Physical Activity, Nutritional Status, and Autonomic Nervous System Activity in Healthy Young Adults with Higher Levels of Depressive Symptoms and Matched Controls without Depressive Symptoms: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Depressive Symptoms
2.3. Exercise Habits
2.4. Nutritional Status Assessment
2.5. Measurement of PA
2.6. Consumption of TRP and/or Vitamin B6-Rich Food
2.7. Blood Investigations
2.8. Measurement of ANS Activity
2.9. Experimental Procedures
2.10. Statistical Analysis
3. Results
3.1. Physical Characteristics
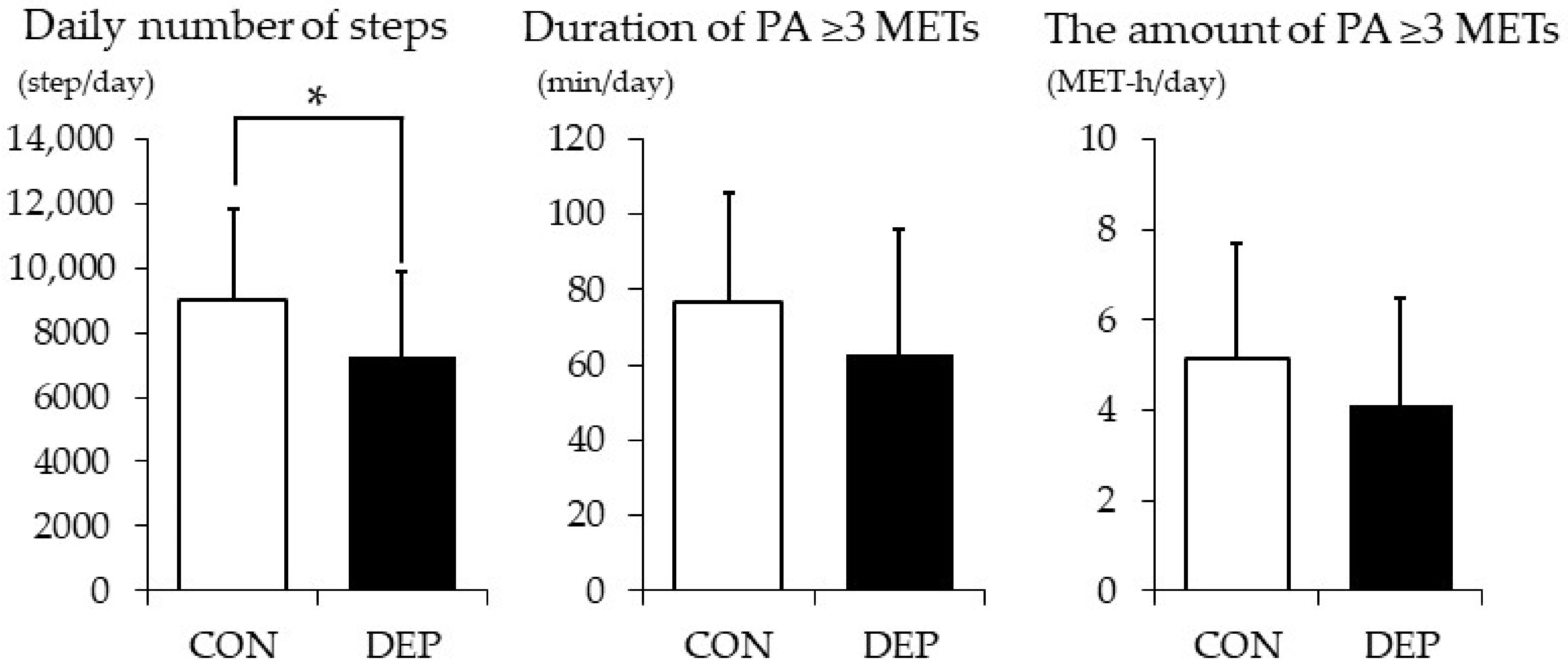
3.2. PA
3.3. Consumption of TRP and/or Vitamin B6-Rich Food
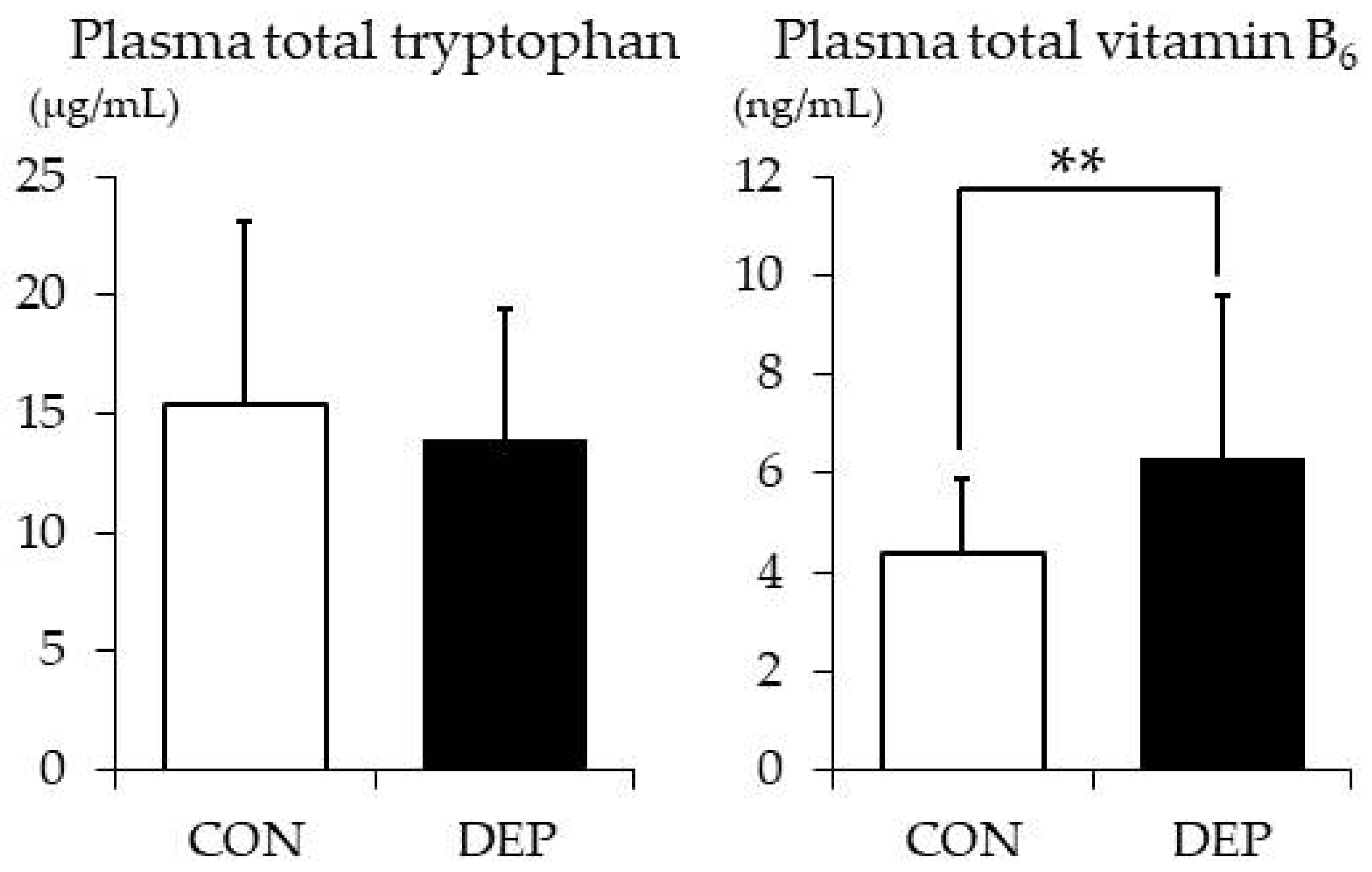
3.4. Plasma TRP and Vitamin B6 Levels
3.5. ANS activity
3.6. Coefficient of Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression. WHO Factsheet. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 14 April 2018).
