Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer
Abstract
1. Introduction
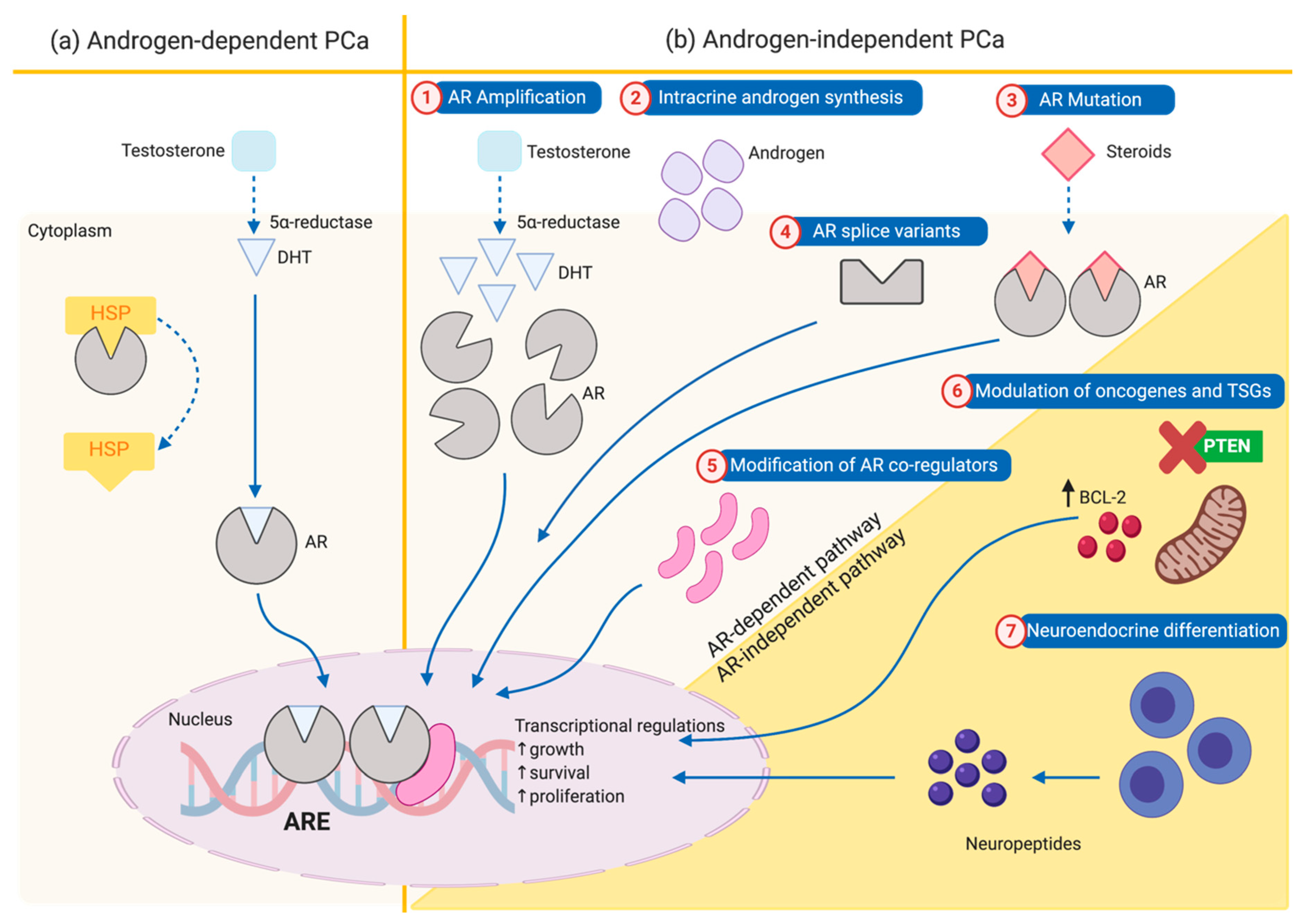
2. Mechanism of Progression from Androgen-Dependent to Androgen-Independent Prostate Cancer
3. Curcumin as a Potential Anticancer Agent for Prostate Cancer
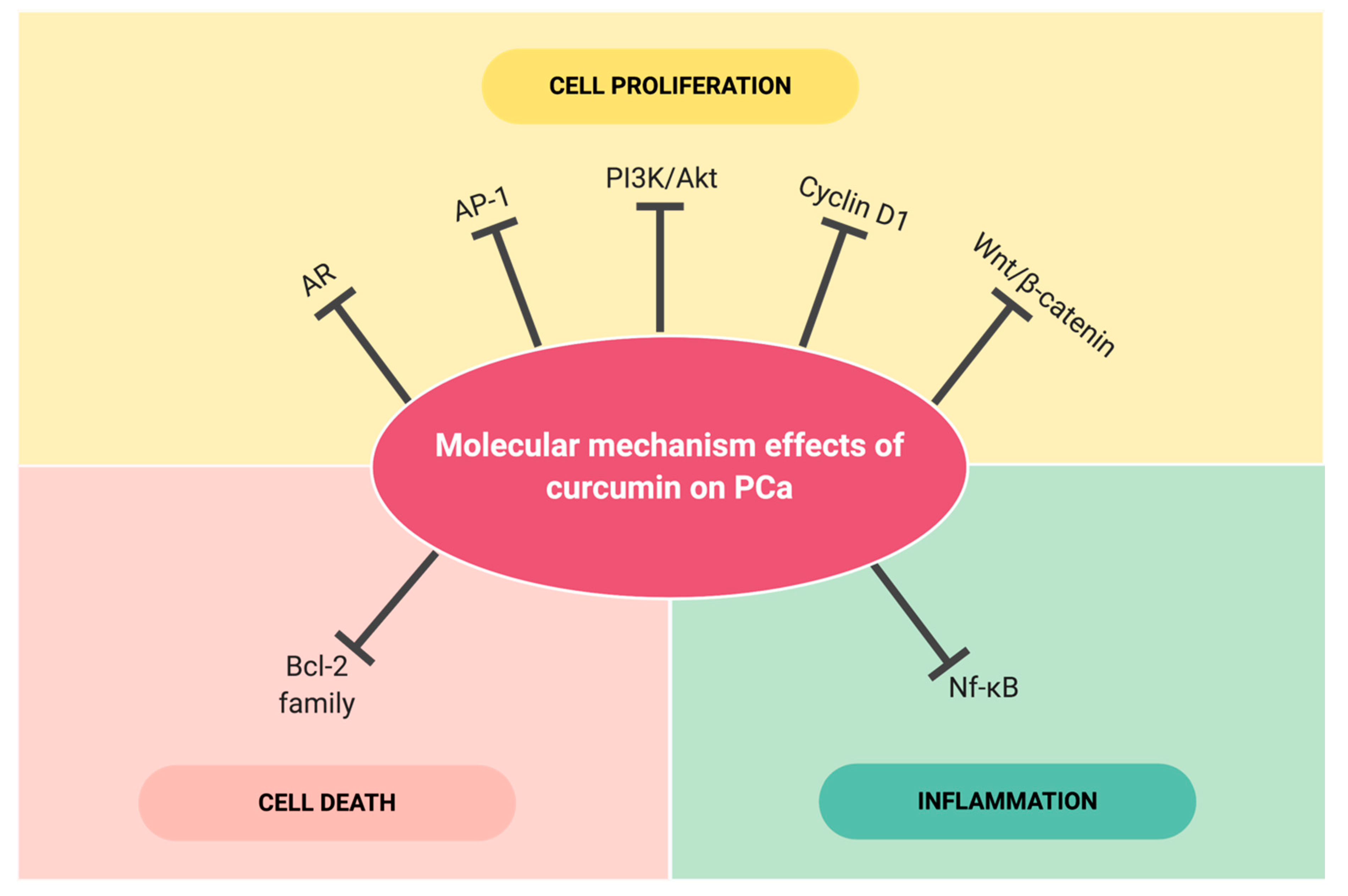
4. Selected Molecular Targets Effected by Curcumin in Prostate Cancer
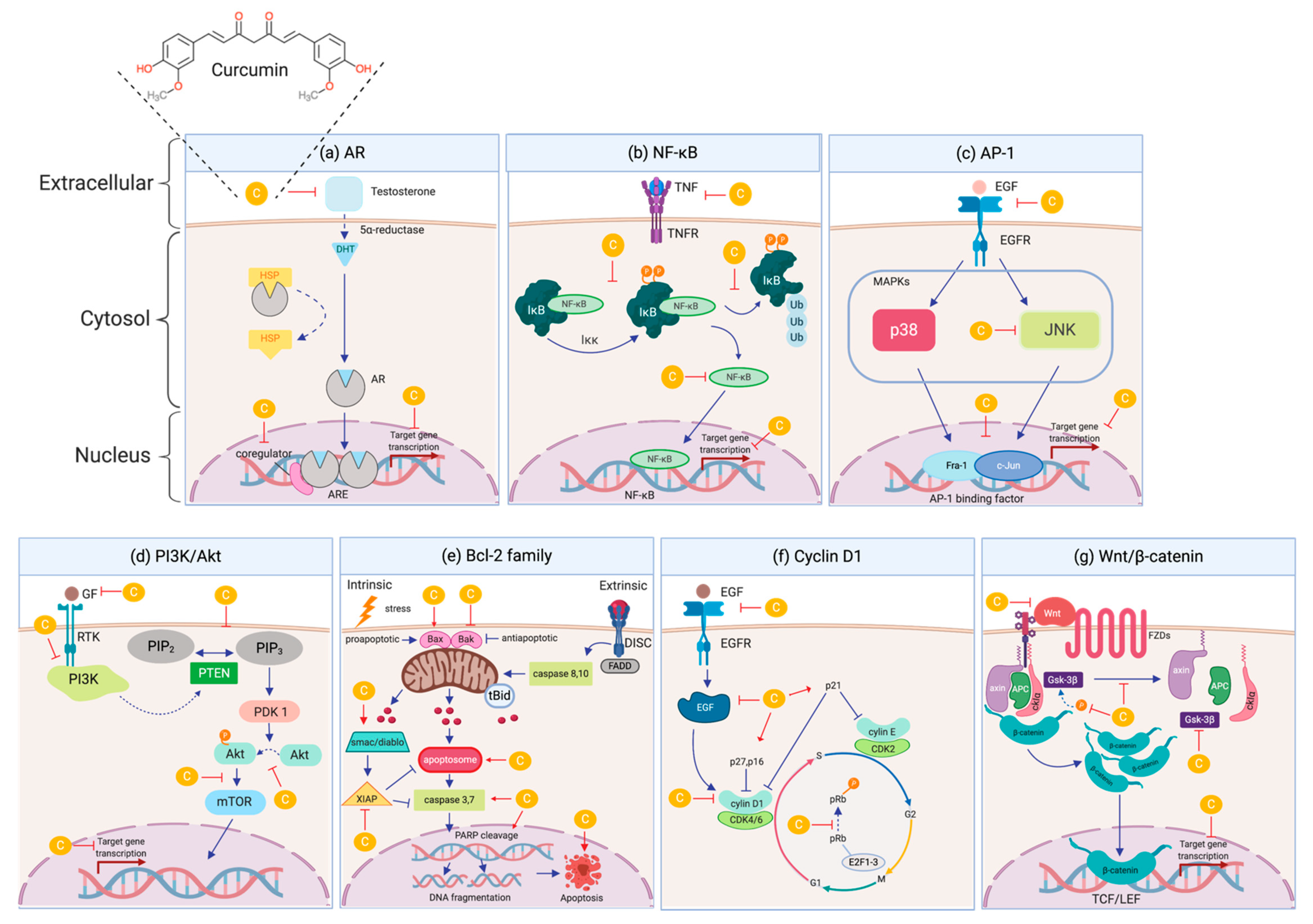
4.1. Androgen Receptor (AR)
4.2. Nuclear Factor kappa-B (NF-κB)
4.3. Activating Protein-1 (AP-1)
4.4. Phosphatidylinositol 3-kinases/the Serine/threonine kinase (PI3K/Akt)
4.5. Bcl-2 family
4.6. Cyclin D1
4.7. Wnt/ß-catenin
4.8. Role of MicroRNA (MiRNA)
5. Clinical Trials
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillen, A.D.; McEwan, I.J. Personalised treatment for prostate cancer patients: Are we there yet? AME Med. J. 2019, 4. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, R.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Pakzad, I.; Salehiniya, H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015, 3, 135–140. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1893–1907. [Google Scholar] [CrossRef]
- Lieberman, R.; Bermejo, C.; Akaza, H.; Greenwald, P.; Fair, W.; Thompson, I. Progress in prostate cancer chemoprevention: Modulators of promotion and progression. Urology 2001, 6, 835–842. [Google Scholar] [CrossRef]
- Montironi, R.; Mazzucchelli, R.; Algaba, F.; Lopez-Beltran, A. Morphological identification of the patterns of prostatic intraepithelial neoplasia and their importance. J Clin Pathol 2000, 53, 655–665. [Google Scholar] [CrossRef]
- Taitt, H.E. Global trends and prostate cancer: A review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am. J. Men’s Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef]
- Allen, N.; Key, T.; Appleby, P.; Travis, R.; Roddam, A.; Tjønneland, A.; Johnsen, N.; Overvad, K.; Linseisen, J.; Rohrmann, S. Animal foods, protein, calcium and prostate cancer risk: The European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2008, 98, 1574–1581. [Google Scholar] [CrossRef]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate cancer progression and mortality: A review of diet and lifestyle factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.; Niedzwiecki, A.; Rath, M. Scientific Evaluation of Dietary Factors in Cancer. J. Nutr. Med. Diet. Care 2018, 4, 029. [Google Scholar]
- Fiñones, R.R.; Yeargin, J.; Lee, M.; Kaur, A.P.; Cheng, C.; Sun, P.; Wu, C.; Nguyen, C.; Wang-Rodriguez, J.; Meyer, A.N. Early human prostate adenocarcinomas harbor androgen-independent cancer cells. PLoS ONE 2013, 8, e74438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaacs, J.T. Role of androgens in prostatic cancer. Vitam. Horm. 1994, 49, 433–502. [Google Scholar]
- Saad, F.; Sternberg, C.N.; Mulders, P.F.A.; Niepel, D.; Tombal, B.F. The role of bisphosphonates or denosumab in light of the availability of new therapies for prostate cancer. Cancer Treat. Rev. 2018, 68, 25–37. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef]
- Savarese, D.M.; Halabi, S.; Hars, V.; Akerley, W.L.; Taplin, M.E.; Godley, P.A.; Hussain, A.; Small, E.J.; Vogelzang, N.J. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: A final report of CALGB 9780. Cancer and Leukemia Group B. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 2509–2516. [Google Scholar] [CrossRef]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62. [Google Scholar]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Aragon-Ching, J.B.; Dahut, W.L. Chemotherapy in Androgen-Independent Prostate Cancer (AIPC): What’s next after taxane progression? Cancer Ther. 2007, 5A, 151–160. [Google Scholar] [PubMed]
- Chang, S.S. Treatment options for hormone-refractory prostate cancer. Rev. Urol. 2007, 9 (Suppl. 2), S13–S18. [Google Scholar]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.; Olmos, D.; De Bono, J. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br. J. Cancer 2009, 100, 671. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.R.; Thomas, G.; Youngren, J.; Foye, A.; Olson, S.; Paris, P.; Beer, T.M.; Ryan, C.J.; Witte, O.; Evans, C.P. Androgen Receptor (AR) Amplification in Patients (pts) with Metastatic Castration Resistant Prostate Cancer (mCRPC) Resistant to Abiraterone (Abi) and Enzalutamide (Enz): Preliminary Results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT); American Society of Clinical Oncology: Alexandria, VA, USA, 2015. [Google Scholar]
- Trapman, J.; Brinkmann, A. The androgen receptor in prostate cancer. Pathol. -Res. Pract. 1996, 192, 752–760. [Google Scholar] [CrossRef]
- Linja, M.J.; Savinainen, K.J.; Saramäki, O.R.; Tammela, T.L.J.; Vessella, R.L.; Visakorpi, T. Amplification and Overexpression of Androgen Receptor Gene in Hormone-Refractory Prostate Cancer. Cancer Res. 2001, 61, 3550. [Google Scholar]
- Nemes, A.; Tomuleasa, C.; Kacso, G. The androgen receptor remains a key player in metastatic hormone-refractory prostate cancer. Implications for new treatments. J. Buon 2014, 19, 357–364. [Google Scholar]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365. [Google Scholar]
