Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases
Abstract
1. Introduction
2. Vitamin D and Inflammatory Arthritis
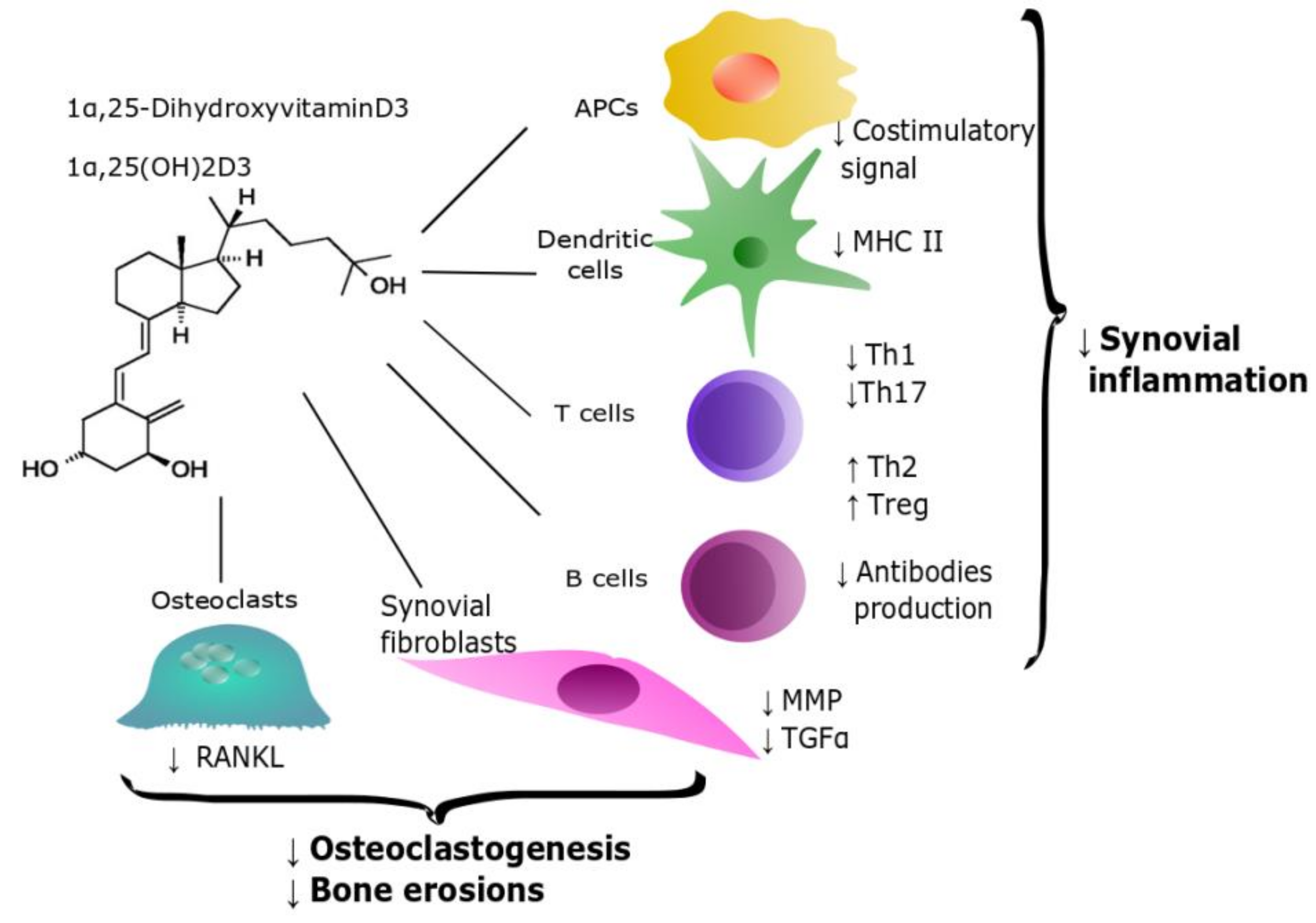
2.1. Rheumatoid Arthritis
- The effect of cholecalciferol supplementation on disease activity is controversial; however, when vitamin D was used at higher doses, the supplementation regimen was generally beneficial [61,62]. This suggests that, possibly, the effect on immune system of vitamin D requires higher plasma levels than those necessary for bone health. Indeed, plasma 25(OH)D3 approximately doubles the synovial concentration [63]; the anti-inflammatory properties of this intermediate have been demonstrated at a 50-100 nM concentration. Thus, the plasma level required for bone health is probably ineffective to elicit immune regulation [37].
2.2. Spondyloarthritis
3. Vitamin D and Autoimmune Connective Tissue Diseases
3.1. Systemic Lupus Erythematosus
3.2. Antiphospholipid Syndrome
4. Vitamin D and Autoimmune Endocrine Diseases
4.1. Type 1 Diabetes Mellitus
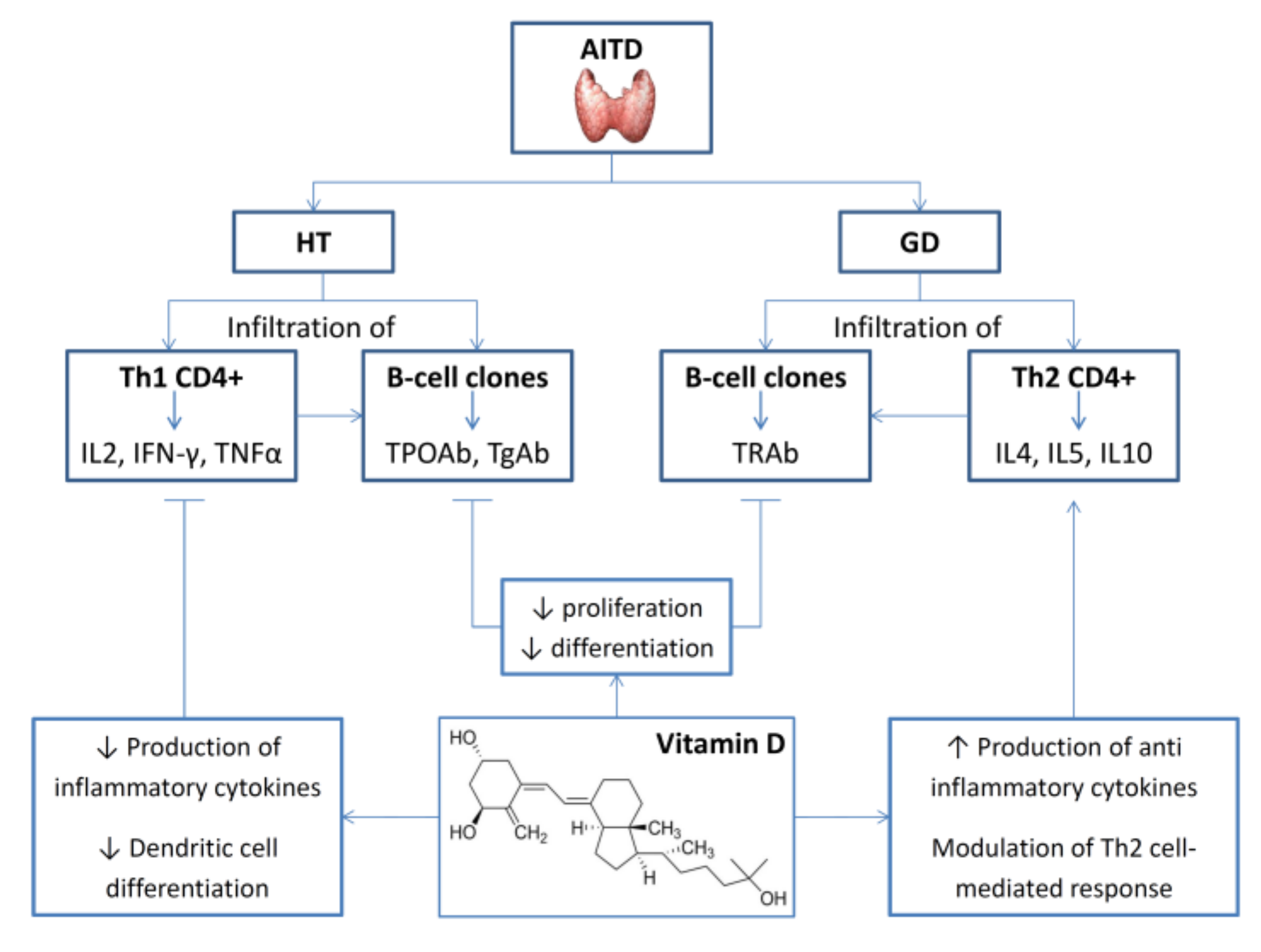
4.2. Thyroid Autoimmunity
4.3. Addison’s Disease
4.4. Autoimmune Polyendocrine Syndromes
5. Vitamin D and Autoimmune Liver Diseases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeLuca, H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Blau, J.E.; Collins, M.T. The PTH-Vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Lieben, L.; Masuyama, R.; Carmeliet, G. Vitamin D endocrine system and the intestine. Bonekey Rep. 2014, 3, 496. [Google Scholar] [CrossRef] [PubMed]
- Demay, M.B.; Kiernan, M.S.; DeLuca, H.F.; Kronenberg, H.M. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1992, 89, 8097–8101. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and Neurological Diseases: An Endocrine View. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Tahawi, Z.; Orolinova, N.; Joshua, I.G.; Bader, M.; Fletcher, E.C. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J. Appl. Physiol. 2001, 90, 2007–2013. [Google Scholar] [CrossRef]
- Talmor, Y.; Golan, E.; Benchetrit, S.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am. J. Physiol. Renal Physiol. 2008, 294, F1059–F1064. [Google Scholar] [CrossRef]
- Molinari, C.; Rizzi, M.; Squarzanti, D.F.; Pittarell, A.P.; Vacca, G.; Renò, F. 1α,25-Dihydroxycholecalciferol (Vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell Physiol. Biochem. 2013, 31, 815–822. [Google Scholar] [CrossRef]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 89–90. [Google Scholar] [CrossRef]
- Bellan, M.; Guzzaloni, G.; Rinaldi, M.; Merlotti, E.; Ferrari, C.; Tagliaferri, A.; Pirisi, M.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc. Diabetol. 2014, 13, 57. [Google Scholar] [CrossRef]
- Grübler, M.R.; März, W.; Pilz, S.; Grammer, T.B.; Trummer, C.; Müllner, C.; Schwetz, V.; Pandis, M.; Verheyen, N.; Tomaschitz, A.; et al. Vitamin-D concentrations, cardiovascular risk and events—A review of epidemiological evidence. Rev. Endocr. Metab. Disord. 2017, 18, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019, 11, 2384. [Google Scholar] [CrossRef] [PubMed]
- Novershtern, N.; Subramanian, A.; Lawton, L.N.; Mak, R.H.; Haining, W.N.; McConkey, M.E.; Habib, N.; Yosef, N.; Chang, C.Y.; Shay, T.; et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 2011, 144, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Gyetko, M.R.; Hsu, C.H.; Wilkinson, C.C.; Patel, S.; Young, E. Monocyte 1 alpha-hydroxylase regulation: Induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J. Leukoc. Biol. 1993, 54, 17–22. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Shin, D.M.; Yuk, J.M.; Lee, H.M.; Lee, S.H.; Son, J.W.; Harding, C.V.; Kim, J.M.; Modlin, R.L.; Jo, E.K. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell. Microbiol. 2010, 12, 1648–1665. [Google Scholar] [CrossRef]
- Sly, L.M.; Lopez, M.; Nauseef, W.M.; Reiner, N.E. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001, 276, 35482–35493. [Google Scholar] [CrossRef]
- Agraz-Cibrian, J.M.; Giraldo, D.M.; Urcuqui-Inchima, S. 1,25-Dihydroxyvitamin D3 induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: A pilot study. Steroids 2019, 141, 14–22. [Google Scholar] [CrossRef]
- Tokuda, N.; Levy, R.B. 1,25-dihydroxyvitamin D3 stimulates phagocytosis but suppresses HLA-DR and CD13 antigen expression in human mononuclear phagocytes. Proc. Soc. Exp. Biol. Med. 1996, 211, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Soruri, A.; Gieseler, R.K.H.; Peters, J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Andressen, R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood 1990, 76, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Griffin, M.D.; Lutz, W.H.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem. Biophys. Res. Commun. 2000, 270, 701–708. [Google Scholar] [CrossRef]
- Gauzzi, M.C.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.C.; Maghazachi, A.A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Illuminating vitamin D effects on B cells—The multiple sclerosis perspective. Immunology 2016, 147, 275–284. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Cippitelli, M.; Fionda, C.; Di Bona, D.; Di Rosa, F.; Lupo, A.; Piccoli, M.; Frati, L.; Santoni, A. Negative regulation of CD95 ligand gene expression by vitamin D3 in T lymphocytes. J. Immunol. 2002, 168, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, J.; Zheng, C.; Wu, J.; Cheng, Y.; Zhu, S.; Lin, C.; Cao, Q.; Zhu, J.; Jin, T. 1,25-dihydroxyvitamin D3 -induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology 2017, 152, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Rigby, W.F.; Denome, S.; Fanger, M.W. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J. Clin. Investig. 1987, 79, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Colin, E.M.; Asmawidjaja, P.S.; van Hamburg, J.P.; Mus, A.M.C.; van Driel, M.; Hazes, J.M.W.; van Leeuwen, J.P.T.M.; Lubberts, E. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010, 62, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Woodward, W.D.; Hayes, C.E.; DeLuca, H.F. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J. Immunol. 1998, 160, 5314–5319. [Google Scholar]
- Sloka, S.; Silva, C.; Wang, J.; Yong, V.W. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J. Neuroinflammation 2011, 8, 56. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J. Immunol 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, S.; Zhang, J.; Zhon, L.; Pen, Z.L.; Xing, T. 1,25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol. Immunol. 2017, 91, 156–164. [Google Scholar] [CrossRef]
- Zwerina, K.; Baum, W.; Axmann, R.; Heiland, G.R.; Distler, J.H.; Smolen, J.; Hayer, S.; Zwerina, J.; Schett, G. Vitamin D receptor regulates TNF-mediated arthritis. Ann. Rheum. Dis. 2011, 70, 1122–1129. [Google Scholar] [CrossRef]
- Tsuji, M.; Fujii, K.; Nakano, T.; Nishii, Y. 1 alpha-hydroxyvitamin D3 inhibits type II collagen-induced arthritis in rats. FEBS Lett. 1994, 337, 248–250. [Google Scholar] [CrossRef]
- Larsson, P.; Mattsson, L.; Klareskog, L.; Johnsson, C. A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin. Exp. Immunol. 1998, 114, 277–283. [Google Scholar] [CrossRef]
- Moghaddami, M.; Mayrhofer, G.; Anderson, P.H.; Morris, H.A.; Van Der Hoek, M.; Cleland, L.G. Efficacy and mechanisms of action of vitamin D in experimental polyarthritis. Immunol. Cell Biol. 2012, 90, 168–177. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin. Exp. Med. 2014, 14, 275–283. [Google Scholar] [CrossRef]
