Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Search Eligibility Criteria
2.3. Selection of Studies and Interventions of Interest
2.4. Data Exctraction
3. Results
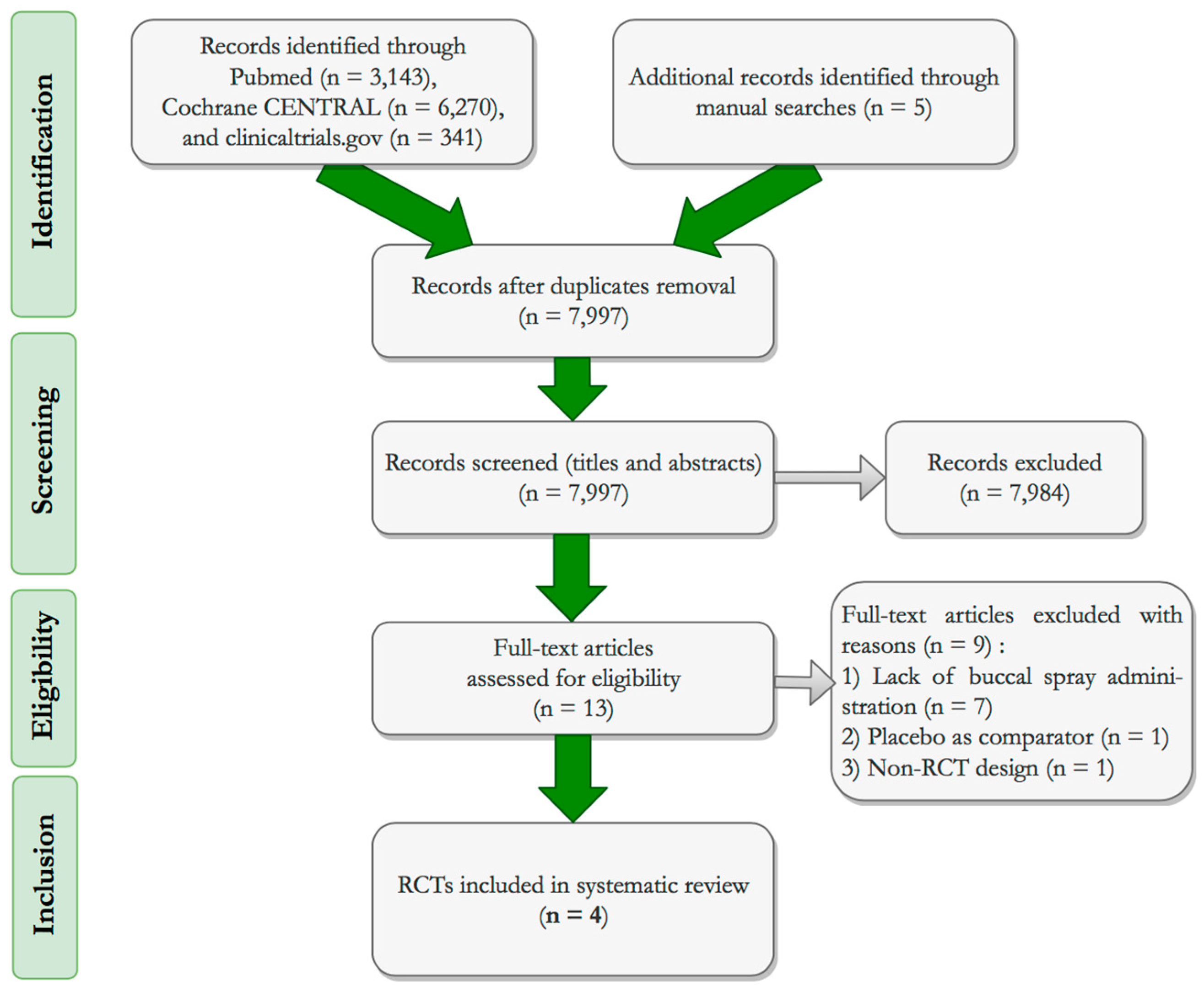
3.1. Study Selection
3.2. Risk of Bias and Quality Assessment of Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiting, S.J.; Calvo, M.S.; Vatanparast, H. Current Understanding of Vitamin D Metabolism, Nutritional Status, and Role in Disease Prevention. In Nutrition in the Prevention and Treatment of Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 937–967. [Google Scholar]
- Park, J.E.; Pichiah, P.B.T.; Cha, Y.-S. Vitamin D and Metabolic Diseases: Growing Roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grübler, M.R.; Verheyen, N.D.; et al. The Effect of Vitamin D Supplementation on its Metabolism and the Vitamin D Metabolite Ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D: A Micronutrient Regulating Genes. Curr. Pharm. Des. 2019, 25, 1740–1746. [Google Scholar] [CrossRef]
- Christakos, S.; Li, S.; De La Cruz, J.; Bikle, D.D. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism 2019, 98, 112–120. [Google Scholar] [CrossRef]
- Anastasiou, A.; Karras, S.N.; Bais, A.; Grant, W.B.; Kotsa, K.; Goulis, D.G. Ultraviolet radiation and effects on humans: The paradigm of maternal vitamin D production during pregnancy. Eur. J. Clin. Nutr. 2017, 71, 1268–1272. [Google Scholar] [CrossRef]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Kim, H.K.; Isakovic, A.F.; Gater, D.L.; Kim, Y.-C. Therapeutic vitamin delivery: Chemical and physical methods with future directions. J. Control. Release 2019, 298, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Paschou, S.A.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D.G. Hypovitaminosis D in pregnancy in the Mediterranean region: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Anagnostis, P.; Naughton, D.; Annweiler, C.; Petroczi, A.; Goulis, D.G. Vitamin D during pregnancy: Why observational studies suggest deficiency and interventional studies show no improvement in clinical outcomes? A narrative review. J. Endocrinol. Invest. 2015, 38, 1265–1275. [Google Scholar] [CrossRef]
- Karras, S.; Anagnostis, P.; Petroczi, A.; Annweiler, C.; Naughton, D.; Goulis, D. Maternal vitamin D status in pregnancy: A critical appraisal of current analytical data on maternal and neonatal outcomes. Hormones 2015, 14, 224–231. [Google Scholar] [CrossRef]
- Janbek, J.; Specht, I.O.; Heitmann, B.L. Associations between vitamin D status in pregnancy and offspring neurodevelopment: A systematic literature review. Nutr. Rev. 2019, 77, 330–349. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2019. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 10, CD013446. [Google Scholar] [CrossRef]
- Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019, 11, 2384. [Google Scholar] [CrossRef]