- Gregory, C.W.; Johnson, R.T.; Mohler, J.L.; French, F.S.; Wilson, E.M. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001, 61, 2892–2898. [Google Scholar]
- Chen, Y.; Sawyers, C.L.; Scher, H.I. Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 2008, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Steketee, K.; Timmerman, L.; Ziel-van der Made, A.C.; Doesburg, P.; Brinkmann, A.O.; Trapman, J. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int. J. Cancer 2002, 100, 309–317. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, Y.; Zhang, D.; Wang, X.; Zhang, P.; Li, H.; Ejaz, S.; Liang, S. PGK1-mediated cancer progression and drug resistance. Am. J. Cancer Res. 2019, 9, 2280–2302. [Google Scholar] [PubMed]
- Crona, D.J.; Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef]
- Javidan, J.; Deitch, A.D.; Shi, X.-B.; de Vere White, R.W. The androgen receptor and mechanisms for androgen independence in prostate cancer. Cancer Investig. 2005, 23, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H. Neuroendocrine cells in benign and malignant prostate tissue: Morphogenesis, proliferation, and androgen receptor status. Prostate 1998, 36, 18–22. [Google Scholar] [CrossRef]
- Debes, J.D.; Tindall, D.J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1488–1490. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Troncoso, P.; Brisbay, S.M.; Logothetis, C.; Chung, L.W.; Hsieh, J.-T.; Tu, S.-M.; Campbell, M.L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992, 52, 6940–6944. [Google Scholar]
- Ghosh, P.M.; Malik, S.N.; Bedolla, R.G.; Wang, Y.; Mikhailova, M.; Prihoda, T.J.; Troyer, D.A.; Kreisberg, J.I. Signal transduction pathways in androgen-dependent and-independent prostate cancer cell proliferation. Endocr. -Relat. Cancer 2005, 12, 119–134. [Google Scholar] [CrossRef]
- Li, P.; Nicosia, S.V.; Bai, W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J. Biol. Chem. 2001, 276, 20444–20450. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Indira Priyadarsini, K. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Qadir, M.I.; Naqvi, S.; Muhammad, S.A. Curcumin: A polyphenol with molecular targets for cancer control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- Vera-Ramirez, L.; Perez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Teiten, M.-H.; Gaascht, F.; Eifes, S.; Dicato, M.; Diederich, M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Banerji, A.; Chakrabarti, J.; Mitra, A.; Chatterjee, A. Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004, 211, 235–242. [Google Scholar] [CrossRef]
- Goel, A.; Aggarwal, B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Shankar, T.N.; Shantha, N.V.; Ramesh, H.P.; Murthy, I.A.; Murthy, V.S. Toxicity studies on turmeric (Curcuma longa): Acute toxicity studies in rats, guineapigs & monkeys. Indian J. Exp. Biol. 1980, 18, 73–75. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 2. [Google Scholar] [CrossRef] [PubMed]
- Nagabhushan, M.; Bhide, S. Curcumin as an inhibitor of cancer. J. Am. Coll. Nutr. 1992, 11, 192–198. [Google Scholar]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–546. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Liu, H.; Zhu, Z.; Lü, D.; Geng, N.; Chen, Y. Anti-proliferative and anti-metastatic effects of curcumin on oral cancer cells. Hua Xi Kou Qiang Yi Xue Za Zhi= Huaxi Kouqiang Yixue Zazhi= West. China J. Stomatol. 2011, 29, 83–86. [Google Scholar]
- Teiten, M.-H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar]
- Prakobwong, S.; Gupta, S.C.; Kim, J.H.; Sung, B.; Pinlaor, P.; Hiraku, Y.; Wongkham, S.; Sripa, B.; Pinlaor, S.; Aggarwal, B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 2011, 32, 1372–1380. [Google Scholar] [CrossRef]
- Hecht, S.S.; Kenney, P.M.; Wang, M.; Trushin, N.; Agarwal, S.; Rao, A.V.; Upadhyaya, P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo [a] pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999, 137, 123–130. [Google Scholar] [CrossRef]
- Okazaki, Y.; Iqbal, M.; Okada, S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2005, 1740, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Berliner, A.; Fernando, S.S.; Ranasinghe, B.; Ray, I.; Tariq, H.; Banerjee, P. Curcumin blocks brain tumor formation. Brain Res. 2009, 1266, 130–138. [Google Scholar] [CrossRef]
- Swamy, M.V.; Citineni, B.; Patlolla, J.M.; Mohammed, A.; Zhang, Y.; Rao, C.V. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr. Cancer 2008, 60, 81–89. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lou, Y.-R.; Ma, W.; Newmark, H.L.; Reuhl, K.R.; Conney, A.H. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994, 54, 5841–5847. [Google Scholar]