- Harry, R.A.; Anderson, A.E.; Isaacs, J.D.; Hilkens, C.M. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 2042–2050. [Google Scholar] [CrossRef]
- Stoop, J.N.; Harry, R.A.; von Delwig, A.; Isaacs, J.D.; Robinson, J.H.; Hilkens, C.M. Therapeutic effect of tolerogenic dendritic cells in established collagen-induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum. 2010, 62, 3656–3665. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, J.; Li, T.; Chen, Y.; June, R.R.; Zheng, S.G. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells Through miR-124 Mediated Inhibition of IL-6 Signaling. Front. Immunol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Laragione, T.; Shah, A.; Gulko, P.S. The vitamin D receptor regulates rheumatoid arthritis synovial fibroblast invasion and morphology. Mol. Med. 2012, 18, 194–200. [Google Scholar] [CrossRef]
- Gu, X.; Gu, B.; Lv, X.; Yu, Z.; Wang, R.; Zhou, X.; Qiao, W.; Mao, Z.; Zuo, G.; Li, Q.; et al. 1, 25-dihydroxy-vitamin D3 with tumor necrosis factor-alpha protects against rheumatoid arthritis by promoting p53 acetylation-mediated apoptosis via Sirt1 in synoviocytes. Cell Death Dis. 2016, 7, e2423. [Google Scholar] [CrossRef]
- Sun, H.Q.; Yan, D.; Wang, Q.N.; Meng, H.Z.; Zhang, Y.Y.; Yin, L.X.; Yan, X.F.; Li, S.F. 1,25-Dihydroxyvitamin D3 attenuates disease severity and induces synoviocyte apoptosis in a concentration-dependent manner in rats with adjuvant-induced arthritis by inactivating the NF-κB signaling pathway. J. Bone Miner. Metab. 2019, 37, 430–440. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Y.; Li, J.; Wei, D.; Liu, D.; Zhao, F. Inhibitory effect and mechanism of 1,25-dihydroxy vitamin D3 on RANKL expression in fibroblast-like synoviocytes and osteoclast-like cell formation induced by IL-22 in rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 798–805. [Google Scholar]
- Sainaghi, P.P.; Bellan, M.; Carda, S.; Cerutti, C.; Sola, D.; Nerviani, A.; Molinari, R.; Cisari, C.; Avanzi, G.C. Hypovitaminosis D and response to cholecalciferol supplementation in patients with autoimmune and non-autoimmune rheumatic diseases. Rheumatol. Int. 2012, 32, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, M.A.; Manson, J.E.; Costenbader, K.H. Does vitamin D affect risk of developing autoimmune disease? A systematic review. Semin. Arthritis Rheum. 2011, 40, 512–531.e8. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar] [PubMed]
- Cecchetti, S.; Tatar, Z.; Galan, P.; Pereira, B.; Lambert, C.; Mouterde, G.; Sutton, A.; Soubrier, M.; Dougados, M. Prevalence of vitamin D deficiency in rheumatoid arthritis and association with disease activity and cardiovascular risk factors: Data from the COMEDRA study. Clin. Exp. Rheumatol. 2016, 34, 984–990. [Google Scholar] [PubMed]
- Bellan, M.; Sainaghi, P.P.; Pirisi, M. Role of Vitamin D in Rheumatoid Arthritis. Adv. Exp. Med. Biol. 2017, 996, 155–168. [Google Scholar]
- Bellan, M.; Pirisi, M.; Sainaghi, P.P. Osteoporosis in Rheumatoid Arthritis: Role of the vitamin D/parathyroid hormone system. Rev. Bras. Reumatol. 2015, 55, 256–263. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Nerviani, A.; Piantoni, S.; Avanzi, G.C.; Soddu, D.; Hayden, E.; Pirisi, M.; Sainaghi, P.P. Is cholecalciferol a potential disease modifying anti-rheumatic drug for the management of rheumatoid arthritis? Clin. Exp. Rheumatol. 2019, in press. [Google Scholar]
- Yesil, H.; Sungur, U.; Akdeniz, S.; Gurer, G.; Yalcın, B.; Dundar, U. Association between serum vitamin D levels and neuropathic pain in rheumatoid arthritis patients: A cross-sectional study. Int. J. Rheum. Dis. 2018, 21, 431–439. [Google Scholar] [CrossRef]
- Adami, G.; Rossini, M.; Bogliolo, L.; Cantatore, F.P.; Varenna, M.; Malavolta, N.; Del Puente, A.; Muratore, M.; Orsolini, G.; Gatti, D.; et al. An exploratory study on the role of vitamin D supplementation in improving pain and disease activity in rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 1059–1062. [Google Scholar] [CrossRef]
- Chandrashekara, S.; Patted, A. Role of vitamin D supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int. J. Rheum. Dis. 2017, 20, 825–831. [Google Scholar] [CrossRef]
- Brohult, J.; Jonson, B. Effects of large doses of calciferol on patients with rheumatoid arthritis. A double-blind clinical trial. Scand. J. Rheumatol. 1973, 2, 173–176. [Google Scholar] [CrossRef]
- Fairney, A.; Straffen, A.M.; May, C.; Seifert, M.H. Vitamin D metabolites in synovial fluid. Ann. Rheum. Dis. 1987, 46, 370–374. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a high loading dose of cholecalciferol to correct hypovitaminosis d in patients with inflammatory/autoimmune rheumatic diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Antonini, G.; Bellomo, G.; Pirisi, M. Unsuppressed parathyroid hormone in patients with autoimmune/inflammatory rheumatic diseases: Implications for vitamin D supplementation. Rheumatology 2011, 50, 2290–2296. [Google Scholar] [CrossRef]
- Fernandes, S.; Etcheto, A.; van der Heijde, D.; Landewé, R.; van den Bosch, F.; Dougados, M.; Moltó, A. Vitamin D status in spondyloarthritis: Results of the ASAS-COMOSPA international study. Clin. Exp. Rheumatol. 2018, 36, 210–214. [Google Scholar]
- Petho, Z.; Kulcsar-Jakab, E.; Kalina, E.; Balogh, A.; Pusztai, A.; Gulyas, K.; Horvath, A.; Szekanecz, Z.; Bhattoa, H.P. Vitamin D status in men with psoriatic arthritis: A case-control study. Osteoporos. Int. 2015, 26, 1965–1970. [Google Scholar] [CrossRef]
- Orgaz-Molina, J.; Buendía-Eisman, A.; Arrabal-Polo, M.A.; Ruiz, J.C.; Arias-Santiago, S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J. Am. Acad. Dermatol. 2012, 67, 931–938. [Google Scholar] [CrossRef]
- Huckins, D.; Felson, D.T.; Holick, M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study. Arthritis Rheum. 1990, 33, 1723–1727. [Google Scholar] [CrossRef]
- Gaál, J.; Lakos, G.; Szodoray, P.; Kiss, J.; Horváth, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm. Venereol. 2009, 89, 140–144. [Google Scholar]
- Cai, G.; Wang, L.; Fan, D.; Xin, L.; Liu, L.; Hu, Y.; Ding, N.; Xu, S.; Xia, G.; Jin, X.; et al. Vitamin D in ankylosing spondylitis: Review and meta-analysis. Clin. Chim. Acta 2015, 438, 316–322. [Google Scholar] [CrossRef]
- Zhao, S.; Duffield, S.J.; Moots, R.J.; Goodson, N.J. Systematic review of association between vitamin D levels and susceptibility and disease activity of ankylosing spondylitis. Rheumatology 2014, 53, 1595–1603. [Google Scholar] [CrossRef]
- Durmus, B.; Altay, Z.; Baysal, O.; Ersoy, Y. Does vitamin D affect disease severity in patients with ankylosing spondylitis? Chin. Med. J. 2012, 125, 2511–2515. [Google Scholar]
- Zhao, S.Z.; Thong, D.; Duffield, S.; Goodson, N. Vitamin D Deficiency in Axial Spondyloarthritis is Associated with Higher Disease Activity. Arch. Rheumatol. 2017, 32, 209–215. [Google Scholar] [CrossRef]
- Klingberg, E.; Oleröd, G.; Hammarsten, O.; Forsblad-d’Elia, H. The vitamin D status in ankylosing spondylitis in relation to intestinal inflammation, disease activity, and bone health: A cross-sectional study. Osteoporos. Int. 2016, 27, 2027–2033. [Google Scholar] [CrossRef]
- Guła, Z.; Kopczyńska, A.; Hańska, K.; Słomski, M.; Nowakowski, J.; Kwaśny-Krochin, B.; Gąsowski, J.; Korkosz, M. Vitamin D serum concentration is not related to the activity of spondyloarthritis—Preliminary study. Reumatologia 2018, 56, 388–391. [Google Scholar] [CrossRef]