- Ruggiero, C.; Baroni, M.; Bini, V.; Brozzetti, A.; Parretti, L.; Zengarini, E.; Lapenna, M.; Antinolfi, P.; Falorni, A.; Mecocci, P.; et al. Effects of Weekly Supplementation of Cholecalciferol and Calcifediol Among the Oldest-Old People: Findings From a Randomized Pragmatic Clinical Trial. Nutrients 2019, 11, 2778. [Google Scholar] [CrossRef]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina (B. Aires) 2019, 55, 368. [Google Scholar] [CrossRef] [PubMed]
- Bassatne, A.; Chakhtoura, M.; Saad, R.; Fuleihan, G.E.-H. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism 2019, 92, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.C.; Carrilho, T.R.B.; Batalha, M.A.; Farias, D.R.; Barros, E.G.; Kac, G. Association between vitamin D status during pregnancy and total gestational weight gain and postpartum weight retention: A prospective cohort. Eur. J. Clin. Nutr. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Bosdou, J.; Konstantinidou, E.; Anagnostis, P.; Kolibianakis, E.; Goulis, D. Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients 2019, 11, 1455. [Google Scholar] [CrossRef]
- Hosseini Marnani, E.; Mollahosseini, M.; Gheflati, A.; Ghadiri-Anari, A.; Nadjarzadeh, A. The effect of vitamin D supplementation on the androgenic profile in men: A systematic review and meta-analysis of clinical trials. Andrologia 2019, 51, e13343. [Google Scholar] [CrossRef]
- Anagnostis, P.; Paschou, S.A.; Goulis, D.G. Calcium and Vitamin D Supplements and Fractures in Community-Dwelling Adults. JAMA 2018, 319, 2041. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 1716. [Google Scholar] [CrossRef]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Anagnostis, P.; Paschou, S.A.; Goulis, D.G. Vitamin D Supplementation and Cardiovascular Disease Risk. JAMA Cardiol. 2017, 2, 1281. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials. JAMA Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Mavropoulos, A.; Mieli-Vergani, G.; Vergani, D.; Lenzi, M.; Bogdanos, D.P. The Role of Invariant NKT in Autoimmune Liver Disease: Can Vitamin D Act as an Immunomodulator? Can. J. Gastroenterol. Hepatol. 2018, 2018, 8197937. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Orfanidou, T.; Invernizzi, P.; Bogdanos, D.P.; Lenzi, M. Vitamin D in autoimmune liver disease. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Kav, T.; Aydin, C.; Cengiz, M.; Imga, N.N.; Purnak, T.; Smyk, D.S.; Torgutalp, M.; Turhan, T.; Ozenirler, S.; et al. Low Serum Vitamin D Levels Are Associated with Severe Histological Features and Poor Response to Therapy in Patients with Autoimmune Hepatitis. Dig. Dis. Sci. 2014, 59, 3035–3042. [Google Scholar] [CrossRef]
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and Safety of Vitamin D Supplementation in Patients With Systemic Lupus Erythematosus: A Meta-analysis of Randomized Controlled Trials. Am. J. Med. Sci. 2019, 358, 104–114. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst. Rev. 2017, 11, CD011564. [Google Scholar] [CrossRef]
