excluded Studies:
3.3. Effect of Interventions
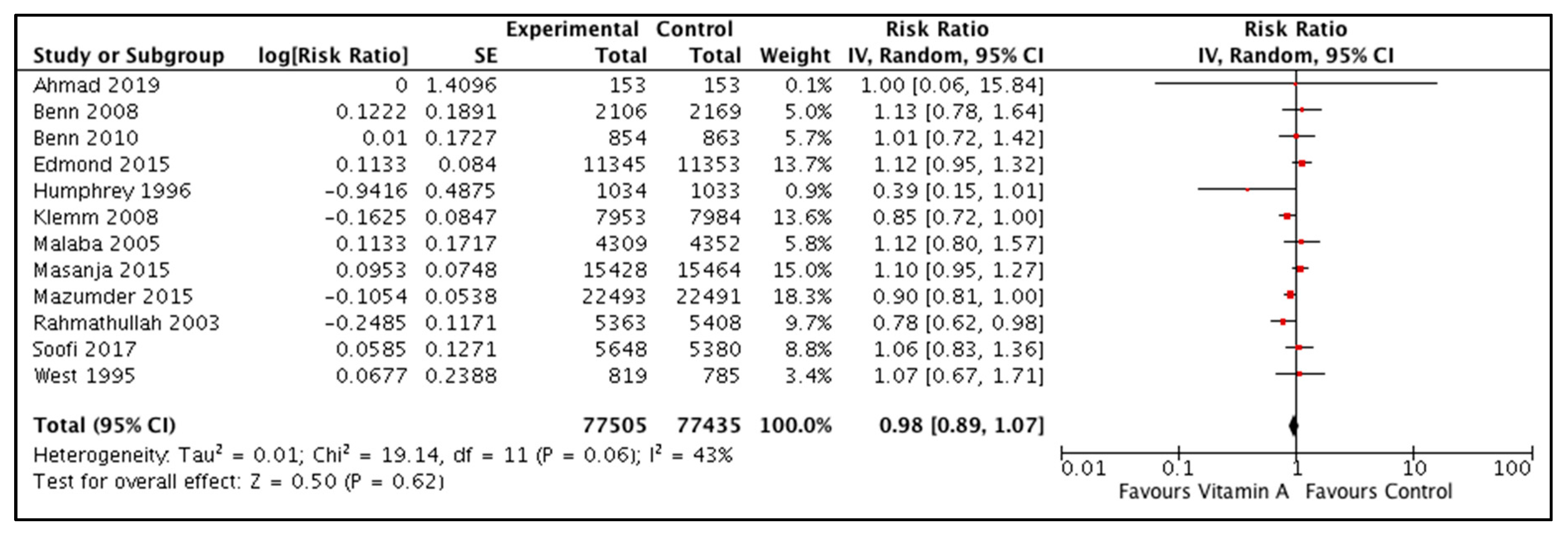
3.3.1. Vitamin A Supplementation during the Neonatal Period
All-cause Mortality
Neurodevelopment Outcomes
Retinopathy of Prematurity
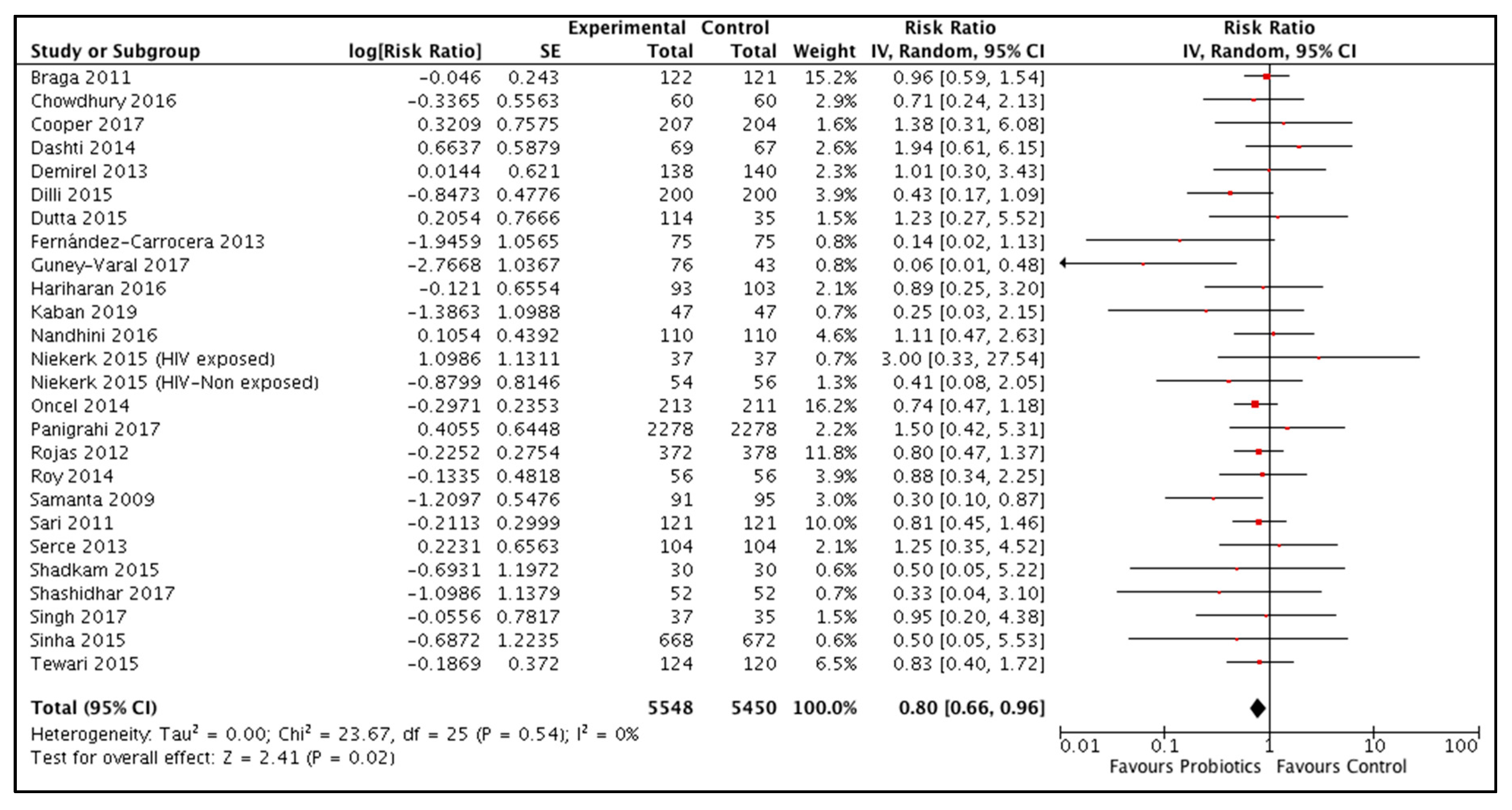
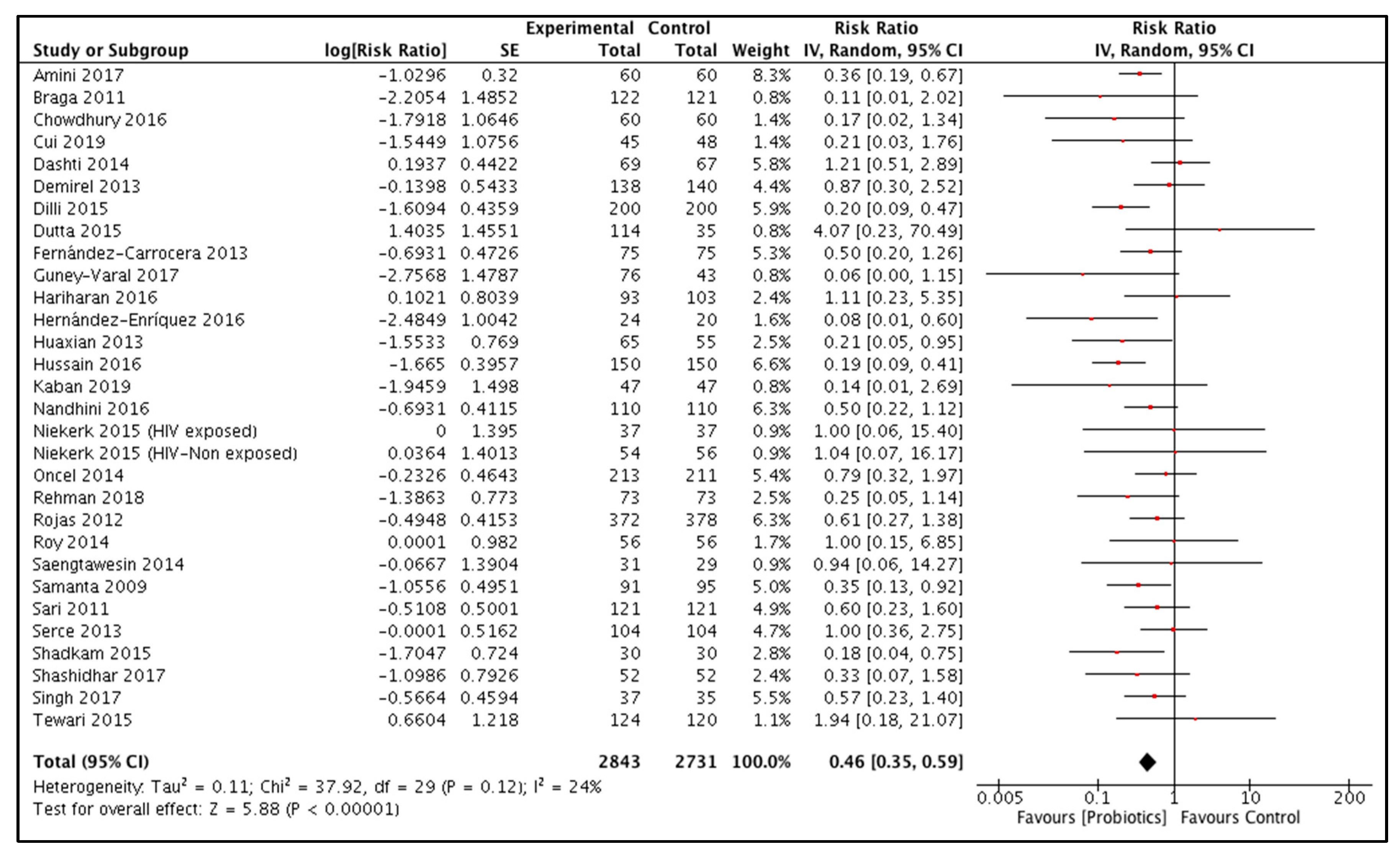
3.3.2. Probiotic Supplementation during the Neonatal Period
All-cause mortality
Necrotizing Enterocolitis
Neonatal Sepsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alkema, L.; Chao, F.; You, D.; Pedersen, J.; Sawyer, C.C. National, regional, and global sex ratios of infant, child, and under-5 mortality and identification of countries with outlying ratios: A systematic assessment. Lancet Glob. Health 2014, 2, e521–e530. [Google Scholar] [CrossRef]
- Wang, H.; A Bhutta, Z.; Coates, M.M.; Coggeshall, M.; Dandona, L.; Diallo, K.; Franca, E.B.; Fraser, M.; Fullman, N.; Gething, P.W.; et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1725–1774. [Google Scholar] [CrossRef]
- WHO. Newborn Care at Birth. WHO, 2017. Available online: http://www.who.int/maternal_child_adolescent/newborns/care_at_birth/en/ (accessed on 17 September 2018).