- Lemire, J.M.; Ince, A.; Takashima, M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 1992, 12, 143–148. [Google Scholar] [CrossRef]
- Vaisberg, M.W.; Kaneno, R.; Franco, M.F.; Mendes, N.F. Influence of cholecalciferol (vitamin D3) on the course of experimental systemic lupus erythematosus in F1 (NZBxW) mice. J. Clin. Lab. Anal. 2000, 14, 91–96. [Google Scholar] [CrossRef][Green Version]
- Yan, L.; Wu, P.; Gao, D.M.; Hu, J.; Wang, Q.; Chen, N.F.; Tong, S.Q.; Rao, L.; Liu, J. The Impact of Vitamin D on Cognitive Dysfunction in Mice with Systemic Lupus Erythematosus. Med. Sci. Monit. 2019, 25, 4716–4722. [Google Scholar] [CrossRef]
- Correa Freitas, E.; Evelyn Karnopp, T.; de Souza Silva, J.M.; Cavalheiro do Espírito Santo, R.; da Rosa, T.H.; de Oliveira, M.S.; da Costa Gonçalves, F.; de Oliveira, F.H.; Guilherme Schaefer, P.; André Monticielo, O. Vitamin D supplementation ameliorates arthritis but does not alleviates renal injury in pristane-induced lupus model. Autoimmunity 2019, 52, 69–77. [Google Scholar] [CrossRef]
- Linker-Israeli, M.; Elstner, E.; Klinenberg, J.R.; Wallace, D.J.; Koeffler, H.P. Vitamin D (3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin. Immunol. 2001, 99, 82–93. [Google Scholar] [CrossRef]
- Lerman, M.; Burnham, J.; Behrens, E. 1,25 Dihydroxyvitamin D3 limits monocyte maturation in lupus sera. Lupus 2011, 20, 749–753. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Aranow, C.; Mackay, M.; Stanevsky, A.; Kamen, D.L.; Marinescu, L.M.; Collins, C.E.; Gilkeson, G.S.; Diamond, B.; Hardin, J.A. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 2010, 5, e9193. [Google Scholar] [CrossRef]
- Piantoni, S.; Andreoli, L.; Scarsi, M.; Zanola, A.; Dall’Ara, F.; Pizzorni, C.; Cutolo, M.; Airò, P.; Tincani, A. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015, 24, 490–498. [Google Scholar] [CrossRef]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Stagi, S.; Rigante, D. Vitamin D and juvenile systemic lupus erythematosus: Lights, shadows and still unresolved issues. Autoimmun. Rev. 2018, 17, 290–300. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Guan, S.Y.; Cai, H.Y.; Wang, P.; Lv, T.T.; Liu, L.N.; Mao, Y.M.; Zhao, C.N.; Wu, Q.; Dan, Y.L.; Sam, N.B.; et al. Association between circulating 25-hydroxyvitamin D and systemic lupus erythematosus: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2019, 22, 1803–1813. [Google Scholar] [CrossRef]
- Wang, X.R.; Xiao, J.P.; Zhang, J.J.; Wu, Y.G. Decreased Serum/Plasma Vitamin D levels in SLE Patients: A Meta-Analysis. Curr. Pharm. Des. 2018, 24, 4466–4473. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Crowe, S.R.; Niewold, T.B.; Kamen, D.L.; Macwana, S.R.; Roberts, V.C.; Dedeke, A.B.; Harley, J.B.; Scofield, R.H.; Guthridge, J.M.; et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2011, 70, 1569–1574. [Google Scholar] [CrossRef]
- Gittoes, N.J. Vitamin D—What is normal according to latest research and how should we deal with it? Clin. Med. 2015, 15 (Suppl. 6), s54–s57. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Jones, G.; Yip, A.; Lohnes, D.; Cohanim, M.; Yendt, E.R. The effects of chloroquine on serum 1, 25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N. Engl. J. Med. 1986, 315, 727–730. [Google Scholar]
- Ruiz-Irastorza, G.; Egurbide, M.V.; Olivares, N.; Martinez-Berriotxoa, A.; Aguirre, C. Vitamin D deficiency in systemic lupus erythematosus: Prevalence, predictors and clinical consequences. Rheumatology 2008, 47, 920–923. [Google Scholar] [CrossRef]
- Toloza, S.; Cole, D.; Gladman, D.; Ibanez, D.; Urowitz, M. Vitamin D insufficiency in a large female SLE cohort. Lupus 2010, 19, 13–19. [Google Scholar] [CrossRef]
- Kamen, D.L. Vitamin D in lupus-new kid on the block? Bull. NYU Hosp. Jt. Dis. 2010, 68, 218–222. [Google Scholar]
- Akeno, N.; Matsunuma, A.; Maeda, T.; Kawane, T.; Horiuchi, N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxy-lase expression by dexamethasone in mouse kidney. J. Endocrinol. 2000, 164, 339–348. [Google Scholar] [CrossRef]
- Sumethkul, K.; Boonyaratavej, S.; Kitumnuaypong, T.; Angthararuk, S.; Cheewasat, P.; Manadee, N.; Sumethkul, V. The predictive factors of low serum 25-hydroxyvitamin D and vitamin D deficiency in patients with systemic lupus erythematosus. Rheumatol. Int. 2013, 33, 1461–1467. [Google Scholar] [CrossRef]
- Yu, Q.; Qiao, Y.; Liu, D.; Liu, F.; Gao, C.; Duan, J.; Liang, L.; Di, X.; Yuan, Y.; Gao, Y.; et al. Vitamin D protects podocytes from autoantibodies induced injury in lupus nephritis by reducing aberrant autophagy. Arthritis Res. Ther. 2019, 21, 19. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Bryant, K.; O’Neill, S.G. Vitamin D in SLE: A role in pathogenesis and fatigue? A review of the literature. Lupus 2018, 27, 2003–2011. [Google Scholar] [CrossRef]
- Sabio, J.M.; Vargas-Hitos, J.A.; Martínez-Bordonado, J.; Mediavilla-García, J.D. Association between non-dipper hypertension and vitamin D deficiency in women with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2019, 37, 286–292. [Google Scholar]
- Mak, A. The Impact of Vitamin D on the Immunopathophysiology, Disease Activity, and Extra-Musculoskeletal Manifestations of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2018, 19, 2355. [Google Scholar] [CrossRef]
- Hussein, H.A.; Daker, L.I.; Fouad, N.A.; Elamir, A.; Mohamed, S.R. Does Vitamin D Deficiency Contribute to Cognitive Dysfunction in Patients with Systemic Lupus Erythematosus? Innov. Clin. Neurosci. 2018, 15, 25–29. [Google Scholar]
- Mok, C.C.; Bro, E.T.; Ho, L.Y.; Singh, R.J.; Jannetto, P.J. Serum 25-hydroxyvitamin D3 levels and flares of systemic lupus erythematosus: A longitudinal cohort analysis. Clin. Rheumatol. 2018, 37, 2685–2692. [Google Scholar] [CrossRef]
- Dutta, C.; Kakati, S.; Barman, B.; Bora, K. Vitamin D status and its relationship with systemic lupus erythematosus as a determinant and outcome of disease activity. Horm. Mol. Biol. Clin. Investig. 2019, 38. [Google Scholar] [CrossRef]
- Aranow, C.; Kamen, D.L.; Dall’Era, M.; Massarotti, E.M.; Mackay, M.C.; Koumpouras, F.; Coca, A.; Chatham, W.W.; Clowse, M.E.; Criscione-Schreiber, L.G.; et al. Randomized, double-blind, placebo- controlled trial of the effect of vitamin D3 on the interferon signature in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015, 67, 1848–1857. [Google Scholar] [CrossRef]
- Lima, G.L.; Paupitz, J.; Aikawa, N.E.; Takayama, L.; Bonfa, E.; Pereira, R.M. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: A randomized, double- blind, placebo-controlled trial. Arthritis Care Res. 2016, 68, 91–98. [Google Scholar] [CrossRef]
- Rifa’I, A.; Kalim, H.; Kusworini, K.; Cesarius, S.W. Effect of vitamin D supplementation on disease activity (SLEDAI) and fatigue in Systemic Lupus Erythematosus patients with hipovitamin D: An open clinical trial. Indones. J. Rheumatol. 2016, 8, 32–37. [Google Scholar]
- Petri, M.; Bello, K.J.; Fang, H.; Magder, L.S. Vitamin D in systemic lupus erythematosus: Modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis Rheum. 2013, 65, 1865–1871. [Google Scholar] [CrossRef]