- Yodoshi, T.; Orkin, S.; Arce-Clachar, A.C.; Bramlage, K.; Liu, C.; Fei, L.; El-Khider, F.; Dasarathy, S.; Xanthakos, S.A.; Mouzaki, M. Vitamin D deficiency: Prevalence and association with liver disease severity in pediatric nonalcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; Bethesda: Rockville, MD, USA, 2011. [Google Scholar]
- Paxton, G.A.; Teale, G.R.; Nowson, C.A.; Mason, R.S.; McGrath, J.J.; Thompson, M.J.; Siafarikas, A.; Rodda, C.P.; Munns, C.F.; Australian and New Zealand Bone and Mineral Society; et al. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: A position statement. Med. J. Aust. 2013, 198, 142–143. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health and Care Excellence. Vitamin D: Supplement Use in Specific Population Groups. Public health guideline. 2014. Available online: https://www.nice.org.uk/guidance/ph56/resources/vitamin-d-supplement-use-in-specific-population-groups-pdf-1996421765317 (accessed on 19 November 2019).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.; del Forno, F.; Piacentini, G.; Pietrobelli, A. Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences. Nutrients 2018, 10, 1934. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2019, 105489. [Google Scholar] [CrossRef]
- Mentaverri, R.; Souberbielle, J.-C.; Brami, G.; Daniel, C.; Fardellone, P. Pharmacokinetics of a New Pharmaceutical Form of Vitamin D3 100,000 IU in Soft Capsule. Nutrients 2019, 11, 703. [Google Scholar] [CrossRef]
- Wagner, C.L.; Shary, J.R.; Nietert, P.J.; Wahlquist, A.E.; Ebeling, M.D.; Hollis, B.W. Bioequivalence Studies of Vitamin D Gummies and Tablets in Healthy Adults: Results of a Cross-Over Study. Nutrients 2019, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Satia, M.; Mukim, A.; Tibrewala, K.; Bhavsar, M. A randomized two way cross over study for comparison of absorption of vitamin D3 buccal spray and soft gelatin capsule formulation in healthy subjects and in patients with intestinal malabsorption. Nutr. J. 2015, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Fineout-Overholt, E.; Johnston, L. Teaching EBP: Asking Searchable, Answerable Clinical Questions. Worldviews Evidence-Based Nurs. 2005, 2, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.; Eldridge, S.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; McSorley, E.M.; Pourshahidi, L.K.; Madigan, S.M.; Laird, E.; Healy, M.; Magee, P.J. Vitamin D3 supplementation in healthy adults: A comparison between capsule and oral spray solution as a method of delivery in a wintertime, randomised, open-label, cross-over study. Br. J. Nutr. 2016, 116, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Borsani, B.; Maruca, K.; Giosia, V.; Bova, S.; Mastrangelo, M.; Zuccotti, G.V.; Mora, S. Short-Term Vitamin D Supplementation in Children with Neurodisabilities: Comparison of Two Delivery Methods. Horm. Res. Paediatr. 2017, 88, 281–284. [Google Scholar] [CrossRef]