- Limtrakul, P.; Lipigorngoson, S.; Namwong, O.; Apisariyakul, A.; Dunn, F. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997, 116, 197–203. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lou, Y.-R.; Xie, J.G.; Ma, W.; Lu, Y.-P.; Yen, P.; Zhu, B.T.; Newmark, H.; Ho, C.-T. Effect of dietary curcumin and dibenzoylmethane on formation of 7, 12-dimethylbenz [a] anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis 1998, 19, 1697–1700. [Google Scholar] [CrossRef]
- Azuine, M.A.; Bhide, S.V. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine-induced hamster oral carcinogenesis. Int. J. Cancer 1992, 51, 412–415. [Google Scholar] [CrossRef]
- Ghalaut, V.S.; Sangwan, L.; Dahiya, K.; Ghalaut, P.; Dhankhar, R.; Saharan, R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2012, 18, 186–190. [Google Scholar] [CrossRef]
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration—A clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Prostate cancer and curcumin: Add spice to your life. Cancer Biol. Ther. 2008, 7, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of Prostate Cancer by Natural Agents: Evidence from Molecular and Epidemiological Studies. Anticancer Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [PubMed]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- Mickey, D.D.; Stone, K.R.; Wunderli, H.; Mickey, G.H.; Vollmer, R.T.; Paulson, D.F. Heterotransplantation of a human prostatic adenocarcinoma cell line in nude mice. Cancer Res. 1977, 37, 4049–4058. [Google Scholar]
- McCarty, M.F. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integr. Cancer Ther. 2004, 3, 349–380. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Bueso-Ramos, C.; Chatterjee, D.; Pantazis, P.; Aggarwal, B.B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001, 20, 7597–7609. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer—I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Ahn, K.; Bae, E.; Jeon, S.; Choi, H. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006, 9, 147. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Hill, D.L.; Wang, H.; Zhang, R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007, 67, 1988–1996. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1. Cell Cycle 2007, 6, 2953–2961. [Google Scholar] [CrossRef]
- Shtivelman, E.; Beer, T.M.; Evans, C.P. Molecular pathways and targets in prostate cancer. Oncotarget 2014, 5, 7217. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From chemoprevention to chemotherapy: Common targets and common goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Tsui, K.H.; Feng, T.H.; Lin, C.M.; Chang, P.L.; Juang, H.H. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J. Androl. 2008, 29, 661–668. [Google Scholar] [CrossRef]
- Shi, Q.; Shih, C.-Y.; Lee, K. Novel anti-prostate cancer curcumin analogues that enhance androgen receptor degradation activity. Anti-Cancer Agents Med. Chem. 2009, 9, 904–912. [Google Scholar] [CrossRef]
- Choi, H.; Lim, J.; Hong, J. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002, 21, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Ramos, Y.; Rodriguez-Valentin, M.; Lopez-Acevedo, S.; Cubano, L.A.; Zou, J.; Zhang, Q.; Wang, G.; Boukli, N.M. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS ONE 2017, 12, e0179587. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.T.; Figg, W.D. The potential role of curcumin in prostate cancer: The importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016, 5, S1107. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, G.; Choi, H.Y. Effect of curcumin on the interaction between androgen receptor and Wnt/β-catenin in LNCaP xenografts. Korean J. Urol. 2015, 56, 656–665. [Google Scholar] [CrossRef]
- Chen, Q.-H. Curcumin-based anti-prostate cancer agents. Anti-Cancer Agents Med. Chem. 2015, 15, 138–156. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Hu, Z.; Zeng, X.; Li, Y.; Su, Y.; Zhang, C.; Ye, Z. Anti-tumor activity of curcumin against androgen-independent prostate cancer cells via inhibition of NF-κB and AP-1 pathway in vitro. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2011, 31, 530. [Google Scholar] [CrossRef]
- Hour, T.C.; Chen, J.; Huang, C.Y.; Guan, J.Y.; Lu, S.H.; Pu, Y.S. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21WAF1/CIP1 and C/EBPβ expressions and suppressing NF-κB activation. Prostate 2002, 51, 211–218. [Google Scholar] [CrossRef]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and-2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef]
- Deeb, D.; Xu, Y.X.; Jiang, H.; Gao, X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Curcumin (Diferuloyl-Methane) Enhances Tumor Necrosis Factor-related Apoptosis-inducing Ligand-induced Apoptosis in LNCaP Prostate Cancer Cells1. Mol. Cancer Ther. 2003, 2, 95–103. [Google Scholar]
- Deeb, D.; Jiang, H.; Gao, X.; Divine, G.; Dulchavsky, S.A.; Gautam, S.C. Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J. Exp. Ther. Oncol. 2005, 5, 81–91. [Google Scholar] [PubMed]