- Tomoda, A.; Mori, K.; Kimura, M.; Takahashi, T.; Kitamura, T. One-year prevalence and incidence of depression among first-year university students in Japan: A preliminary study. Psychiatry Clin. Neurosci. 2000, 54, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Lewinsohn, P.M.; Seeley, J.R. Symptoms versus a diagnosis of depression: Differences in psychosocial functioning. J. Consult. Clin. Psychol. 1995, 63, 90–100. [Google Scholar] [CrossRef]
- Harrington, R.; Fudge, H.; Rutter, M.; Pickles, A.; Hill, J. Adult outcomes of childhood and adolescent depression. I. Psychiatric status. Arch. Gen. Psychiatry 1990, 47, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Slade, T.; Johnston, A.; Oakley Browne, M.A.; Andrews, G.; Whiteford, H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust. N. Z. J. Psychiatry 2009, 43, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Mammen, G.; Faulkner, G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Stubbs, B.; Vancampfort, D.; Lundin, A.; Jaakallio, P.; Forsell, Y. Treatment guidelines for depression: Greater emphasis on physical activity is needed. Eur. Psychiatry 2017, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; De Leon, A.P.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef]
- Klaassen, T.; Riedel, W.J.; Van Someren, A.; Deutz, N.E.; Honig, A.; Van Praag, H.M. Mood effects of 24-hour tryptophan depletion in healthy first-degree relatives of patients with affective disorders. Biol. Psychiatry 1999, 46, 489–497. [Google Scholar] [CrossRef]
- Shabbir, F.; Patel, A.; Mattison, C.; Bose, S.; Krishnamohan, R.; Sweeney, E.; Sandhu, S.; Nel, W.; Rais, A.; Sandhu, R.; et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem. Int. 2013, 62, 324–329. [Google Scholar] [CrossRef]
- Hvas, A.M.; Juul, S.; Bech, P.; Nexo, E. Vitamin B6 level is associated with symptoms of depression. Psychother. Psychosom. 2004, 73, 340–343. [Google Scholar] [CrossRef]
- Tulen, J.H.; Bruijn, J.A.; de Man, K.J.; van der Velden, E.; Pepplinkhuizen, L.; Man in ‘t Veld, A.J. Anxiety and autonomic regulation in major depressive disorder: An exploratory study. J. Affect. Disord. 1996, 40, 61–71. [Google Scholar] [CrossRef][Green Version]
- Veith, R.C.; Lewis, N.; Linares, O.A.; Barnes, R.F.; Raskind, M.A.; Villacres, E.C.; Murburg, M.M.; Ashleigh, E.A.; Castillo, S.; Peskind, E.R.; et al. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch. Gen. Psychiatry 1994, 51, 411–422. [Google Scholar] [CrossRef] [PubMed]
- De Meersman, R.E.; Stein, P.K. Vagal modulation and aging. Biol. Psychol. 2007, 74, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.T.; Giardina, E.G.; Sloan, R.P.; Greenhill, L.; Goldfein, J. Effects of desipramine on autonomic control of the heart. J. Am. Acad. Child. Adolesc. Psychiatry 1994, 33, 191–197. [Google Scholar] [CrossRef]
- Nagai, N.; Hamada, T.; Kimura, T.; Moritani, T. Moderate physical exercise increases cardiac autonomic nervous system activity in children with low heart rate variability. Childs Nerv. Syst. 2004, 20, 209–214. [Google Scholar] [CrossRef]
- Matsumoto, T.; Miyawaki, C.; Ue, H.; Kanda, T.; Yoshitake, Y.; Moritani, T. Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes. Res. 2001, 9, 78–85. [Google Scholar] [CrossRef]
- Oida, E.; Moritani, T.; Yamori, Y. Tone-entropy analysis on cardiac recovery after dynamic exercise. J. Appl. Physiol. 1997, 82, 1794–1801. [Google Scholar] [CrossRef]
- Ue, H.; Masuda, I.; Yoshitake, Y.; Inazumi, T.; Moritani, T. Assessment of Cardiac Autonomic Nervous Activities by Means of ECG R-R Interval Power Spectral Analysis and Cardiac Depolarization-Repolarization Process. Ann. Noninvasive Electrocardiol. 2000, 5, 336–345. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef]
- Hattori, S.; Kishida, I.; Suda, A.; Kawanishi, C.; Miyauchi, M.; Shiraishi, Y.; Fujibayashi, M.; Tsujita, N.; Ishii, C.; Moritani, T.; et al. A return to work program improves parasympathetic activity and psychiatric symptoms in workers on sick leave due to depression. Heliyon 2019, 5, e02151. [Google Scholar] [CrossRef]
- Shima, S.; Shikano, T.; Kitamura, T.; Asai, M. New self-rating scales for depression. Clin. Psychiatry 1985, 27, 717–723. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psych. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Takahashi, K.; Yoshimura, Y.; Kaimoto, T.; Kunii, D.; Komatsu, T.; Yamamoto, S. Validation of a Food Frequency Questionnaire based on food groups for estimating individual nutrient intake. Jpn. J. Nutr. Diet. 2001, 59, 221–232. [Google Scholar] [CrossRef]
- Science and Technology Agency. Standard Tables of Food Composition in Japan; 7th Revis. ed.; Printing Bureau of the Ministry of Finance: Tokyo, Japan, 2015.