- Williams, C.E.; Williams, E.A.; Corfe, B.M. Rate of change of circulating 25-hydroxyvitamin D following sublingual and capsular vitamin D preparations. Eur. J. Clin. Nutr. 2019, 73, 1630–1635. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. coNSorT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 332. [Google Scholar] [CrossRef]
- Secnn, S.; D’Angelo, G.; Potvin, D. Carry-over in cross-over trials in bioequivalence: Theoretical concerns and empirical evidence. Pharm. Stat. 2004, 3, 133–142. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioequivalence Testing; Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2006. [Google Scholar]
- Food and Drug Administration. Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA; Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2013. [Google Scholar]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Jones, G. Interpreting vitamin D assay results: Proceed with caution. Clin. J. Am. Soc. Nephrol. 2015, 10, 331–334. [Google Scholar] [CrossRef]
- Farrell, C.-J.L.; Martin, S.; McWhinney, B.; Straub, I.; Williams, P.; Herrmann, M. State-of-the-Art Vitamin D Assays: A Comparison of Automated Immunoassays with Liquid Chromatography–Tandem Mass Spectrometry Methods. Clin. Chem. 2012, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Anderson, D.; Clarke, M.W.; Ponsonby, A.-L.; Lucas, R.M.; Group, A.I. Analytical Bias in the Measurement of Serum 25-Hydroxyvitamin D Concentrations Impairs Assessment of Vitamin D Status in Clinical and Research Settings. PLoS ONE 2015, 10, e0135478. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Mott, L.A.; Barry, E.L.; Baron, J.A.; Bostick, R.M.; Figueiredo, J.C.; Bresalier, R.S.; Robertson, D.J.; Peacock, J.L. Lifestyle and Other Factors Explain One-Half of the Variability in the Serum 25-Hydroxyvitamin D Response to Cholecalciferol Supplementation in Healthy Adults. J. Nutr. 2016, 146, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Genetic and environmental determinants of vitamin D status. Lancet 2010, 376, 148–149. [Google Scholar] [CrossRef]
- Paschou, S.A.; Anagnostis, P.G.; Muscogiuri, G.; Goulis, D.G.; Vryonidou, A. Letter to the Editor: Genetics and Vitamin D Supplementation in Pregnancy. J. Clin. Endocrinol. Metab. 2017, 102, 3563–3564. [Google Scholar] [CrossRef][Green Version]
- Moon, R.J.; Harvey, N.C.; Cooper, C.; D’Angelo, S.; Curtis, E.M.; Crozier, S.R.; Barton, S.J.; Robinson, S.M.; Godfrey, K.M.; Graham, N.J.; et al. Response to Antenatal Cholecalciferol Supplementation Is Associated With Common Vitamin D–Related Genetic Variants. J. Clin. Endocrinol. Metab. 2017, 102, 2941–2949. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Why Secondary Analyses in Vitamin D Clinical Trials Are Important and How to Improve Vitamin D Clinical Trial Outcome Analyses—A Comment on “Extra-Skeletal Effects of Vitamin D, Nutrients 2019, 11, 1460”. Nutrients 2019, 11, 2182. [Google Scholar] [CrossRef]