- Andreoli, L.; Dall’Ara, F.; Piantoni, S.; Zanola, A.; Piva, N.; Cutolo, M.; Tincani, A. A 24-month prospective study on the efficacy and safety of two different monthly regimens of vitamin D supplementation in pre-menopausal women with systemic lupus erythematosus. Lupus 2015, 24, 499–506. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Gordo, S.; Olivares, N.; Egurbide, M.V.; Aguirre, C. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arthritis Care Res. 2010, 62, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, H.; Shirzadi, M.; Karimifar, M. The effect of vitamin D supplementation in disease activity of systemic lupus erythematosus patients with vitamin D deficiency: A randomized clinical trial. J. Res. Med. Sci. 2017, 22, 4. [Google Scholar] [PubMed]
- Bultink, I.E.; Lems, W.F. Lupus and fractures. Curr. Opin. Rheumatol. 2016, 28, 426–432. [Google Scholar] [CrossRef]
- Edens, C.; Robinson, A.B. Systemic lupus erythematosus, bone health, and osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 422–431. [Google Scholar] [CrossRef]
- Roman, M.J.; Crown, M.K.; Lockshin, M.D.; Devereux, R.B.; Paget, S.A.; Sammaritano, L.; Levine, D.M.; Davis, A.; Salmon, J.E. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 3412–3419. [Google Scholar] [CrossRef]
- Mellor-Pita, S.; Tutor-Ureta, P.; Rosado, S.; Alkadi, K.; Granado, F.; Jimenez-Ortiz, C.; Castejon, R. Calcium and vitamin D Supplement intake may increase arterial stiffness in systemic lupus erythematosus patients. Clin. Rheumatol. 2019, 38, 1177–1186. [Google Scholar] [CrossRef]
- Dall’Ara, F.; Cutolo, M.; Andreoli, L.; Tincani, A.; Paolino, S. Vitamin D and systemic lupus erythematous: A review of immunological and clinical aspects. Clin. Exp. Rheumatol. 2018, 36, 153–162. [Google Scholar]
- Hollis, B.W. Editorial: The determination of circulating 25- hydroxyvitamin D: No easy task. J. Clin. Endocrinol. Metab. 2004, 89, 3149–3151. [Google Scholar] [CrossRef]
- Klack, K.; Carvalho, J.F. High frequency of vitamin D insufficiency in primary antiphospholipid syndrome. Jt. Bone Spine 2010, 77, 489–490. [Google Scholar] [CrossRef]
- Andreoli, L.; Piantoni, S.; Dall’Ara, F.; Allegri, F.; Meroni, P.L.; Tincani, A. Vitamin D and antiphospholipid syndrome. Lupus 2012, 21, 736–740. [Google Scholar] [CrossRef]
- Riancho-Zarrabeitia, L.; Cubería, M.; Muñoz, P.; López-Hoyos, M.; García-Canale, S.; García-Unzueta, M.; Hernández, J.L.; Martínez-Taboada, V.M. Vitamin D and antiphospholipid syndrome: A retrospective cohort study and meta-analysis. Semin. Arthritis Rheum. 2018, 47, 877–882. [Google Scholar] [CrossRef]
- Piantoni, S.; Andreoli, L.; Allegri, F.; Meroni, P.L.; Tincani, A. Low levels of vitamin D are common in primary antiphospholipid syndrome with thrombotic disease. Reumatismo 2012, 64, 307–313. [Google Scholar] [CrossRef]
- García-Carrasco, M.; Jiménez-Herrera, E.A.; Gálvez-Romero, J.L.; Mendoza-Pinto, C.; Méndez-Martínez, S.; Etchegaray-Morales, I.; Munguía-Realpozo, P.; Vázquez de Lara-Cisneros, L.; Santa Cruz, F.J.; Cervera, R. The anti-thrombotic effects of vitamin D and their possible relationship with antiphospholipid syndrome. Lupus 2018, 27, 2181–2189. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Blank, M.; Zandman-Goddard, M.; Orbach, H.; Meroni, P.L.; Tincani, A.; Doria, A.; Cervera, R.; Miesbach, W.; Stojanovich, L.; et al. Vitamin D: An instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann. Rheum. Dis. 2011, 70, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Epstein, E.; Olsson, H. Does an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesis. J. Thromb. Haemost. 2009, 7, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Venner, P.M.; Ryan, C.W.; Petrylak, D.P.; Chatta, G.; Dean Ruether, J.; Chi, K.N.; Curd, J.G.; DeLoughery, T.G. High dose calcitriol may reduce thrombosis in cancer patients. Br. J. Haematol. 2006, 135, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Wu-Wong, J.R. Are vitamin D receptor activators useful for the treatment of thrombosis? Curr. Opin. Investig. Drugs 2009, 10, 919–927. [Google Scholar] [PubMed]
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y.; Hansen, J.B. Parameters of the thrombogram are associated with serum 25- hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thromb. Res. 2010, 125, e210–e213. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Dambaeva, S.; Han, A.R.; Beaman, K.; Gilman-Sachs, A.; Kwak-Kim, J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum. Reprod. 2014, 29, 208–219. [Google Scholar] [CrossRef]
- Barrera, D.; Avila, E.; Hernandez, G.; Halhali, A.; Biruete, B.; Larrea, F.; Diaz, L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J. Steroid Biochem. Mol. Biol. 2007, 103, 529–532. [Google Scholar] [CrossRef]
- Diaz, L.; Noyola-Martinez, N.; Barrera, D.; Hernandez, G.; Avila, E.; Halhali, A.; Larrea, F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J. Reprod. Immunol. 2009, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, L.; Bertsias, G.K.; Agmon-Levin, N.; Brown, S.; Cervera, R.; Costedoat-Chalumeau, N.; Doria, A.; Fischer-Betz, R.; Forger, F.; Moraes-Fontes, M.F.; et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 2017, 76, 476–485. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Noble, J.A.; Erlich, H.A. Genetics of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018, 7, 38–46. [Google Scholar] [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, S2213–S8587, 30307–30412. [Google Scholar] [CrossRef]
- Kahles, H.; Morahan, G.; Todd, J.A.; Badenhoop, K.; Type I Diabetes Genetics Consortium. Association analyses of the vitamin D receptor gene in 1654 families with type I diabetes. Genes Immun. 2009, 10 (Suppl. 1), S60–S63. [Google Scholar] [CrossRef]
- Ban, Y.; Taniyama, M.; Yanagawa, T.; Yamada, S.; Maruyama, T.; Kasuga, A. Vitamin D receptor initiation codon polymorphism influences genetic susceptibility to type 1 diabetes mellitus in the Japanese population. BMC Med. Genet. 2001, 2, 7. [Google Scholar] [CrossRef]
- Van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Liu, J.; Wu, W.; Ouyang, H.; Zhang, Q.; Wang, Y.; Liu, L.; Yang, R.; Liu, X.; et al. Polymorphisms in the vitamin D receptor gene and type 1 diabetes mellitus risk: An update by meta-analysis. Mol. Cell. Endocrinol. 2012, 355, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Batool, A.; Wajid, A.; Qayyum, I. Vitamin D Receptor Gene Polymorphisms Influence T1D Susceptibility among Pakistanis. Int. J. Genom. 2017, 2017, 4171254. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Fawzy, I.; Mohsen, I.; Settin, A. Evaluation of vitamin D receptor gene polymorphisms (Fok-I and Bsm-I) in T1DM Saudi children. J. Clin. Lab. Anal. 2018, 32, e22397. [Google Scholar] [CrossRef] [PubMed]
- Kirac, D.; Dincer Yazan, C.; Gezmis, H.; Yaman, A.; Haklar, G.; Sirikci, O.; Altunok, E.C.; Deyneli, O. VDBP, VDR Mutations and Other Factors Related With Vitamin D Metabolism May Be Associated With Type 1 Diabetes Mellitus. Cell. Mol. Biol. 2018, 64, 11–16. [Google Scholar] [PubMed]
- Tapia, G.; Mårild, K.; Dahl, S.R.; Lund-Blix, N.A.; Viken, M.K.; Lie, B.A.; Njølstad, P.R.; Joner, G.; Skrivarhaug, T.; Cohen, A.S.; et al. Maternal and Newborn Vitamin D-Binding Protein, Vitamin D Levels, Vitamin D Receptor Genotype, and Childhood Type 1 Diabetes. Diabetes Care 2019, 42, 553–559. [Google Scholar] [CrossRef]
- Habibian, N.; Amoli, M.M.; Abbasi, F.; Rabbani, A.; Alipour, A.; Sayarifard, F.; Rostami, P.; Dizaji, S.P.; Saadati, B.; Setoodeh, A. Role of vitamin D and vitamin D receptor gene polymorphisms on residual beta cell function in children with type 1 diabetes mellitus. Pharmacol. Rep. 2019, 71, 282–288. [Google Scholar] [CrossRef]