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Ahishakiye, A.; Abimana, M.C.; Beck, K.; Miller, A.C.; Betancourt, T.S.; Magge, H.; Mutaganzwa, C.; Kirk, C.M. Developmental outcomes of preterm and low birth weight toddlers and term peers in rwanda. Ann. Glob. Health 2019, 85, 147. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: Analysis of CHERG datasets. BMJ 2017, 358, j3677. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Rind, F.; Irfan, O.; Hadi, R.; Das, J.K.; Bhutta, Z.A. Impact of infant and young child feeding (iycf) nutrition interventions on breastfeeding practices, growth and mortality in low- and middle-income countries: systematic review. Nutrients 2020, 12, 722. [Google Scholar] [CrossRef]
- Imdad, A.; Mayo-Wilson, E.; Herzer, K.; Bhutta, Z.A. Vitamin a supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. 2017, 3, CD008524. [Google Scholar] [CrossRef]
- Haider, B.A.; Sharma, R.; Bhutta, Z.A. Neonatal vitamin a supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst. Rev. 2017, 2, CD006980. [Google Scholar] [CrossRef]
- Weston, P.J.; Harris, D.L.; Battin, M.; Brown, J.; Hegarty, J.E.; Harding, J.E. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database Syst. Rev. 2016, CD011027. [Google Scholar] [CrossRef]
- Hegarty, J.E.; Harding, J.E.; Gamble, G.D.; Crowther, C.A.; Edlin, R.; Alsweiler, J.M. Prophylactic oral dextrose gel for newborn babies at risk of neonatal hypoglycaemia: A randomised controlled dose-finding trial (the Pre-hPOD study). PLoS Med. 2016, 13, e1002155. [Google Scholar] [CrossRef]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, CD005496. [Google Scholar] [CrossRef]
- Deshpande, G.; Rao, S.; Athalye-Jape, G.; Conway, P.; Patole, S. Probiotics in very preterm infants: The PiPS trial. Lancet 2016, 388, 655. [Google Scholar] [CrossRef]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic supplementation and late-onset sepsis in preterm infants: A meta-analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Ranjit, D.; Surin, G.S.; Lawler, S.; Smith, A.A.; Bhutta, Z.A. Effects of neonatal nutrition interventions on neonatal mortality and child health and development outcomes: A systematic review. Campbell Syst. Rev. 2019, 15, e1021. [Google Scholar] [CrossRef]
- Bank, W. Low and Middle income Countries. World Bank, 2017. Available online: https://data.worldbank.org/income-level/low-and-middle-income (accessed on 29 May 2018).
- Samanta, M.; Sarkar, M.; Ghosh, P.; Ghosh, J.; Sinha, M.; Chatterjee, S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J. Trop. Pediatr. 2009, 55, 128–131. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Collaboration. The Nordic Cochrane Centre RevMan, Version 5.3; The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Neonatal Vitamin A Supplementation Evidence Group; West, K.P. Early neonatal vitamin A supplementation and infant mortality: An individual participant data meta-analysis of randomised controlled trials. Arch. Dis. Child. 2019, 104, 217–226. [Google Scholar] [CrossRef]
- Soofi, S.; Ariff, S.; Sadiq, K.; Habib, A.; Bhatti, Z.; Ahmad, I.; Hussain, M.; Ali, N.; Cousens, S.; Bhutta, Z.A. Evaluation of the uptake and impact of neonatal vitamin a supplementation delivered through the lady health worker programme on neonatal and infant morbidity and mortality in rural pakistan: An effectiveness trial. Arch. Dis. Child. 2017, 102, 216–223. [Google Scholar] [CrossRef]
- Benn, C.S.; Diness, B.R.; Roth, A.; Nante, E.; Fisker, A.B.; Lisse, I.M.; Yazdanbakhsh, M.; Whittle, H.; Rodrigues, A.; Aaby, P. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: Randomised placebo controlled trial. BMJ 2008, 336, 1416–1420. [Google Scholar] [CrossRef]