- Pritchard, L.; Lewis, S.; Hickson, M. Comparative effectiveness of vitamin D supplementation via buccal spray versus oral supplements on serum 25-hydroxyvitamin D concentrations in humans: A systematic review protocol. JBI database Syst. Rev. Implement. reports 2019, 17, 487–499. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Saldanha, I.J.; Li, T.; Yang, C.; Owczarzak, J.; Williamson, P.R.; Dickersin, K. Clinical trials and systematic reviews addressing similar interventions for the same condition do not consider similar outcomes to be important: A case study in HIV/AIDS. J. Clin. Epidemiol. 2017, 84, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Hernandez-Boussard, T.; Ioannidis, J.P.A. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ 2013, 347, f4501. [Google Scholar] [CrossRef] [PubMed]

| PICO | Description |
|---|---|
| Population | Any population, healthy or not |
| Intervention | Vitamin D3 buccal spray supplementation |
| Comparison | Other modes of vitamin D3 supplementation delivery (capsules, drops, etc.) |
| Outcome | Change in serum 25(OH)D concentrations |
| First Author: | Satia [51] | Todd [57] | Penagini [58] | Williams [59] |
|---|---|---|---|---|
| Implementation year: | NR | 2015–2016 | 2015–2016 | 2017 |
| Publication year: | 2015 | 2016 | 2017 | 2019 |
| Design: | Cross-over | Cross-over | Parallel | Parallel |
| Masking: | Single-blinded | Open-label | Open-label | Double-blind |
| Multicenter: | √ | - | - | - |
| Origin: | India | U.K. | Italy | UK |
| Registry: | CTRI/2013/06/003770 | NCT02608164 | NR | NR |
| Funding: | (1) Buccal spray provided by Pharma Base SA. | (1) Dept of Employment & Learning, N. Ireland (2) Translational Research Group, Public Health Agency, Belfast (3) Buccal spray provided by BetterYou Ltd. | NR | (1) BetterYou Ltd. (2) University of Sheffield |
| Ethical approval: | Spandan–Ethics | University of Ulster | University of Milan | University of Sheffield |
| Participant recruitment: | Two different hospitals, one physician’s site (healthy subjects) and a gastroenterologist’s site (patients with intestinal malabsorption) | The university and local area through circular emails and online advertisements | V. Buzzi Children’s Hospital | University of Sheffield |
| Participants (n): | N = 40 (healthy subjects and patients with malabsorption syndrome, ♂/♀ ratio = 1) Patients n = 14 ‡ Healthy controls n = 14 ‡ | N = 22 healthy adults (♀ = 12) | N = 24 children (5–17 years old, ♀ = 14, with neuro-disabilities and vitamin D deficiency (cerebral palsy n = 7, symptomatic or genetic epilepsy n = 5, epileptic encephalopathy n = 9, genetic syndromes n = 3) | N = 50 ¥ non-obese, apparently healthy adults (18–50 years old, ♀ = 29) |
| Participant age (years): | Patients: 39.9 ± 11.7 Healthy controls: 36.2 ± 10 | 25.2 ± 6.5 | Intervention: 7.8 (5–17) † Comparator: 9.4 (7–16) † | Intervention: 21.7 ± 3.1 Comparator: 22.9 ± 4.8 |
| BMI (kg/m2): | Patients: 21.5 ± 2.8 Healthy controls: 23.4 ± 3.9 | Intervention: 24.2 ± 3.5 § Comparator: 24.4 ± 3.6 § | Intervention: 18.2 (12.5–25.5) † Comparator: 16.9 (11.8–24.6) † | Intervention: 23.8 ± 2.6 Comparator: 23.6 ± 3 |