- Deeb, D.; Jiang, H.; Gao, X.; Al-Holou, S.; Danyluk, A.L.; Dulchavsky, S.A.; Gautam, S.C. Curcumin [1, 7-bis (4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3, 5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-κB via inhibition of the prosurvival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007, 321, 616–625. [Google Scholar] [PubMed]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor–related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-κB through suppression of IκBα phosphorylation. Mol. Cancer Ther. 2004, 3, 803–812. [Google Scholar] [PubMed]
- Andrzejewski, T.; Deeb, D.; Gao, X.; Danyluk, A.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Therapeutic efficacy of curcumin/TRAIL combination regimen for hormone-refractory prostate cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2008, 17, 257–267. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.-M.; Ye, Z.-Q.; Yu, J.-H.; Hu, X.-Y. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IκBα, c-Jun and androgen receptor. Die Pharm. -Int. J. Pharm. Sci. 2013, 68, 431–434. [Google Scholar]
- Zhao, W.; Zhou, X.; Qi, G.; Guo, Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J. Biochem. Mol. Toxicol. 2018, 32, e22049. [Google Scholar] [CrossRef]
- Polytarchou, C.; Hatziapostolou, M.; Papadimitriou, E. Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J. Biol. Chem. 2005, 280, 40428–40435. [Google Scholar] [CrossRef]
- Katta, S.; Srivastava, A.; Thangapazham, R.L.; Rosner, I.L.; Cullen, J.; Li, H.; Sharad, S. Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4891. [Google Scholar] [CrossRef]
- Shankar, S.; Srivastava, R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007, 30, 905–918. [Google Scholar] [CrossRef]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.-H.; Kong, A.-N.T. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef]
- Beevers, C.S.; Li, F.; Liu, L.; Huang, S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer 2006, 119, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, B.; Chung, S.; Wu, Y.; Henning, S.M.; Vadgama, J.V. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv. 2014, 4, 35242–35250. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Dev. Ther. 2017, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ning, J.; Peng, L.; He, D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9272–9278. [Google Scholar]
- Valentini, A.; Conforti, F.; Crispini, A.; De Martino, A.; Condello, R.; Stellitano, C.; Rotilio, G.; Ghedini, M.; Federici, G.; Bernardini, S. Synthesis, oxidant properties, and antitumoral effects of a heteroleptic palladium (II) complex of curcumin on human prostate cancer cells. J. Med. Chem. 2008, 52, 484–491. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Furlong, S.J.; Sutton, K.; Richardson, A.; Robichaud, M.R.; Giacomantonio, C.A.; Ridgway, N.D.; Hoskin, D.W. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr. Cancer 2010, 62, 379–389. [Google Scholar] [CrossRef]
- Shenouda, N.S.; Zhou, C.; Browning, J.D.; Ansell, P.J.; Sakla, M.S.; Lubahn, D.B.; MacDonald, R.S. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr. Cancer 2004, 49, 200–208. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin sensitizes TRAIL-resistant xenografts: Molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer 2008, 7, 16. [Google Scholar] [CrossRef]
- Kim, J.-H.; Xu, C.; Keum, Y.-S.; Reddy, B.; Conney, A.; Kong, A.-N.T. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with β-phenylethyl isothiocyanate and curcumin. Carcinogenesis 2005, 27, 475–482. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Shaheduzzaman, S.; Kim, K.-H.; Passi, N.; Tadese, A.; Vahey, M.; Dobi, A.; Srivastava, S.; Maheshwari, R.K. Androgen responsive and refractory prostate cancer cells exhibit distinct curcumin regulated transcriptome. Cancer Biol. Ther. 2008, 7, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.-J.; Cho, M.; Song, J.-Y.; Yun, Y.-S.; Choi, I.-W.; Kim, D.-E.; Park, B.-S.; Oh, S. Natural derivatives of curcumin attenuate the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem. Biophys. Res. Commun. 2008, 377, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-L.; Lu, S.-X.; Li, M.; Li, L.-Z.; Fu, J.; Hu, W.; Yang, Y.-Z.; Luo, R.-Z.; Zhang, C.Z.; Yun, J.-P. FoxD3-regulated microRNA-137 suppresses tumour growth and metastasis in human hepatocellular carcinoma by targeting AKT2. Oncotarget 2014, 5, 5113–5124. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Enokida, H.; Yoshino, H.; Itesako, T.; Chiyomaru, T.; Kinoshita, T.; Fuse, M.; Nishikawa, R.; Goto, Y.; Naya, Y. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J. Hum. Genet. 2014, 59, 78–87. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, W.-G.; Li, X.-W. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am. J. Cancer Res. 2015, 5, 2056. [Google Scholar]
- Chang, C.; Kokontis, J.; Liao, S. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Joseph, D.R.; Sullivan, P.M.; Willard, H.F.; French, F.S.; Wilson, E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988, 240, 327–330. [Google Scholar] [CrossRef]