- Benn, C.S.; Fisker, A.B.; Napirna, B.M.; Roth, A.; Diness, B.R.; Lausch, K.R.; Ravn, H.; Yazdanbakhsh, M.; Rodrigues, A.; Whittle, H.; et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: Two by two factorial randomised controlled trial. BMJ 2010, 340, c1101. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Martins, C.L.; Fisker, A.B.; Diness, B.R.; Garly, M.L.; Balde, I.; Rodrigues, A.; Whittle, H.; Aaby, P. Interaction between neonatal vitamin A supplementation and timing of measles vaccination: A retrospective analysis of three randomized trials from Guinea-Bissau. Vaccine 2014, 32, 5468–5474. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.; Hurt, L.; Fenty, J.; Amenga-Etego, S.; Zandoh, C.; Hurt, C.; Danso, S.; Tawiah, C.; Hill, Z.; Ten Asbroek, A.H.; et al. Effect of vitamin A supplementation in women of reproductive age on cause-specific early and late infant mortality in rural Ghana: ObaapaVitA double-blind, cluster-randomised, placebo-controlled trial. BMJ Open 2012, 2, e000658. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.H.; Agoestina, T.; Wu, L.; Usman, A.; Nurachim, M.; Subardja, D.; Hidayat, S.; Tielsch, J.; West, K.P., Jr. Sommer, a impact of neonatal vitamin a supplementation on infant morbidity and mortality. J. Pediatr. 1996, 128, 489–496. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Raqib, R.; Qadri, F.; Stephensen, C.B. The effect of newborn vitamin A supplementation on infant immune functions: Trial design, interventions, and baseline data. Contemp. Clin. Trials 2014, 39, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Khanna, P.; Srivastava, R.; Kumar, A. Oral vitamin a supplementation in very low birth weight neonates: A randomized controlled trial. Eur. J. Pediatr. 2019, 178, 1255–1265. [Google Scholar] [CrossRef]
- Klemm, R.D.; Labrique, A.B.; Christian, P.; Rashid, M.; Shamim, A.A.; Katz, J.; Sommer, A.; West, K.P., Jr. Newborn vitamin a supplementation reduced infant mortality in rural Bangladesh. Pediatrics 2008, 122, e242–e250. [Google Scholar] [CrossRef]
- Malaba, L.C.; Iliff, P.J.; Nathoo, K.J.; Marinda, E.; Moulton, L.H.; Zijenah, L.S.; Zvandasara, P.; Ward, B.J.; Humphrey, J.H. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am. J. Clin. Nutr. 2005, 81, 454–460. [Google Scholar] [CrossRef]
- Masanja, H.; Smith, E.R.; Muhihi, A.; Briegleb, C.; Mshamu, S.; Ruben, J.; Noor, R.A.; Khudyakov, P.; Yoshida, S.; Martines, J.; et al. Effect of neonatal vitamin a supplementation on mortality in infants in Tanzania (Neovita): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 1324–1332. [Google Scholar] [CrossRef]
- Mazumder, S.; Taneja, S.; Bhatia, K. Efficacy of early neonatal supplementation with vitamin a to reduce mortality in infancy in Haryana, India: A randomised, double-blind, placebo- controlled trial. Lancet 2015, 385, 1333–1342. [Google Scholar] [CrossRef]
- Rahmathullah, L.; Tielsch, J.M.; Thulasiraj, R.D.; Katz, J.; Coles, C.; Devi, S.; John, R.; Prakash, K.; Sadanand, A.V.; Edwin, N.; et al. Impact of supplementing newborn infants with vitamin a on early infant mortality: Community based randomised trial in southern India. BMJ Br. Med. J. (Int. Ed.) 2003, 327, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cheng, R.; Wang, Z. Iearly vitamin a supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina 2019. [Google Scholar] [CrossRef] [PubMed]