- Rasoul, M.A.; Haider, M.Z.; Al-Mahdi, M.; Al-Kandari, H.; Dhaunsi, G.S. Relationship of four vitamin D receptor gene polymorphisms with type 1 diabetes mellitus susceptibility in Kuwaiti children. BMC Pediatr. 2019, 19, 71. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Sakhr, H.M.; Hassan, M.H.; El-Amir, M.I.; Ameen, H.H. Vitamin D receptor rs7975232, rs731236 and rs1544410 single nucleotide polymorphisms, and 25-hydroxyvitamin D levels in Egyptian children with type 1 diabetes mellitus: Effect of vitamin D co-therapy. Diabetes Metab. Syndr. Obes. 2019, 12, 703–716. [Google Scholar] [CrossRef]
- Sahin, O.A.; Goksen, D.; Ozpinar, A.; Serdar, M.; Onay, H. Association of vitamin D receptor polymorphisms and type 1 diabetes susceptibility in children: A meta-analysis. Endocr. Connect. 2017, 6, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wolden-Kirk, H.; Rondas, D.; Bugliani, M.; Korf, H.; Van Lommel, L.; Brusgaard, K.; Christesen, H.T.; Schuit, F.; Proost, P.; Masini, M.; et al. Discovery of molecular pathways mediating 1,25-dihydroxyvitamin D3 protection against cytokine-induced inflammation and damage of human and male mouse islets of Langerhans. Endocrinology 2014, 155, 736–747. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Moltchanova, E.V.; Schreier, N.; Lammi, N.; Karvonen, M. Seasonal variation of diagnosis of type 1 diabetes mellitus in children worldwide. Diabet. Med. 2009, 26, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Cadario, F.; Prodam, F.; Savastio, S.; Monzani, A.; Balafrej, A.; Bellomo, G.; Bona, G. Vitamin D status and type 1 diabetes in children: Evaluation according to latitude and skin color. Minerva Pediatr. 2015, 67, 263–267. [Google Scholar] [PubMed]
- Xia, Y.; Xie, Z.; Huang, G.; Zhou, Z. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab. Res. Rev. 2019, 35, e3075. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Brunborg, C.; Torjesen, P.A.; Stene, L.C. Vitamin D-binding protein and 25-hydroxyvitamin D during pregnancy in mothers whose children later developed type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 883–890. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Li, G.; Li, Z.; Zhang, Y.; Li, Q.; Sun, C. Lower serum 25 (OH) D concentrations in type 1 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2015, 108, e71–e75. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Xia, X.; Wang, J.; Wan, Y.; He, L.; Li, M. Correlation of serum vitamin D level with type 1 diabetes mellitus in children: A meta-analysis. Nutr. Hosp. 2015, 32, 1591–1594. [Google Scholar]
- Shen, L.; Zhuang, Q.S.; Ji, H.F. Assessment of vitamin D levels in type 1 and type 2 diabetes patients: Results from metaanalysis. Mol. Nutr. Food Res. 2016, 60, 1059–1067. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Szalecki, M.; Pludowski, P.; Jaworski, M.; Brzozowska, A. Vitamin D status, body composition and glycemic control in Polish adolescents with type 1 diabetes. Minerva Endocrinol. 2016, 41, 445–455. [Google Scholar]
- Al Sawah, S.; Compher, C.W.; Hanlon, A.L.; Lipman, T.H. 25-Hydroxyvitamin D and glycemic control: A cross-sectional study of children and adolescents with type 1 diabetes. Diabetes Res. Clin. Pract. 2016, 115, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rasoul, M.A.; Al-Mahdi, M.; Al-Kandari, H.; Dhaunsi, G.S.; Haider, M.Z. Low serum vitamin-D status is associated with high prevalence and early onset of type-1 diabetes mellitus in Kuwaiti children. BMC Pediatr. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Savastio, S.; Cadario, F.; Genoni, G.; Bellomo, G.; Bagnati, M.; Secco, G.; Picchi, R.; Giglione, E.; Bona, G. Vitamin D Deficiency and Glycemic Status in Children and Adolescents with Type 1 Diabetes Mellitus. PLoS ONE 2016, 11, e0162554. [Google Scholar] [CrossRef] [PubMed]
- Talaat, I.M.; Nasr, A.; Alsulaimani, A.A.; Alghamdi, H.; Alswat, K.A.; Almalki, D.M.; Abushouk, A.; Saleh, A.M.; Allam, G. Association between type 1, type 2 cytokines, diabetic autoantibodies and 25-hydroxyvitamin D in children with type 1 diabetes. J. Endocrinol. Investig. 2016, 39, 1425–1434. [Google Scholar] [CrossRef]
- Ziaei-Kajbaf, T.; Aminzadeh, M.; Fatahinezhad, E.; Aletayeb, S.M. Vitamin D status in diabetic children and adolescents. Diabetes Metab. Syndr. 2018, 12, 849–852. [Google Scholar] [CrossRef]
- Jacobsen, R.; Thorsen, S.U.; Cohen, A.S.; Lundqvist, M.; Frederiksen, P.; Pipper, C.B.; Pociot, F.; Thygesen, L.C.; Ascherio, A.; Svensson, J.; et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: Results from two large Danish population-based studies. Diabetologia 2016, 59, 1871–1881. [Google Scholar] [CrossRef]
- Wood, J.R.; Connor, C.G.; Cheng, P.; Ruedy, K.J.; Tamborlane, W.V.; Klingensmith, G.; Schatz, D.; Gregg, B.; Cengiz, E.; Willi, S.; et al. Vitamin D status in youth with type 1 and type 2 diabetes enrolled in the Pediatric Diabetes Consortium (PDC) is not worse than in youth without diabetes. Pediatr. Diabetes 2016, 17, 584–591. [Google Scholar] [CrossRef]
- Thorsen, S.U.; Mårild, K.; Olsen, S.F.; Holst, K.K.; Tapia, G.; Granström, C.; Halldorsson, T.I.; Cohen, A.S.; Haugen, M.; Lundqvist, M.; et al. Lack of Association Between Maternal or Neonatal Vitamin D Status and Risk of Childhood Type 1 Diabetes: A Scandinavian Case-Cohort Study. Am. J. Epidemiol. 2018, 187, 1174–1181. [Google Scholar] [CrossRef]
- Dogan, B.; Oner, C.; Feyizoglu, G.; Yoruk, N.; Oguz, A. Vitamin D status of Turkish type 1 diabetic patients. Diabetes Metab. Syndr. 2019, 13, 2037–2039. [Google Scholar] [CrossRef]
- Pitocco, D.; Crinò, A.; Di Stasio, E.; Manfrini, S.; Guglielmi, C.; Spera, S.; Anguissola, G.B.; Visalli, N.; Suraci, C.; Matteoli, M.C.; et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet. Med. 2006, 23, 920–923. [Google Scholar] [CrossRef]
- Papadimitriou, D.T.; Marakaki, C.; Fretzayas, A.; Nicolaidou, P.; Papadimitriou, A. Negativation of type 1 diabetes-associated autoantibodies to glutamic acid decarboxylase and insulin in children treated with oral calcitriol. J. Diabetes 2013, 5, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liao, L.; Yan, X.; Huang, G.; Lin, J.; Lei, M.; Wang, X.; Zhou, Z. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes/Met. Res. Rev. 2009, 25, 411–416. [Google Scholar] [CrossRef]
- Walter, M.; Kaupper, T.; Adler, K.; Foersch, J.; Bonifacio, E.; Ziegler, A.G. No effect of the 1alpha, 25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care 2010, 33, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, E.; Mamais, I.; Tzanetakou, I.; Lavranos, G.; Chrysostomou, S. The Effects of Vitamin D supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials. Rev. Diabet. Stud. 2017, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Felício, K.M.; de Souza, A.C.C.B.; Neto, J.F.A.; de Melo, F.T.C.; Carvalho, C.T.; Arbage, T.P.; de Rider Brito, H.A.; Peixoto, A.S.; de Oliveira, A.F.; de Souza Resende, F.; et al. Glycemic Variability and Insulin Needs in Patients with Type 1 Diabetes Mellitus supplemented with Vitamin D: A Pilot Study Using Continuous Glucose Monitoring System. Curr. Diabetes Rev. 2018, 14, 395–403. [Google Scholar] [CrossRef]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef]