- Yoshiuchi, K.; Nakahara, R.; Kumano, H.; Kuboki, T.; Togo, F.; Watanabe, E.; Yasunaga, A.; Park, H.; Shephard, R.J.; Aoyagi, Y. Yearlong physical activity and depressive symptoms in older Japanese adults: Cross-sectional data from the Nakanojo study. Am. J. Geriatr. Psychiatry 2006, 14, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Van Ravenswaaij-Arts, C.M.; Kollee, L.A.; Hopman, J.C.; Stoelinga, G.B.; Van Geijn, H.P. Heart rate variability. Ann. Intern. Med. 1993, 118, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Rompelman, O.; Coenen, A.J.; Kitney, R.I. Measurement of heart-rate variability: Part 1-Comparative study of heart-rate variability analysis methods. Med. Biol. Eng. Comput. 1977, 15, 233–239. [Google Scholar] [CrossRef]
- Moritani, T.; Hayashi, T.; Shinohara, M.; Mimasa, F.; Shibata, M. Comparison of Sympatho-Vagal Function among Diabetic patients, Normal Controls and Endurance Athletes by Heart Rate Spectral Analysis. J. Sports Sci. Med. 1993, 7, 31–39. [Google Scholar]
- Anderson, I.M.; Parry-Billings, M.; Newsholme, E.A.; Poortmans, J.R.; Cowen, P.J. Decreased plasma tryptophan concentration in major depression: Relationship to melancholia and weight loss. J. Affect. Disord. 1990, 20, 185–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare. Exercise and Physical Activity Reference for Health Promotion 2013 [in Japanese]. Available online: http://www.mhlw.go.jp/stf/houdou/2r9852000002xple-att/2r9852000002xpqt.pdf (accessed on 20 October 2018).
- McKercher, C.M.; Schmidt, M.D.; Sanderson, K.A.; Patton, G.C.; Dwyer, T.; Venn, A.J. Physical activity and depression in young adults. Am. J. Prev. Med. 2009, 36, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S., Jr.; Lee, I.M.; Leung, R. Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatr. Scand. Suppl. 1994, 377, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.L.; Trivedi, M.H.; Kampert, J.B.; Clark, C.G.; Chambliss, H.O. Exercise treatment for depression: Efficacy and dose response. Am. J. Prev. Med. 2005, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aaron, D.J.; Jekal, Y.S.; LaPorte, R.E. Epidemiology of physical activity from adolescence to young adulthood. World Rev. Nutr. Diet. 2005, 94, 36–41. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Oldendorf, W.H. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim. Biophys. Acta 1975, 401, 128–136. [Google Scholar] [CrossRef]
- Lyons, P.M.; Truswell, A.S. Serotonin precursor influenced by type of carbohydrate meal in healthy adults. Am. J. Clin. Nutr. 1988, 47, 433–439. [Google Scholar] [CrossRef]
- Ogawa, S.; Koga, N.; Hattori, K.; Matsuo, J.; Ota, M.; Hori, H.; Sasayama, D.; Teraishi, T.; Ishida, I.; Yoshida, F.; et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case-control sample sets. J. Psychiatr. Res. 2018, 96, 23–32. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Verkerk, R.; Rief, W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro Endocrinol. Lett. 2011, 32, 264–273. [Google Scholar]