- West, K.P., Jr.; Katz, J.; Shrestha, S.R.; LeClerq, S.C.; Khatry, S.K.; Pradhan, E.K.; Adhikari, R.; Wu, L.S.; Pokhrel, R.P.; Sommer, A. Mortality of infants <6 mo of age supplemented with vitamin a: A randomized, double-masked trial in Nepal. Am. J. Clin. Nutr. 1995, 62, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, S.; Kumar, J.; Attri, S.V.; Dutta, S.; Kumar, P. Intramuscular followed by oral vitamin a supplementation in neonates with birth weight from 750 to 1250 g: A randomized controlled trial. Indian J. Clin. Biochem. 2019, 1–6. [Google Scholar] [CrossRef]
- Amini, E.; Dalili, H.; Niknafs, N.; Shariat, M.; Nakhostin, M.; Jedari-Attari, S. The effect of probiotics in prevention of necrotising enterocolitis in preterm neonates in comparison with control group. Iranian J. Pediatr. 2017, 27, 1–4. [Google Scholar] [CrossRef]
- Braga, T.D.; da Silva, G.A.; de Lira, P.I.; de Carvalho Lima, M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2011, 93, 81–86. [Google Scholar] [CrossRef]
- Chowdhury, T.; Ali, M.M.; Hossain, M.M.; Singh, J.; Yousuf, A.N.; Yasmin, F.; Chowdhury, F.R. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: A double-blind randomized controlled trial. J. College Phys. Surg. Pak. JCPSP 2016, 26, 770–774. [Google Scholar]
- Cooper, P.; Bolton, K.D.; Velaphi, S.; de Groot, N.; Emady-Azar, S.; Pecquet, S.; Steenhout, P. Early benefits of a starter formula enriched in prebiotics and probiotics on the gut microbiota of healthy infants born to HIV+ mothers: A randomized double-blind controlled trial. Clin. Med. Insights Pediatrics 2016, 10, 119–130. [Google Scholar] [CrossRef]
- Cui, X.; Shi, Y.; Gao, S.; Xue, X.; Fu, J. Effects of Lactobacillus reuteri DSM 17938 in preterm infants: A double-blinded randomized controlled study. Ital. J. Pediatr. 2019, 45, 140. [Google Scholar] [CrossRef]
- Dashti, A.S.; Afjeh, S.A.; Basiry, A.; Shirvani, F.; Seifi, K.; Taheri, Z.M. Prophylactic probiotics for prevention of necrotizing enterocolitis (NEC) in low birth weight neonates. Arch. Pediatr. Infect. Dis. 2014, 2, 174–179. [Google Scholar] [CrossRef]
- Demirel, G.; Erdeve, O.; Celik, I.H.; Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr. (Oslo Nor.) 2013, 102, e560–e565. [Google Scholar] [CrossRef] [PubMed]
- Dilli, D.; Aydin, B.; Fettah, N.D.; Ozyazici, E.; Beken, S.; Zenciroglu, A.; Okumus, N.; Ozyurt, B.M.; Ipek, M.S.; Akdag, A.; et al. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 2015, 166, 545–551.e541. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ray, P.; Narang, A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: A randomized controlled trial. Am. J. Perinatol. 2015, 32, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Carrocera, L.A.; Solis-Herrera, A.; Cabanillas-Ayon, M.; Gallardo-Sarmiento, R.B.; Garcia-Perez, C.S.; Montano-Rodriguez, R.; Echaniz-Aviles, M.O. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F5–F9. [Google Scholar] [CrossRef]
- Guney-Varal, I.; Koksal, N.; Ozkan, H.; Bagci, O.; Dogan, P. The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: A randomized controlled trial in a tertiary care unit. Turk. J. Pediatr. 2017, 59, 13–19. [Google Scholar] [CrossRef]
- Hariharan, D.; Balasubramanian, L.; Kannappan, V.; Veluswami, G. Probiotic supplementation in VLBW preterm infants improves feeding tolerance and reduces risk of gram negative sepsis. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 655. [Google Scholar] [CrossRef]
- Hernández-Enríquez, N.P.; Rosas-Sumano, A.B.; Monzoy-Ventre, M.A.; Galicia-Flores, L. Lactobacillus reuteri DSM 17938 in preventing necrotizing enterocolitis in preterm newborns. Pilot study of efficacy and safety. Rev. Mex. de Pediatr. 2016, 83, 37–43. [Google Scholar]