- Trapman, J.; Klaassen, P.; Kuiper, G.; Van der Korput, J.; Faber, P.; Van Rooij, H.; Van Kessel, A.G.; Voorhorst, M.; Mulder, E.; Brinkmann, A. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem. Biophys. Res. Commun. 1988, 153, 241–248. [Google Scholar] [CrossRef]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and androgen receptor signaling in prostate tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef]
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-l. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3. [Google Scholar] [CrossRef]
- Gregory, C.W.; Hamil, K.G.; Kim, D.; Hall, S.H.; Pretlow, T.G.; Mohler, J.L.; French, F.S. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998, 58, 5718–5724. [Google Scholar]
- Brooke, G.; Bevan, C. The role of androgen receptor mutations in prostate cancer progression. Curr. Genom. 2009, 10, 18–25. [Google Scholar] [CrossRef]
- Yuan, X.; Cai, C.; Chen, S.; Yu, Z.; Balk, S. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene 2014, 33, 2815. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tindall, D.J. The role of the androgen receptor in prostate cancer. Crit. Rev. ™ Eukaryot. Gene Expr. 2002, 12, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.A.; Guns, E.S.; Lubik, A.A.; Adomat, H.H.; Hendy, S.C.; Wood, C.A.; Ettinger, S.L.; Gleave, M.E.; Nelson, C.C. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008, 68, 6407–6415. [Google Scholar] [CrossRef]
- Mao, H.L.; Zhu, Z.Q.; Chen, C.D. The androgen receptor in hormone-refractory prostate cancer. Asian J. 2009, 11, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Krishna, N.S.; Grigor, K.M.; Bartlett, J.M.S. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 2003, 89, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Li, R.; Kuri, B.; Lotan, Y.; Roehrborn, C.G.; Liu, J.; Vessella, R.; Nelson, P.S.; Kapur, P.; Guo, X.; et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 2013, 154, 1074–1084. [Google Scholar] [CrossRef]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR 1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef]
- Zhang, H.-n.; Yu, C.-x.; Zhang, P.-j.; Chen, W.-w.; Jiang, A.-l.; Kong, F.; Deng, J.-t.; Zhang, J.-y.; Young, C.Y. Curcumin downregulates homeobox gene NKX3. 1 in prostate cancer cell LNCaP. Acta Pharmacol. Sin. 2007, 28, 423. [Google Scholar] [CrossRef]
- Bieberich, C.J.; Fujita, K.; He, W.-W.; Jay, G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J. Biol. Chem. 1996, 271, 31779–31782. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Khan, N.; Afaq, F.; Mukhtar, H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol. Prev. Biomark. 2007, 16, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49. [Google Scholar] [CrossRef]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef]
- Lessard, L.; Bégin, L.R.; Gleave, M.E.; Mes-Masson, A.M.; Saad, F. Nuclear localisation of nuclear factor-kappaB transcription factors in prostate cancer: An immunohistochemical study. Br. J. Cancer 2005, 93, 1019–1023. [Google Scholar] [CrossRef]
- Thapa, D.; Ghosh, R. Chronic inflammatory mediators enhance prostate cancer development and progression. Biochem. Pharmacol. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Chi, N.; Tan, Z.; Ma, K.; Bao, L.; Yun, Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and-6 in prostate cancer. Int. J. Clin. Exp. Med. 2014, 7, 3181. [Google Scholar]
- Chen, C.D.; Sawyers, C.L. NF-κB activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol. Cell. Biol. 2002, 22, 2862–2870. [Google Scholar] [CrossRef]
- Jin, R.J.; Lho, Y.; Connelly, L.; Wang, Y.; Yu, X.; Saint Jean, L.; Case, T.C.; Ellwood-Yen, K.; Sawyers, C.L.; Bhowmick, N.A.; et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008, 68, 6762–6769. [Google Scholar] [CrossRef] [PubMed]
- da Silva, H.B.; Amaral, E.P.; Nolasco, E.L.; de Victo, N.C.; Atique, R.; Jank, C.C.; Anschau, V.; Zerbini, L.F.; Correa, R.G. Dissecting major signaling pathways throughout the development of prostate cancer. Prostate Cancer 2013, 2013, 920612. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.; Beyaert, R. Inflammation and NF-kappaB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Patel, J.; Marengo, S.R.; Gupta, S. Nuclear factor-κB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 2004, 6, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; MacLennan, G.T.; Marengo, S.R.; Resnick, M.I.; Gupta, S. Constitutive activation of PI3K-Akt and NF-κB during prostate cancer progression in autochthonous transgenic mouse model. Prostate 2005, 64, 224–239. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Shanmugam, M.; Rane, G.; Kanchi, M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.; Tan, B.; Kumar, A.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Philip, S.; Kundu, G.C. Osteopontin induces nuclear factor κB-mediated promatrix metalloproteinase-2 activation through IκBα/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J. Biol. Chem. 2003, 278, 14487–14497. [Google Scholar] [CrossRef]