- Kim, D. The role of vitamin D in thyroid diseases. Int. J. Mol. Sci 2017, 18, 1949. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Tirabassi, G.; Bizzaro, G.; Orio, F.; Paschou, S.A.; Vryonidou, A.; Balercia, G.; Shoenfeld, Y.; Colao, A. Vitamin D and thyroid disease: To D or not to D? Eur. J. Clin. Nutr. 2015, 69, 291–296. [Google Scholar] [CrossRef]
- Gallo, D.; Mortara, L.; Gariboldi, M.B.; Cattaneo, S.A.M.; Rosetti, S.; Gentile, L.; Noonan, D.M.; Premoli, P.; Cusini, C.; Tanda, M.L.; et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: A narrative review. J. Endocrinol. Investig. 2019, in press. [Google Scholar] [CrossRef]
- Fournier, C.; Gepner, P.; Sadouk, M.; Charreire, J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin. Immunol. Immunopathol 1990, 4, 53–63. [Google Scholar] [CrossRef]
- Borgogni, E.; Sarchielli, E.; Sottili, M.; Santarlasci, V.; Cosmi, L.; Gelmini, S.; Lombardi, A.; Cantini, G.; Perigli, G.; Luconi, M.; et al. Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology 2008, 149, 3626–3634. [Google Scholar] [CrossRef]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapoport, B.; McLachlan, S.M. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009, 50, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. Effects of 1,25-dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis. J. South. Med. Univ. 2010, 30, 1573–1576. [Google Scholar]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Marwaha, R.K.; Gupta, N.; Tandon, N.; Sreenivas, V.; Tomar, N.; Ray, D.; Kanwar, R.; Agarwal, R. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br. J. Nutr. 2009, 102, 382–386. [Google Scholar] [CrossRef]
- Camurdan, O.M.; Döğer, E.; Bideci, A.; Celik, N.; Cinaz, P. Vitamin D status in children with Hashimoto thyroiditis. J. Pediatr. Endocrinol. Metab. 2012, 25, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, K.J.; Kim, D.; Hwang, S.; Lee, E.J. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med. J. 2014, 55, 476–481. [Google Scholar] [CrossRef]
- Unal, A.D.; Tarcin, O.; Parildar, H.; Cigerli, O.; Eroglu, H.; Demirag, N.G. Vitamin D deficiency is related to thyroid antibodies in autoimmune thyroiditis. Cent. Eur. J. Immunol. 2014, 39, 493–497. [Google Scholar] [CrossRef]
- Wang, X.; Zynat, J.; Guo, Y.; Osiman, R.; Tuhuti, A.; Zhao, H.; Abdunaimu, M.; Wang, H.; Jin, X.; Xing, S. Low serum vitamin D is associated with anti-thyroid-globulin antibody in female individuals. Int. J. Endocrinol. 2015, 2015, 285290. [Google Scholar] [CrossRef]
- ElRawi, H.A.; Ghanem, N.S.; ElSayed, N.M.; Ali, H.M.; Rashed, L.A.; Mansour, M.M. Study of Vitamin D Level and Vitamin D Receptor Polymorphism in Hypothyroid Egyptian Patients. J. Thyroid Res. 2019, 2019, 3583250. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, Y.J.; Choi, J.H.; Lee, S.Y.; Lee, H.Y.; Jeong, D.H.; Choi, Y.J. The Association between Low Vitamin D Status and Autoimmune Thyroid Disease in Korean Premenopausal Women: The 6th Korea National Health and Nutrition Examination Survey, 2013–2014. Korean J. Fam. Med. 2019, 40, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 2011, 21, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, W.G.; Kim, T.Y.; Bae, S.J.; Kim, H.K.; Jang, E.K.; Jeon, M.J.; Han, J.M.; Lee, S.H.; Baek, J.H.; et al. Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid 2014, 24, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Evliyaoğlu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: A critical vitamin D level for this association? J. Clin Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Mari, D.; Prolo, S.; Fatti, L.M.; Cantone, M.C.; Garagnani, P. 25 Hydroxyvitamin D deficiency and its relationship to autoimmune thyroid disease in the elderly. Int. J. Environ. Res. Public Health 2016, 13, 850. [Google Scholar] [CrossRef]
- Kim, M.; Song, E.; Oh, H.S.; Park, S.; Kwon, H.; Jeon, M.J.; Kim, W.G.; Kim, W.B.; Shong, Y.K.; Kim, T.Y. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine 2017, 58, 332–339. [Google Scholar] [CrossRef]
- Gao, X.R.; Yu, Y.G. Meta-Analysis of the Association between Vitamin D Receptor Polymorphisms and the Risk of Autoimmune Thyroid Disease. Int. J. Endocrinol. 2018, 2018, 2846943. [Google Scholar] [CrossRef]
- Simsek, Y.; Cakır, I.; Yetmis, M.; Dizdar, O.S.; Baspinar, O.; Gokay, F. Effects of vitamin D treatment on thyroid autoimmunity. J. Res. Med. Sci. 2016, 21, 85. [Google Scholar] [PubMed]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.A.; Kumar, A.; Mukhopadhyay, S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J. Endocrinol. Metab. 2016, 20, 391–398. [Google Scholar] [PubMed]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto’s thyroiditis and low vitamin D status. Pharmacol. Rep. 2018, 71, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef]
- Chahardoli, R.; Saboor-Yaraghi, A.A.; Amouzegar, A.; Khalili, D.; Vakili, A.Z.; Azizi, F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, Randomized clinical trial. Horm. Metab. Res. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Menconi, F.; Marcocci, C.; Marino, M. Diagnosis and classification of Graves’ disease. Autoimmun. Rev. 2014, 13, 398–402. [Google Scholar] [CrossRef]
- Cooper, G.S.; Stroehla, B.C. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2003, 2, 119–125. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef]
- Yasuda, T.; Okamoto, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 2013, 43, 230–232. [Google Scholar] [CrossRef]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur. Thyroid J. 2018, 7, 27–33. [Google Scholar] [CrossRef]
- Mangaraj, S.; Choudhury, A.K.; Swain, B.M.; Sarangi, P.K.; Mohanty, B.K.; Baliarsinha, A.K. Evaluation of vitamin D status and its impact on thyroid related parameters in new onset Graves’ disease- A cross-sectional observational study. Indian J. Endocrinol. Metab. 2019, 23, 35–39. [Google Scholar] [PubMed]
- Xu, M.Y.; Cao, B.; Yin, J.; Wang, D.F.; Chen, K.L.; Lu, Q.B. Vitamin D and Graves’ disease: A meta-analysis update. Nutrients 2015, 7, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Chung, Y.J. Vitamin D supplementation does not prevent the recurrence of Graves’ disease. Sci. Rep. 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, C.; Gu, M. Vitamin D receptor (VDR) gene polymorphisms and Graves’ disease: A meta-analysis. Clin. Endocrinol. 2009, 70, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, M.M.; Løvås, K.; Fougner, K.J.; Svartberg, J.; Hauge, E.R.; Bollerslev, J.; Berg, J.P.; Mella, B.; Husebye, E.S. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur. J. Endocrinol. 2009, 160, 233–237. [Google Scholar] [CrossRef]
- Meyer, G.; Neumann, K.; Badenhoop, K.; Linder, R. Increasing prevalence of Addison’s disease in German females: Health insurance data 2008-2012. Eur. J. Endocrinol. 2014, 170, 367–373. [Google Scholar] [CrossRef]
- Barthel, A.; Benker, G.; Berens, K.; Diederich, S.; Manfras, B.; Gruber, M.; Kanczkowski, W.; Kline, G.; Kamvissi-Lorenz, V.; Hahner, S.; et al. An Update on Addison’s Disease. Exp. Clin. Endocrinol. Diabetes 2019, 127, 165–175. [Google Scholar] [CrossRef]
- Bratland, E.; Husebye, E.S. Cellular immunity and immunopathology in autoimmune Addison’s disease. Mol. Cell. Endocrinol. 2011, 336, 180–190. [Google Scholar] [CrossRef]
- Kraus, A.U.; Penna-Martinez, M.; Meyer, G.; Badenhoop, K. Vitamin D effects on monocytes’ CCL-2, IL6 and CD14 transcription in Addison’s disease and HLA susceptibility. J. Steroid Biochem. Mol. Biol. 2018, 177, 53–58. [Google Scholar] [CrossRef]