- Huaxian, L. Analysis of the effect of probiotics combined with early minimal enteral feeding on prevention of neonatal necrotizing enterocolitis. J. Pediatr. Pharm. 2013, 6, 18–20. [Google Scholar]
- Hussain, M.; Jabeen, S.; Subhani, R.U.H. Role of probiotics in prevention of nectrotizing enterocolitis in preterm low birth weight neonates. Pak. J. Med. Health Sci. 2016, 10, 455–459. [Google Scholar]
- Kaban, R.K.; Wardhana, H.B.; Rohsiswatmo, R.; Handryastuti, S.; Amelia, N.; Muktiarti, D.; Indrio, F.; Vandenplas, Y. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 545–553. [Google Scholar] [CrossRef]
- Nandhini, L.P.; Biswal, N.; Adhisivam, B.; Mandal, J.; Bhat, B.V.; Mathai, B. Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates—A randomized controlled trial. J. Mater. Fetal Neonatal Med. 2016, 29, 821–825. [Google Scholar] [CrossRef]
- Niekerk, E.; Nel, D.G.; Blaauw, R.; Kirsten, G.F. Probiotics reduce necrotizing enterocolitis severity in hiv-exposed premature infants. J. Trop. Pediatr. 2015, 61, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Sari, F.N.; Arayici, S.; Guzoglu, N.; Erdeve, O.; Uras, N.; Oguz, S.S.; Dilmen, U. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F110–F115. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Rojas, M.A.; Lozano, J.M.; Rojas, M.X.; Rodriguez, V.A.; Rondon, M.A.; Bastidas, J.A.; Perez, L.A.; Rojas, C.; Ovalle, O.; Garcia-Harker, J.E.; et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012, 130, e1113–e1120. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chaudhuri, J.; Sarkar, D.; Ghosh, P.; Chakraborty, S. Role of enteric supplementation of probiotics on late-onset sepsis by candida species in preterm low birth weight neonates: A randomized, double blind, placebo-controlled trial. N. Am. J. Med. Sci. 2014, 6, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Saengtawesin, V.; Tangpolkaiwalsak, R.; Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2014, 97 (Suppl. 6), S20–S25. [Google Scholar]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 2011, 65, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Serce, O.; Benzer, D.; Gursoy, T.; Karatekin, G.; Ovali, F. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum. Dev. 2013, 89, 1033–1036. [Google Scholar] [CrossRef]
- Shadkam, M.N.; Jalalizadeh, F.; Nasiriani, K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iranian J. Neonatol. 2015, 6, 15–20. [Google Scholar]
- Shashidhar, A.; Suman Rao, P.N.; Nesargi, S.; Bhat, S.; Chandrakala, B.S. Probiotics for promoting feed tolerance in very low birth weight neonates—A randomized controlled trial. Indian Pediatr. 2017, 54, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Gupta, S.S.; Chellani, H.; Maliye, C.; Kumari, V.; Arya, S.; Garg, B.S.; Gaur, S.D.; Gaind, R.; Deotale, V.; et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: A randomised controlled trial. BMJ Open 2015, 5. [Google Scholar] [CrossRef]
- Tewari, V.V.; Dubey, S.K.; Gupta, G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J. Trop. Pediatr. 2015, 61, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Wang, Y.; Fu, J.; Sun, M.; Mao, Z.; Vandenplas, Y. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J. Pediatr. 2016, 92, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.S.S.; Klobassa, D.S.; Resch, B.; Urlesberger, B.; Shrestha, R.P. Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at dhulikhel hospital in nepal. Kathmandu Univ. Med. J. (KUMJ) 2017, 15, 319–323. [Google Scholar]