- Anderson, G.; Berk, M.; Maes, M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr. Scand. 2014, 129, 83–97. [Google Scholar] [CrossRef]
- Tsujita, N.; Akamatsu, Y.; Nishida, M.M.; Hayashi, T.; Moritani, T. Effect of Tryptophan, Vitamin B6, and Nicotinamide-Containing Supplement Loading between Meals on Mood and Autonomic Nervous System Activity in Young Adults with Subclinical Depression: A Randomized, Double-Blind, and Placebo-Controlled Study. J. Nutr. Sci. Vitaminol. 2019, 65, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Lehofer, M.; Hoehn-Saric, R.; McLeod, D.R.; Hildebrandt, G.; Steinbrenner, B.; Voica, M.; Liebmann, P.; Zapotoczky, H.G. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? J. Affect. Disord. 1998, 48, 115–124. [Google Scholar] [CrossRef]
- Rechlin, T.; Claus, D.; Weis, M. Heart rate analysis in 24 patients treated with 150 mg amitriptyline per day. Psychopharmacology 1994, 116, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.W.; Stoney, C.M. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosom. Med. 2000, 62, 796–803. [Google Scholar] [CrossRef]
- Mestanikova, A.; Mestanik, M.; Ondrejka, I.; Hrtanek, I.; Cesnekova, D.; Jurko, A., Jr.; Visnovcova, Z.; Sekaninova, N.; Tonhajzerova, I. Complex cardiac vagal regulation to mental and physiological stress in adolescent major depression. J. Affect. Disord. 2019, 249, 234–241. [Google Scholar] [CrossRef]
| CON | DEP | t Value | Cohen’s d | p Value | |||
|---|---|---|---|---|---|---|---|
| n = 35 | n = 35 | ||||||
| Age (years) | 20.3 | (1.6) | 20.5 | (2.1) | −0.575 | −0.11 | 0.567 |
| Body mass (kg) | 54.3 | (9.2) | 54.5 | (10.8) | −0.050 | 0.02 | 0.960 |
| BMI (kg/m2) | 20.3 | (3.2) | 20.0 | (2.1) | 0.484 | −0.11 | 0.630 |
| Body fat (%) | 21.8 | (6.7) | 22.1 | (6.6) | −0.210 | 0.05 | 0.834 |
| Exercise (times/week) | 1.2 | (1.8) | 1.5 | (2.2) | −0.685 | 0.16 | 0.496 |
| Resting heart rate (bpm) | 69.8 | (9.6) | 69.3 | (9.6) | 0.224 | −0.06 | 0.823 |
| Energy intake (kcal/day) | 1815 | (433) | 1839 | (452) | −0.251 | 0.06 | 0.820 |
| CES-D scores | 3.9 | (2.2) | 25.1 | (7.4) | −16.125 | 3.86 | 0.000 |
| Tryptophan Contents (mg) | Vitamin B6 Contents (mg) | Almost Never | Twice in a Week | Once in Two Days | Almost Every Day | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooked rice | 35 | 0.02 | CON | 0 | (0%) | 1 | (3%) | 3 | (9%) | 31 | (89%) | 0.236 |
| DEP | 0 | (0%) | 3 | (9%) | 7 | (20%) | 25 | (71%) | ||||
| Bread | 95 | 0.03 | CON | 7 | (20%) | 15 | (43%) | 5 | (14%) | 8 | (23%) | 0.461 |
| DEP | 8 | (23%) | 9 | (26%) | 7 | (20%) | 11 | (31%) | ||||
| Soybean curd (Tofu) | 98 | 0.05 | CON | 3 | (9%) | 14 | (40%) | 13 | (37%) | 5 | (14%) | 0.930 |
| DEP | 3 | (9%) | 12 | (34%) | 16 | (46%) | 4 | (11%) | ||||
| Soy milk | 53 | 0.06 | CON | 30 | (86%) | 2 | (6%) | 2 | (6%) | 1 | (3%) | 0.536 |