- Pani, M.A.; Seissler, J.; Usadel, K.H.; Badenhoop, K. Vitamin D receptor genotype is associated with Addison’s disease. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2002, 147, 635–640. [Google Scholar] [CrossRef]
- Lopez, E.R.; Zwermann, O.; Segni, M.; Meyer, G.; Reincke, M.; Seissler, J.; Herwig, J.; Usadel, K.H.; Badenhoop, K. A promoter polymorphism of the CYP27B1 gene is associated with Addison’s disease, Hashimoto’s thyroiditis, Graves’ disease, and type 1 diabetes mellitus in Germans. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc 2004, 151, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.E.; Owen, C.J.; Wilson, V.; Pearce, S.H. A haplotype of the CYP27B1 promoter is associated with autoimmune Addison’s disease but not with Graves’ disease in a UK population. J. Mol. Endocrinol. 2005, 34, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Fichna, M.; Zurawek, M.; Januszkiewicz-Lewandowska, D.; Gryczynska, M.; Fichna, P.; Sowinski, J. Association of the CYP27B1 C(-1260)A polymorphism with autoimmune Addison’s disease. Exp. Clin. Endocrinol. Diabetes 2010, 118, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Goldacre, R.; Disanto, G.; Giovannoni, G.; Goldacre, M.J. Hospital admissions for vitamin D related conditions and subsequent immune-mediated disease: Record-linkage studies. BMC Med. 2013, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Pazderska, A.; Fichna, M.; Mitchell, A.L.; Napier, C.M.; Gan, E.; Ruchała, M.; Santibanez-Koref, M.; Pearce, S.H. Impact of Month of Birth on the Risk of Development of Autoimmune Addison’s Disease. J. Clin. Endocrinol. Metab. 2016, 101, 4214–4218. [Google Scholar] [CrossRef] [PubMed]
- Penna-Martinez, M.; Filmann, N.; Bogdanou, D.; Shoghi, F.; Huenecke, S.; Schubert, R.; Herrmann, E.; Koehl, U.; Husebye, E.S.; Badenhoop, K. High-dose vitamin D in Addison’s disease regulates T-cells and monocytes: A pilot trial. Nutrition 2018, 49, 66–73. [Google Scholar] [CrossRef]
- Cutolo, M. Autoimmune polyendocrine syndromes. Autoimmun. Rev. 2014, 13, 85–89. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Husebye, E.S.; Anderson, M.S.; Kämpe, O. Autoimmune Polyendocrine Syndromes. N. Engl. J. Med. 2018, 378, 1132–1141. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; Petrizzo, M.; De Bellis, A.; Capuano, A.; Esposito, K.; Giugliano, D. Vitamin D and autoimmunity: What happens in autoimmune polyendocrine syndromes? J. Endocrinol. Investig. 2015, 38, 629–633. [Google Scholar] [CrossRef]
- Lohse, A.W.; Chazouillères, O.; Dalekos, G.; Drenth, J.; Heneghan, M.; Hofer, H.; Lammert, F.; Lenzi, M. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hep. 2015, 63, 971–1004. [Google Scholar]
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Grønbæk, L.; Vilstrup, H. Worldwide Incidence of Autoimmune Liver Disease. Dig. Dis. 2015, 33 (Suppl. 2), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary sclerosing cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef]
- Baran, D.T.; Milne, M.L. 1,25Dihydroxyvitamin D increases hepatocyte cytosolic calcium levels. A potential regulator of vitamin D-25-hydroxylase. J. Clin. Investig. 1986, 77, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Rixon, R.H.; Isaacs, R.J.; Whitfield, J.F. Control of DNA polymerase-alpha activity in regenerating rat liver by calcium and 1 alpha,25(OH)2D3. J. Cell. Physiol. 1989, 139, 354–360. [Google Scholar] [CrossRef]
- Drocourt, L.; Ourlin, J.C.; Pascussi, J.M.; Maurel, P.; Vilarem, M.J. Expression of CYP3A4, CYP2D6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J. Biol. Chem. 2002, 277, 25125–25132. [Google Scholar] [CrossRef]
- Lin, C.J.; Dardis, A.; Wijesuriya, S.D.; Abdullah, M.A.; Casella, S.J.; Miller, W.L. Lack of mutations in CYP2D6 and CYP27 in patients with apparent deficiency of vitamin D 25-hydroxylase. Mol. Genet. Metab. 2003, 80, 469–472. [Google Scholar] [CrossRef]
- Sutti, S.; Rigamonti, C.; Vidali, M.; Albano, E. CYP2E1 autoantibodies in liver diseases. Redox. Biol. 2014, 3, 72–78. [Google Scholar] [CrossRef]
- Lapierre, P.; Djilali-Saiah, I.; Vitozzi, S.; Alvarez, F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology 2004, 39, 1066–1074. [Google Scholar] [CrossRef]
- Luong, K.V.; Nguyen, L.T. The role of vitamin d in autoimmune hepatitis. J. Clin. Med. Res. 2013, 5, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.D.; Jiang, S.L.; Liu, C.H.; Hu, Y.Y.; Liu, C.; Sun, M.Y.; Chen, G.F.; Liu, P. Preventive effects of 1,25-(OH)2VD3 against ConA-induced mouse hepatitis through promoting vitamin D receptor gene expression. Acta Pharmacol. Sin. 2010, 31, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Q.; Shi, Y.; Tang, H. New insight of vitamin D in chronic liver diseases. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 580–585. [Google Scholar] [CrossRef]
- Czaja, A.J.; Montano-Loza, A.J. Evolving Role of Vitamin D in Immune-Mediated Disease and Its Implications in Autoimmune Hepatitis. Dig. Dis. Sci. 2019, 64, 324–344. [Google Scholar] [CrossRef]
- Bachelet, M.; Bader, C.; Merlot, A.M.; Laborde, K.; Snarska, J.; Ulmann, A. Cellular utilization of cytosolic NADPH in kidney and liver cells from rats fed a normal or a vitamin D-deficient diet. Cell Biochem. Funct. 1983, 1, 25–29. [Google Scholar] [CrossRef]
- Kumar, V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J. Hepatol. 2013, 59, 618–620. [Google Scholar] [CrossRef]
- Lan, P.; Fan, Y.; Zhao, Y.; Lou, X.; Monsour, H.P.; Zhang, X.; Choi, Y.; Dou, Y.; Ishii, N.; Ghobrial, R.M.; et al. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury. J. Clin. Investig. 2017, 127, 2222–2234. [Google Scholar] [CrossRef]
- Smyk, D.S.; Mavropoulos, A.; Mieli-Vergani, G.; Vergani, D.; Lenzi, M.; Bogdanos, D.P. The Role of Invariant NKT in Autoimmune Liver Disease: Can Vitamin D Act as an Immunomodulator? Can. J. Gastroenterol. Hepatol. 2018, 2018, 8197937. [Google Scholar] [CrossRef]
- Yu, S.; Cantorna, M.T. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J. Immunol. 2011, 186, 1384–1390. [Google Scholar] [CrossRef]
- Bhalla, A.K.; Amento, E.P.; Clemens, T.L.; Holick, M.F.; Krane, S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983, 57, 1308–1310. [Google Scholar] [CrossRef]
- Gascon-Barré, M.; Demers, C.; Mirshahi, A.; Néron, S.; Zalzal, S.; Nanci, A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 2003, 37, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef]
- Firrincieli, D.; Zúñiga, S.; Rey, C.; Wendum, D.; Lasnier, E.; Rainteau, D.; Braescu, T.; Falguières, T.; Boissan, M.; Cadoret, A.; et al. Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice. Hepatology 2013, 58, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Nishida, S.; Ishizawa, M.; Sakurai, K.; Shimizu, M.; Matsuo, S.; Amano, S.; Uno, S.; Makishima, M. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J. Pharmacol. Exp. Ther. 2009, 328, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Goleva, E. Vitamin D enhances glucocorticoid action in human monocytes: Involvement of granulocyte-macrophage colony-stimulating factor and mediator complex subunit 14. J. Biol. Chem. 2013, 288, 14544–14553. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: Molecular mechanisms for gene repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, S.; Dahan-Bachar, L.; Sharvit, E.; Weisman, Y.; Ben Tov, A.; Brazowski, E.; Reif, S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 2011, 60, 1728–1737. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef]