- Zheng, M.; Ekmekcioglu, S.; Walch, E.T.; Tang, C.-H.; Grimm, E.A. Inhibition of nuclear factor-κB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004, 14, 165–171. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Hardegen, N.J.; Aggarwal, B.B. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem. Pharm. 1998, 55, 775–783. [Google Scholar] [CrossRef]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [PubMed]
- Garces de los Fayos Alonso, I.; Liang, H.-C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef]
- Andreucci, J.J.; Grant, D.; Cox, D.M.; Tomc, L.K.; Prywes, R.; Goldhamer, D.J.; Rodrigues, N.; Bédard, P.-A.; McDermott, J.C. Composition and function of AP-1 transcription complexes during muscle cell differentiation. J. Biol. Chem. 2002, 277, 16426–16432. [Google Scholar] [CrossRef]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Surh, Y.-J. Transcription factors in the cellular signaling network as prime targets of chemopreventive phytochemicals. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2004, 36, 275. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.-g.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Zerbini, L.; Wang, Y.; Cho, J.; Gu, X.; Jones, J.; Inan, M.; Bailey, C.; Joseph, M.; Zhou, J.; Libermann, T. 1075. Transcription Factors NF-kB and AP-1 as Targets for Prostate Cancer Gene Therapy. Mol. Ther. 2003, 7, S415. [Google Scholar]
- Kavya, K.; Kumar, M.N.; Patil, R.H.; Hegde, S.M.; Kiran Kumar, K.M.; Nagesh, R.; Babu, R.L.; Ramesh, G.T.; Chidananda Sharma, S. Differential expression of AP-1 transcription factors in human prostate LNCaP and PC-3 cells: Role of Fra-1 in transition to CRPC status. Mol. Cell. Biochem. 2017, 433, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kajanne, R.; Miettinen, P.; Tenhunen, M.; Leppa, S. Transcription factor AP-1 promotes growth and radioresistance in prostate cancer cells. Int. J. Oncol. 2009, 35, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.E.-F.; Abudu, A.; Jonhson, E.; Aftab, N.; Conrad, S.; Fluck, M. The role of AP-1 in self-sufficient proliferation and migration of cancer cells and its potential impact on an autocrine/paracrine loop. Oncotarget 2018, 9, 34259–34278. [Google Scholar] [CrossRef] [PubMed]
- Millena, A.C.; Vo, B.T.; Khan, S.A. JunD is required for proliferation of prostate cancer cells and plays a role in transforming growth factor-β (TGF-β)-induced inhibition of cell proliferation. J. Biol. Chem. 2016, 291, 17964–17976. [Google Scholar] [CrossRef]
- Chen, S.; Cai, C.; Fisher, C.; Zheng, Z.; Omwancha, J.; Hsieh, C.; Shemshedini, L. c-Jun enhancement of androgen receptor transactivation is associated with prostate cancer cell proliferation. Oncogene 2006, 25, 7212. [Google Scholar] [CrossRef]
- Ricote, M.; Garcia-Tunon, I.; Bethencourt, F.; Fraile, B.; Onsurbe, P.; Paniagua, R.; Royuela, M. The p38 transduction pathway in prostatic neoplasia. J. Pathol. 2006, 208, 401–407. [Google Scholar] [CrossRef]
- Edwards, J.; Krishna, N.S.; Mukherjee, R.; Bartlett, J.M. The role of c-Jun and c-Fos expression in androgen-independent prostate cancer. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 204, 153–158. [Google Scholar] [CrossRef]
- Ouyang, X.; Jessen, W.J.; Al-Ahmadie, H.; Serio, A.M.; Lin, Y.; Shih, W.-J.; Reuter, V.E.; Scardino, P.T.; Shen, M.M.; Aronow, B.J. Activator protein-1 transcription factors are associated with progression and recurrence of prostate cancer. Cancer Res. 2008, 68, 2132–2144. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bartlett, J.; Krishna, N.; Underwood, M.; Edwards, J. Raf-1 expression may influence progression to androgen insensitive prostate cancer. Prostate 2005, 64, 101–107. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Bierhaus, A.; Zhang, Y.; Quehenberger, P.; Luther, T.; Haase, M.; Muller, M.; Mackman, N.; Ziegler, R.; Nawroth, P.P. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb. Haemost. 1997, 77, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Tan, T.-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-T.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991, 51, 813–819. [Google Scholar] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Georgescu, M.-M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef]
- Ferraldeschi, R.; Welti, J.; Luo, J.; Attard, G.; de Bono, J.S. Targeting the androgen receptor pathway in castration-resistant prostate cancer: Progresses and prospects. Oncogene 2015, 34, 1745–1757. [Google Scholar] [CrossRef]
- Sun, X.; Huang, J.; Homma, T.; Kita, D.; Klocker, H.; Schafer, G.; Boyle, P.; Ohgaki, H. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009, 29, 1739–1743. [Google Scholar]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. -Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.-K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725. [Google Scholar] [CrossRef] [PubMed]