| DEP | 25 | (71%) | 3 | (9%) | 4 | (11%) | 3 | (9%) | ||||
| Fermented soybeans | 240 | 0.24 | CON | 23 | (66%) | 9 | (26%) | 1 | (3%) | 2 | (6%) | 0.386 |
| DEP | 17 | (49%) | 11 | (31%) | 4 | (11%) | 3 | (9%) | ||||
| Nuts | 360 | 0.36 | CON | 29 | (83%) | 5 | (14%) | 1 | (3%) | 0 | (0%) | 0.355 |
| DEP | 23 | (66%) | 10 | (29%) | 2 | (6%) | 0 | (0%) | ||||
| Chestnuts | 57 | 0.37 | CON | 33 | (94%) | 2 | (6%) | 0 | (0%) | 0 | (0%) | 0.151 |
| DEP | 28 | (80%) | 6 | (17%) | 1 | (3%) | 0 | (0%) | ||||
| Spinach | 41 | 0.14 | CON | 14 | (40%) | 17 | (49%) | 4 | (11%) | 0 | (0%) | 0.444 |
| DEP | 9 | (26%) | 21 | (60%) | 5 | (14%) | 0 | (0%) | ||||
| Green soybeans | 150 | 0.15 | CON | 27 | (77%) | 7 | (20%) | 1 | (3%) | 0 | (0%) | 1.000 |
| DEP | 26 | (74%) | 8 | (23%) | 1 | (3%) | 0 | (0%) | ||||
| Green peppers | 11 | 0.19 | CON | 10 | (29%) | 16 | (46%) | 9 | (26%) | 0 | (0%) | 0.403 |
| DEP | 14 | (40%) | 13 | (37%) | 8 | (23%) | 0 | (0%) | ||||
| Avocado | 34 | 0.32 | CON | 26 | (74%) | 7 | (20%) | 2 | (6%) | 0 | (0%) | 1.000 |
| DEP | 26 | (74%) | 6 | (17%) | 2 | (6%) | 1 | (3%) | ||||
| Kiwi fruit | 14 | 0.12 | CON | 27 | (77%) | 7 | (20%) | 1 | (3%) | 0 | (0%) | 0.752 |
| DEP | 30 | (86%) | 4 | (11%) | 1 | (3%) | 0 | (0%) | ||||
| Bananas | 10 | 0.38 | CON | 19 | (54%) | 8 | (23%) | 6 | (17%) | 2 | (6%) | 0.747 |
| DEP | 19 | (54%) | 10 | (29%) | 3 | (9%) | 3 | (9%) | ||||
| Bonito | 300 | 0.76 | CON | 29 | (83%) | 4 | (11%) | 2 | (6%) | 0 | (0%) | 0.006 |
| DEP | 17 | (49%) | 14 | (40%) | 4 | (11%) | 0 | (0%) | ||||
| Tuna | 300 | 0.85 | CON | 26 | (74%) | 8 | (23%) | 1 | (3%) | 0 | (0%) | 0.104 |
| DEP | 18 | (51%) | 15 | (43%) | 2 | (6%) | 0 | (0%) | ||||
| Salmon | 250 | 0.64 | CON | 20 | (57%) | 14 | (40%) | 1 | (3%) | 0 | (0%) | 0.319 |
| DEP | 21 | (60%) | 10 | (29%) | 4 | (11%) | 0 | (0%) | ||||
| Prawns | 190 | 0.12 | CON | 23 | (66%) | 9 | (26%) | 3 | (9%) | 0 | (0%) | 1.000 |
| DEP | 22 | (63%) | 9 | (26%) | 3 | (9%) | 1 | (3%) | ||||
| Pacific saury | 220 | 0.51 | CON | 27 | (77%) | 7 | (20%) | 1 | (3%) | 0 | (0%) | 0.022 |
| DEP | 17 | (49%) | 17 | (49%) | 1 | (3%) | 0 | (0%) | ||||
| Horse mackerel | 220 | 0.30 | CON | 30 | (86%) | 4 | (11%) | 1 | (3%) | 0 | (0%) | 0.038 |
| DEP | 21 | (60%) | 12 | (34%) | 2 | (6%) | 0 | (0%) | ||||
| Mackerel | 230 | 0.59 | CON | 30 | (86%) | 4 | (11%) | 1 | (3%) | 0 | (0%) | 0.001 |
| DEP | 16 | (46%) | 16 | (46%) | 3 | (9%) | 0 | (0%) | ||||
| Beef round | 240 | 0.44 | CON | 14 | (40%) | 12 | (34%) | 8 | (23%) | 1 | (3%) | 0.108 |
| DEP | 8 | (23%) | 21 | (60%) | 6 | (17%) | 0 | (0%) | ||||
| Beef liver | 290 | 0.89 | CON | 34 | (97%) | 0 | (0%) | 1 | (3%) | 0 | (0%) | 0.025 |
| DEP | 29 | (83%) | 6 | (17%) | 0 | (0%) | 0 | (0%) | ||||
| Pork ham | 240 | 0.37 | CON | 4 | (11%) | 14 | (40%) | 14 | (40%) | 3 | (9%) | 0.559 |
| DEP | 2 | (6%) | 11 | (31%) | 20 | (57%) | 2 | (6%) | ||||
| Chicken breast | 230 | 0.35 | CON | 5 | (14%) | 18 | (51%) | 11 | (31%) | 1 | (3%) | 0.735 |