- Abramovitch, S.; Sharvit, E.; Weisman, Y.; Bentov, A.; Brazowski, E.; Cohen, G.; Volovelsky, O.; Reif, S. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G112–G120. [Google Scholar] [CrossRef]
- Potter, J.J.; Liu, X.; Koteish, A.; Mezey, E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1 (I) collagen expression and type I collagen formation. Liver Int. 2013, 33, 677–686. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Meyer, C.; Li, J.; Nadalin, S.; Königsrainer, A.; Weng, H.; Dooley, S.; ten Dijke, P. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J. Biol. Chem. 2013, 288, 30708–30719. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, T.; Du, G.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxyvitamin D3 on the expression of connective tissue growth factor and transforming growth factor-β1 in the myocardium of rats with diabetes. Diabetes Res. Clin. Pract. 2014, 104, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Honsawek, S.; Udomsinprasert, W.; Chirathaworn, C.; Anomasiri, W.; Vejchapipat, P.; Poovorawan, Y. Correlation of connective tissue growth factor with liver stiffness measured by transient elastography in biliary atresia. Hepatol. Res. 2013, 43, 795–800. [Google Scholar] [CrossRef]
- Hochrath, K.; Stokes, C.S.; Geisel, J.; Pollheimer, M.J.; Fickert, P.; Dooley, S.; Lammert, F. Vitamin D modulates biliary fibrosis in ABCB4-deficient mice. Hepatol. Int. 2014, 8, 443–452. [Google Scholar] [CrossRef]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Lytvyak, E.; Mason, A.; Czaja, A.J.; Montano-Loza, A.J. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis. Aliment. Pharmacol. Ther. 2019, 49, 173–182. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Kopilov, R.; Selmi, C.; Nussinovitch, U.; Sánchez-Castañón, M.; López-Hoyos, M.; Amital, H.; Kivity, S.; Gershwin, E.M.; Shoenfeld, Y. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol. Res. 2015, 61, 141–146. [Google Scholar] [CrossRef]
- Kempinska-Podhorodecka, A.; Milkiewicz, M.; Jabłonski, D.; Milkiewicz, P.; Wunsch, E. ApaI polymorphism of vitamin D receptor affects health-related quality of life in patients with primary sclerosing cholangitis. PLoS ONE 2017, 12, e0176264. [Google Scholar] [CrossRef]
- Stokes, C.S.; Krawczyk, M.; Reichel, C.; Lammert, F.; Grünhage, F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur. J. Clin. Investig. 2014, 44, 176–183. [Google Scholar] [CrossRef]
- Efe, C.; Kav, T.; Aydin, C.; Cengiz, M.; Imga, N.N.; Purnak, T.; Smyk, D.S.; Torgutalp, M.; Turhan, T.; Ozenirler, S.; et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig. Dis. Sci. 2014, 59, 3035–3042. [Google Scholar] [CrossRef]
- Guo, G.Y.; Shi, Y.Q.; Wang, L.; Ren, X.; Han, Z.Y.; Guo, C.C.; Cui, L.N.; Wang, J.B.; Zhu, J.; Wang, N.; et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2015, 42, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Strassburg, C.P.; Manns, M.P. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002, 35, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tu, X.; Zhu, Y.; Zhou, L.; Pfeiffer, T.; Feltens, R.; Stoecker, W.; Zhong, R. Genetic association of vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J. Gastroenterol. Hepatol. 2005, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.E.; Cole, D.E.; Rubin, L.A.; Cauch-Dudek, K.; Harewood, L.; Evrovski, J.; Peltekova, V.D.; Heathcote, E.J. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology 2000, 118, 145–151. [Google Scholar] [CrossRef]
- Kempinska-Podhorodecka, A.; Milkiewicz, M.; Wasik, U.; Ligocka, J.; Zawadzki, M.; Krawczyk, M.; Milkiewicz, P. Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. Int. J. Mol. Sci. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Epigenetic changes and their implications in autoimmune hepatitis. Eur. J. Clin. Investig. 2018, 48. [Google Scholar] [CrossRef]
- Fisher, L.; Fisher, A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 513–520. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst. Rev. 2017, 11, CD011564. [Google Scholar] [CrossRef]
| Author (Publication Year) | Type of Study | Number of Enrolled Patients | Type of Supplementation | Main Findings |
|---|---|---|---|---|
| Ruiz Irastorza et al. (2010) | Longitudinal observational | 80 | Cholecalciferol, 600-800 IU day p.o. (24 mos) | Improved fatigue symptoms, no correlation with SLEDAI or SDI. Side effects: not reported |
| Terrier et al. (2012) | Prospective | 20 | Cholecalciferol, 100.000 IU/wk p.o. (4 wks) | Improved naïve CD4+ T cells, regulatory T cells; reduced Th1 and Th17 cells, memory B cells, anti-DNA antibodies. No cases of hypercalcemia |
| Petri et al. (2013) | Prospective | 1006 | Ergocalciferol, 50.000 IU/wk p.o., calcium/vitamin D 200 IU/twice daily p.o. | Reduced SELENA-SLEDAI, decreased urine protein-to-creatinine ratio. Hypercalcemia rate, 0.002% |
| Andreoli et al. (2015) | Prospective, cross-over | 34 | Cholecalciferol Intensive Regimen: 300.000 IU bolus plus 50.000 IU/mo p.o. (850.000 IU/yr). Standard Regimen: 25.000 IU/mo p.o. (300.000 IU/yr) for 12 mos. Regimens switched in the second year. | No effect on disease activity and SLE serology. No cases of hypercalcemia. Slight transient hypercalciuria in 3 |
| Piantoni et al. (2015) | Prospective, cross-over | 34 | Cholecalciferol Intensive Regimen: 300.000 IU bolus plus 50.000 IU/mo p.o. (850.000 IU/yr). Standard Regimen: 25.000 IU/mo p.o. (300.000 IU/yr) for 12 mos. Regimens switched in the second year. | Enhancement of T-reg cells and Th2 cytokines. No cases of hypercalcemia |
| Aranow et al. (2015) | Randomized, double blind, placebo controlled | 57 | Cholecalciferol, 2.000 or 4.000 IU/d p.o. | Well-tolerated. No effect on IFN-alpha. No cases of hypercalcemia |
| Lima et al. (2016) | Randomized, double blind, placebo controlled | 40 (JoSLE) | Cholecalciferol, 5000 IU/wk p.o. | Decreased disease activity and improved fatigue symptoms in JoSLE patients. No cases of hypercalcemia |
| Rifa’i et al. (2016) | Randomized, placebo controlled | 39 | Cholecalciferol, 1.200 IU/d p.o. | Decreased SLE disease activity and fatigue symptoms. Side effects: not reported |
| Karimzadeh et al. (2017) | Randomized, double blind, placebo controlled | 90 | Cholecalciferol, 50.000 IU/wk p.o. for 12 wks and 50.000 IU/mo p.o. for 6 mos. | No effect on SLE disease activity. Side effects: not reported |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. https://doi.org/10.3390/nu12030789
Bellan M, Andreoli L, Mele C, Sainaghi PP, Rigamonti C, Piantoni S, De Benedittis C, Aimaretti G, Pirisi M, Marzullo P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients. 2020; 12(3):789. https://doi.org/10.3390/nu12030789
Chicago/Turabian StyleBellan, Mattia, Laura Andreoli, Chiara Mele, Pier Paolo Sainaghi, Cristina Rigamonti, Silvia Piantoni, Carla De Benedittis, Gianluca Aimaretti, Mario Pirisi, and Paolo Marzullo. 2020. "Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases" Nutrients 12, no. 3: 789. https://doi.org/10.3390/nu12030789
APA StyleBellan, M., Andreoli, L., Mele, C., Sainaghi, P. P., Rigamonti, C., Piantoni, S., De Benedittis, C., Aimaretti, G., Pirisi, M., & Marzullo, P. (2020). Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients, 12(3), 789. https://doi.org/10.3390/nu12030789