- Hammers, H.J.; Antonarakis, E.S. Targeting the PI3K/AKT/mTOR Pathway in Prostate Cancer. In Management of Castration Resistant Prostate Cancer; Springer: Berlin, Germany, 2014; pp. 249–252. [Google Scholar]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Novel targets for prostate cancer chemoprevention. Endocr. -Relat. Cancer 2010, 17, R195–R212. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.S.; Kim, D.Y.; So, I.; Jeon, J.-H. PI3K pathway in prostate cancer: All resistant roads lead to PI3K. Biochim. Et Biophys. Acta (Bba)-Rev. Cancer 2018, 1870, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Stadelman, K.M.; Chappell, W.H.; Horn, S.; Bäsecke, J.; Cervello, M.; Nicoletti, F.; Libra, M.; Stivala, F.; Martelli, A.M. Akt as a therapeutic target in cancer. Expert Opin. Ther. Targets 2008, 12, 1139–1165. [Google Scholar] [CrossRef]
- Crumbaker, M.; Khoja, L.; Joshua, A.M. AR Signaling and the PI3K Pathway in Prostate Cancer. Cancers 2017, 9, 34. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, G.; Getzenberg, R.H.; Veltri, R.W. The Upregulation of PI3K/Akt and MAP Kinase Pathways is Associated with Resistance of Microtubule-Targeting Drugs in Prostate Cancer. J. Cell. Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G 2/M cell cycle arrest. BMC Cancer 2006, 6, 107. [Google Scholar] [CrossRef]
- Qiao, Q.; Jiang, Y.; Li, G. Inhibition of the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced apoptosis in human Burkitt’s lymphoma. J. Pharmacol. Sci. 2013, 121, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.S.; Abel, P.D.; Lalani, E.-N. Role of the Bcl-2 gene family in prostate cancer progression and its implications for therapeutic intervention. Environ. Health Perspect. 1999, 107, 49–57. [Google Scholar] [PubMed]
- Fernandez, A.; Udagawa, T.; Schwesinger, C.; Beecken, W.-D.; Achilles-Gerte, E.; McDonnell, T.J.; D’Amato, R.J. Angiogenic Potential of Prostate Carcinoma Cells Overexpressing bcl-2. JNCI J. Natl. Cancer Inst. 2001, 93, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Anvari, K.; Toussi, M.S.; Kalantari, M.; Naseri, S.; Shahri, M.K.; Ahmadnia, H.; Katebi, M.; Pashaki, A.S.; Dayani, M.; Broumand, M. Expression of Bcl-2 and Bax in advanced or metastatic prostate carcinoma. Urol. J. 2012, 9, 381–388. [Google Scholar] [PubMed]
- Castilla, C.; Congregado, B.n.; Chinchón, D.; Torrubia, F.J.; Japón, M.A.; Sáez, C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology 2006, 147, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, H.; Shin, E.A.; Kim, D.H.; Choi, J.B.; Kim, S.-H. Implications of Bcl-2 and its interplay with other molecules and signaling pathways in prostate cancer progression. Expert Opin. Ther. Targets 2017, 21, 911–920. [Google Scholar] [CrossRef]
- Syng-ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar]
- Kim, M.S.; Kang, H.J.; Moon, A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch. Pharmacal Res. 2001, 24, 349–354. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006, 8, E443. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. A J. Virtual Libr. 2008, 13, 2191–2202. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. The Cell Cycle: Principles of Control; New Science Press: London, UK, 2007. [Google Scholar]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Das, T. Anti cancer effects of curcumin: Cycle of life and death. Cell Div. 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298. [Google Scholar] [CrossRef]
- Donnellan, R.; Chetty, R. Cyclin D1 and human neoplasia. Mol. Pathol. 1998, 51, 1. [Google Scholar] [CrossRef]
- Pereira, R.; Ravinal, R.; Costa, R.S.; Lima, M.; Tucci, S.; Muglia, V.; Dos Reis, R.; Silva, G.E.B. Cyclin D1 expression in prostate carcinoma. Braz. J. Med. Biol. Res. 2014, 47, 515–521. [Google Scholar] [CrossRef]
- Agus, D.B.; Cordon-Cardo, C.; Fox, W.; Drobnjak, M.; Koff, A.; Golde, D.W.; Scher, H.I. Prostate Cancer Cell Cycle Regulators: Response to Androgen Withdrawal and Development of Androgen Independence. JNCI J. Natl. Cancer Inst. 1999, 91, 1869–1876. [Google Scholar] [CrossRef]
- Drobnjak, M.; Osman, I.; Scher, H.I.; Fazzari, M.; Cordon-Cardo, C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin. Cancer Res. 2000, 6, 1891–1895. [Google Scholar]
- Teo, K.; McVitty, C.; Mitchell, T.; McCall, P.; Edwards, J. 385 Cyclin D1 Expression Influences Overall Survival in Androgen Independent Prostate Cancer. J. Urol. 2010, 183, e152–e153. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The effect of curcumin on breast cancer cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Poch, B.; Gansauge, F.; Schwarz, A.; Seufferlein, T.; Schnelldorfer, T.; Ramadani, M.; Beger, H.G.; Gansauge, S. Epidermal growth factor induces cyclin D1 in human pancreatic carcinoma: Evidence for a cyclin D1–dependent cell cycle progression. Pancreas 2001, 23, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.D.; RABEN, D.M.; Phillips, P.J.; Baldassare, J.J. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J. 1997, 326, 61–68. [Google Scholar] [CrossRef]
- Perry, J.E.; Grossmann, M.E.; Tindall, D.J. Epidermal growth factor induces cyclin D1 in a human prostate cancer cell line. Prostate 1998, 35, 117–124. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127. [Google Scholar] [CrossRef]