- Rehman, S.; Iqbal, A.; Ali, W. Role of probiotics in reducing frequency of necrotizing enterocolitis in preterm neonates. Pak. Paediatr. J. 2018, 42, 172–177. [Google Scholar]
- Alfaleh, K.; Anabrees, J.; Bassler, D.; Al-Kharfi, T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2011, CD005496. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Braegger, C.P.; Decsi, T.; Kolacek, S.; Lanzinger, H.; Mayer, B.; Moreno, L.A.; Pohlandt, F.; Puntis, J.; Shamir, R.; et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin. Nutr. 2012, 31, 6–15. [Google Scholar] [CrossRef]
- Das, R.R. Probiotics in necrotizing enterocolitis: More questions than answers? Clin. Nutr. 2012, 31, 431. [Google Scholar] [CrossRef]
- Mihatsch, W.A. What is the power of evidence recommending routine probiotics for necrotizing enterocolitis prevention in preterm infants? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 302–306. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M.; ProPrems Study, G. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Probiotics in preterm infants study collaborative, g. bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Rebello, C.; Tarnow-Mordi, W. Probiotics in very preterm infants: The PiPS trial. Lancet 2016, 388, 655. [Google Scholar] [CrossRef][Green Version]
- van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef]
- Ofek, S.N.; Deshpande, G.; Rao, S.; Patole, S. Probiotics for preterm neonates: What will it take to change clinical practice? Neonatology 2014, 105, 64–70. [Google Scholar] [CrossRef]
| Outcomes | Relative Effect (95% CI) | № of Participants (Studies) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| All-cause neonatal mortality | RR 0.99 (0.90 to 1.07) | 126242 (5 RCTs) | ⊕⊕⊕⊕ |
| HIGH | |||
| All-cause mortality at 6 months of age | RR 0.98 (0.89 to 1.08) | 154940 (12 RCTs) | ⊕⊕⊕⊕ |
| HIGH | |||
| All-cause mortality at 12 months of age | RR 1.04 (0.95 to 1.14) | 118376 (8 RCTs) | ⊕⊕⊕⊕ |
| HIGH | |||
| Adverse Events: Bulging Fontanelle 48–72 hours | RR 1.53 (1.12 to 2.09) | 100562 (6 RCTs) | ⊕⊕⊕⊕ |
| HIGH |
| No. of Participants (Studies) Follow up | Relative Effect (95% CI) | Certainty of the Evidence (GRADE) | |
|---|---|---|---|
| All-cause Mortality | 10904 (25 RCTs) | RR 0.80 (0.66 to 0.96) | ⊕⊕⊕⊕ HIGH 1,2,3,4 |
| Neonatal Sepsis | 8918 (21 RCTs) | RR 0.78 (0.70 to 0.86) | ⊕⊕⊕⊕ HIGH 1,4,5 |
| Necrotizing Enterocolitis | 55574 (29 RCTs) | RR 0.46 (0.35 to 0.61) | ⊕⊕⊕⊕ HIGH 1,4,6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imdad, A.; Rehman, F.; Davis, E.; Attia, S.; Ranjit, D.; Surin, G.S.; Lawler, S.; Smith, A.; Bhutta, Z.A. Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries. Nutrients 2020, 12, 791. https://doi.org/10.3390/nu12030791
Imdad A, Rehman F, Davis E, Attia S, Ranjit D, Surin GS, Lawler S, Smith A, Bhutta ZA. Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries. Nutrients. 2020; 12(3):791. https://doi.org/10.3390/nu12030791
Chicago/Turabian StyleImdad, Aamer, Faseeha Rehman, Evan Davis, Suzanna Attia, Deepika Ranjit, Gamael Saint Surin, Sarah Lawler, Abigail Smith, and Zulfiqar A. Bhutta. 2020. "Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries" Nutrients 12, no. 3: 791. https://doi.org/10.3390/nu12030791
APA StyleImdad, A., Rehman, F., Davis, E., Attia, S., Ranjit, D., Surin, G. S., Lawler, S., Smith, A., & Bhutta, Z. A. (2020). Effect of Synthetic Vitamin A and Probiotics Supplementation for Prevention of Morbidity and Mortality during the Neonatal Period. A Systematic Review and Meta-Analysis of Studies from Low- and Middle-Income Countries. Nutrients, 12(3), 791. https://doi.org/10.3390/nu12030791