| Participant Groups (n): | Healthy participants: n = 14 * Patients: n = 14 * | Intervention: n = 22 Comparator: n = 22 | Intervention: n = 12 (♀ = 7) Comparator: n = 12 (♀ = 7) | Intervention: n = 25 (♀ = 15) Comparator: n = 25 (♀ = 14) (1) Active caps + placebo spray: n = 25 (2) Active spray + placebo caps: n = 25 (3) Double placebo: n = 25 |
| Randomization: | Block, by statistician | MINIM software | NR | Block (size of 9), computer-generated |
| Vitamin D status definition: | Νone | Clinical deficiency: 25(OH)D < 30 nmol/L Insufficiency: 25(OH)D 31–49 nmol/L Sufficiency: 25(OH)D > 50 nmol/L | Deficiency: 25(OH)D ≤ 20 ng/mL | Deficiency: 25(OH)D < 30 nmol/L Insufficiency: 25(OH)D 31–46 nmol/L Sufficiency: 25(OH)D > 50 mmol/L ˆ |
| 25(OH)D assay: | ECLIA | LC-MS/MS | Immunoassay | LC-MS |
| Kit: | Roche diagnostics (GmbH, Germany) | API 4000; AB SCIEX, Chromsystems Instruments and Mass-Chrom 25-OH vitamin D3/D2; Chromsystems Instruments & Chemicals (GmbH) | 25-Hydroxy Vitamin D EIA, Immunodiagnostic System, Ltd. | finger-prick blood spot |
| Assay laboratory: | Independent lab (APL Institute of Clinical Laboratory & Research Pvt. Ltd., Ahmedabad, IN) | Independent lab (Biochemistry Dept of St. James’ Hospital, Dublin, IE) | Pediatric Endocrinology Lab, Division of Genetics and Cell Biology, IRCCS San Raffaele Scientific Institute, Milan, IT | City Assays, Department of Pathology, Birmingham Sand-well Hospitals NHS Trust, UK |
| Exclusion criteria: | √ | √ | √ | √ |
| Intervention: | Buccal spray 2 shots x 500 IU vitamin D3/d | Buccal spray 3000 IU/d (75 μg) vitamin D3 | Buccal spray 800 IU/d vitamin D3 | Active vitamin D3 buccal spray 3000 IU (75 μg) + placebo caps |
| Comparators: | (1) soft caps (1000 IU) vitamin D3/d (2) none | 3 × 1000 IU (25 μg) vitamin D3 caps/d, with water | Oral drops 750 IU/d vitamin D3 | Active vitamin D3 caps 3000 IU (75 μg) + placebo spray |
| Intervention duration: | 30 days | 4 weeks | 3 months | 6 weeks |
| Season: | NR | Winter | Winter | Spring |
| Skin-tone evaluation: | NR | NR | NR | √ |
| Washout duration: | 30 days | 10 weeks | NR | NR |
| Compliance assessment: | √ | √ | √ | √ |
| Dietary intake: | Recorded at baseline | Recorded at baseline | NR | NR |
| Analyses: | PP | ITT and PP | NR | ITT |
| Outcomes: | Δ in 25(OH)D levels | Δ in levels of 25(OH)D, creatinine, PTH, Ca, eGFR | Δ in levels of 25(OH)D, Ca, P, PTH, BAP, CTx | Δ in 25(OH)D levels |
| Dropouts: | n = 2 (low compliance) | n = 4 (3 went for a sun holiday, no longer wished to participate and 1 had illness unrelated to the intervention) | NR (flowchart lacking) | NR (flowchart lacking) n = 1 stopped due to adverse events without information on the allocation group |
| Baseline data (intervention group): | Healthy subjects: 18.9 ± 4.3 ng/mL (n = 13) Patients: 10 ± 4.3 ng/mL (n = 13) | 25(OH)D: 59.6 ± 24.4 nmol/L (n = 22) Dietary vitamin D intake: 6.3 ± 6.2 μg/d PTH: 50.1 ± 26 pg/mL (n = 22) Ca: 2.2 ± 0.1 mmol/L (n = 22) | 25(OH)D: 15.5 (8–20) † ng/mL PTH: 72.5 (31.4–145.8) † pg/mL Ca: 9.6 (9.1–9.8) † mg/dL | 25(OH)D: 54.9 ± 27.8 nmol/L (n = 25) |