| DEP | 3 | (9%) | 17 | (49%) | 12 | (34%) | 3 | (9%) | ||||
| Chicken liver | 270 | 0.65 | CON | 33 | (94%) | 2 | (6%) | 0 | (0%) | 0 | (0%) | 0.023 |
| DEP | 25 | (71%) | 10 | (29%) | 0 | (0%) | 0 | (0%) | ||||
| Milk | 45 | 0.03 | CON | 9 | (26%) | 7 | (20%) | 5 | (14%) | 14 | (40%) | 0.336 |
| DEP | 7 | (20%) | 10 | (29%) | 9 | (26%) | 8 | (23%) | ||||
| Yoghurt | 48 | 0.04 | CON | 4 | (11%) | 16 | (46%) | 9 | (26%) | 6 | (17%) | 0.339 |
| DEP | 9 | (26%) | 11 | (31%) | 11 | (31%) | 4 | (11%) | ||||
| Cheese | 290 | 0.01 | CON | 10 | (29%) | 15 | (43%) | 7 | (20%) | 3 | (9%) | 0.763 |
| DEP | 7 | (20%) | 14 | (40%) | 10 | (29%) | 4 | (11%) | ||||
| Potato chips | 58 | 0.54 | CON | 29 | (83%) | 6 | (17%) | 0 | (0%) | 0 | (0%) | 0.486 |
| DEP | 26 | (74%) | 7 | (20%) | 2 | (6%) | 0 | (0%) | ||||
| Fried potato | 34 | 0.35 | CON | 27 | (77%) | 7 | (20%) | 1 | (3%) | 0 | (0%) | 0.678 |
| DEP | 24 | (69%) | 9 | (26%) | 2 | (6%) | 0 | (0%) | ||||
| CON | DEP | t Value | Cohen’s d | p Value | |||
|---|---|---|---|---|---|---|---|
| n = 35 | n = 35 | ||||||
| LF power (ln ms2) | 5.84 | (1.00) | 6.28 | (1.12) | −1.71 | 0.37 | 0.092 |
| HF power (ln ms2) | 5.48 | (1.01) | 5.87 | (1.08) | −1.49 | 0.34 | 0.142 |
| Total power (HF + LF) (ln ms2) | 6.46 | (0.91) | 6.88 | (1.03) | −1.78 | 0.40 | 0.080 |
| LF/HF | 2.04 | (1.74) | 2.19 | (1.88) | −0.346 | 0.04 | 0.730 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujita, N.; Akamatsu, Y.; Nishida, M.M.; Hayashi, T.; Moritani, T. Physical Activity, Nutritional Status, and Autonomic Nervous System Activity in Healthy Young Adults with Higher Levels of Depressive Symptoms and Matched Controls without Depressive Symptoms: A Cross-Sectional Study. Nutrients 2020, 12, 690. https://doi.org/10.3390/nu12030690
Tsujita N, Akamatsu Y, Nishida MM, Hayashi T, Moritani T. Physical Activity, Nutritional Status, and Autonomic Nervous System Activity in Healthy Young Adults with Higher Levels of Depressive Symptoms and Matched Controls without Depressive Symptoms: A Cross-Sectional Study. Nutrients. 2020; 12(3):690. https://doi.org/10.3390/nu12030690
Chicago/Turabian StyleTsujita, Natsuki, Yasunori Akamatsu, Márcio Makoto Nishida, Tatsuya Hayashi, and Toshio Moritani. 2020. "Physical Activity, Nutritional Status, and Autonomic Nervous System Activity in Healthy Young Adults with Higher Levels of Depressive Symptoms and Matched Controls without Depressive Symptoms: A Cross-Sectional Study" Nutrients 12, no. 3: 690. https://doi.org/10.3390/nu12030690
APA StyleTsujita, N., Akamatsu, Y., Nishida, M. M., Hayashi, T., & Moritani, T. (2020). Physical Activity, Nutritional Status, and Autonomic Nervous System Activity in Healthy Young Adults with Higher Levels of Depressive Symptoms and Matched Controls without Depressive Symptoms: A Cross-Sectional Study. Nutrients, 12(3), 690. https://doi.org/10.3390/nu12030690