| Baseline data (comparator group): | Healthy subjects: 18.7 ± 5.9 ng/mL (n = 13) Patients: 11 ± 6.4 ng/mL (n = 13) | 25(OH)D: 60 ± 26.3 nmol/L (n = 22) PTH: 50.3 ± 25.5 pg/mL (n = 22) Ca: 2.2 ± 0.1 mmol/L (n = 22) | 25(OH)D: 11.5 (8–19) † ng/mL PTH: 65.9 (46–98.8) † pg/mL Ca: 9.4 (8.9–10.4) † mg/dL | 25(OH)D: 50.7 ± 19.7 nmol/L (n = 25) |
| Results (intervention group): | Healthy subjects: 26.9 ± 5.7 ng/mL (n = 13) Patients: 20.5 ± 7.9 ng/mL (n = 13) | 25(OH)D: 85.8 ± 19.4 nmol/L (n = 22) PTH: 48.2 ± 27.3 pg/mL (n = 22) Ca: 2.2 ± 0.1 mmol/L (n = 22) | 25(OH)D: 26.5 (13.6–39) † ng/mL PTH: 48.9 (23.2–89.6) † Ca: 9.27 (8.7–10) † mg/dL | 25(OH)D: 95.8 ± 28.0 nmol/L (n = 25) |
| Results (comparator group): | Healthy subjects: 22.8 ± 6.8 ng/mL (n = 13) Patients: 15 ± 9 ng/mL (n = 13) | 25(OH)D: 90.4 ± 21 nmol/L (n = 22) PTH: 52.2 ± 19.3 pg/mL (n = 22) Ca: 2.2 ± 0.1 mmol/L (n = 22) | 25(OH)D: 34.5 (22–49) † ng/mL PTH: 53.5 (30.6–98.4) † pg/mL Ca: 9.19 (8.6–9.8) † mg/dL | 25(OH)D: 91.4 ± 19.8 nmol/L (n = 25) |
| Results overall: | The buccal spray significantly increased serum 25(OH)D levels as compared to the caps, in both healthy subjects and patients with malabsorption syndrome | No difference between buccal spray and caps | Vitamin D3 supplementation with buccal spray and oral drops are equally effective | Vitamin D3 supplementation via capsules and sublingual spray are equally effective |
| Adverse events: | NR | NR | NR | n = 2 small blisters on cheek and tongue |
| RCT Issues: | NR | NR | The dosage could not be matched precisely between the two interventions. | Dose inconsistency: The spray/caps content was prepared to 97.5 μg/dose in order to maintain shelf life and guarantee dose, however, each capsule and spray contained 3000 IU (75 μg) of vitamin D3 per dose. |
| Manuscript issues: | - | ITT and PP were not separated | No flowchart, no detailed n in each stage | No flowchart |
| Jadad [55] score: | 2 | 2 | −1 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grammatikopoulou, M.G.; Gkiouras, K.; Nigdelis, M.P.; Bogdanos, D.P.; Goulis, D.G. Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials. Nutrients 2020, 12, 691. https://doi.org/10.3390/nu12030691
Grammatikopoulou MG, Gkiouras K, Nigdelis MP, Bogdanos DP, Goulis DG. Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials. Nutrients. 2020; 12(3):691. https://doi.org/10.3390/nu12030691
Chicago/Turabian StyleGrammatikopoulou, Maria G., Konstantinos Gkiouras, Meletios P. Nigdelis, Dimitrios P. Bogdanos, and Dimitrios G. Goulis. 2020. "Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials" Nutrients 12, no. 3: 691. https://doi.org/10.3390/nu12030691
APA StyleGrammatikopoulou, M. G., Gkiouras, K., Nigdelis, M. P., Bogdanos, D. P., & Goulis, D. G. (2020). Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials. Nutrients, 12(3), 691. https://doi.org/10.3390/nu12030691