- Lo, H.-W.; Hsu, S.-C.; Ali-Seyed, M.; Gunduz, M.; Xia, W.; Wei, Y.; Bartholomeusz, G.; Shih, J.-Y.; Hung, M.-C. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 2005, 7, 575–589. [Google Scholar] [CrossRef]
- Jura, N.; Zhang, X.; Endres, N.F.; Seeliger, M.A.; Schindler, T.; Kuriyan, J. Catalytic control in the EGF receptor and its connection to general kinase regulatory mechanisms. Mol. Cell 2011, 42, 9–22. [Google Scholar] [CrossRef]
- Hernes, E.; Fosså, S.; Berner, A.; Otnes, B.; Nesland, J. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br. J. Cancer 2004, 90, 449. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Miller, J.R.; Hocking, A.M.; Brown, J.D.; Moon, R.T. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene 1999, 18, 7860–7872. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ashihara, E.; Maekawa, T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin. Ther. Targets 2011, 15, 873–887. [Google Scholar] [CrossRef]
- Camilli, T.C.; Weeraratna, A.T. Striking the target in Wnt-y conditions: Intervening in Wnt signaling during cancer progression. Biochem. Pharmacol. 2010, 80, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Logan, S.K. Revisiting the role of Wnt/β-catenin signaling in prostate cancer. Mol. Cell. Endocrinol. 2018, 462, 3–8. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar] [CrossRef]
- Verras, M.; Sun, Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006, 237, 22–32. [Google Scholar] [CrossRef]
- Robinson, D.R.; Zylstra, C.R.; Williams, B.O. Wnt signaling and prostate cancer. Curr Drug Targets 2008, 9, 271–580. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Sadar, M.D. Crosstalk between the androgen receptor and β-catenin in castrate-resistant prostate cancer. Cancer Res. 2008, 68, 9918–9927. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Jiang, M.; Bierie, B.; Roy-Burman, P.; Shen, M.M.; Taketo, M.M.; Wills, M.; Matusik, R.J. Activation of β-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 2009, 69, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Chesire, D.; Isaacs, W.B. Beta-catenin signaling in prostate cancer: An early perspective. Endocr. -Relat. Cancer 2003, 10, 537–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tinsley, H.N.; Keeton, A.; Qu, Z.; Piazza, G.A.; Li, Y. Suppression of Wnt/β-catenin signaling inhibits prostate cancer cell proliferation. Eur. J. Pharmacol. 2009, 602, 8–14. [Google Scholar] [CrossRef]
- Lee, E.; Ha, S.; Logan, S.K. Divergent Androgen Receptor and Beta-Catenin Signaling in Prostate Cancer Cells. PLoS ONE 2015, 10, e0141589. [Google Scholar] [CrossRef]
- Park, C.H.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS letters 2005, 579, 2965–2971. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.-N. Curcumin: A therapeutic strategy in cancers by inhibiting the canonical WNT/β-catenin pathway. Journal of Experimental & Clinical Cancer Research 2019, 38, 323. [Google Scholar] [CrossRef]
- Chesire, D.R.; Ewing, C.M.; Gage, W.R.; Isaacs, W.B. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene 2002, 21, 2679. [Google Scholar] [CrossRef]
- Masiello, D.; Chen, S.-Y.; Xu, Y.; Verhoeven, M.C.; Choi, E.; Hollenberg, A.N.; Balk, S.P. Recruitment of β-Catenin by Wild-Type or Mutant Androgen Receptors Correlates with Ligand-Stimulated Growth of Prostate Cancer Cells. Molecular Endocrinology 2004, 18, 2388–2401. [Google Scholar] [CrossRef]
- Truica, C.I.; Byers, S.; Gelmann, E.P. β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000, 60, 4709–4713. [Google Scholar]
- McCubrey, J.A.; Steelman, L.; Bertrand, F.E.; Davis, N.M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; Maestro, R. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 2014, 28, 15. [Google Scholar] [CrossRef]
- Jaggi, M.; Chauhan, S.C.; Du, C.; Balaji, K. Bryostatin 1 modulates β-catenin subcellular localization and transcription activity through protein kinase D1 activation. Mol. Cancer Ther. 2008, 7, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Du, C.; Zhang, W.; Balaji, K.C. Protein kinase D1: A protein of emerging translational interest. Front. Biosci. 2007, 12, 3757–3767. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Rao, P.S.; Smith, D.J.; Hemstreet, G.P.; Balaji, K. Protein kinase C μ is down-regulated in androgen-independent prostate cancer. Biochem. Biophys. Res. Commun. 2003, 307, 254–260. [Google Scholar] [CrossRef]
- Sundram, V.; Chauhan, S.C.; Ebeling, M.; Jaggi, M. Curcumin attenuates β-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS ONE 2012, 7, e35368. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.-G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef]
- Richardsen, E.; Andersen, S.; Melbø-Jørgensen, C.; Rakaee, M.; Ness, N.; Al-Saad, S.; Nordby, Y.; Pedersen, M.I.; Dønnem, T.; Bremnes, R.M. MicroRNA 141 is associated to outcome and aggressive tumor characteristics in prostate cancer. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Li, F.; Mahato, R.I. MicroRNAs and drug resistance in prostate cancers. Mol. Pharm. 2014, 11, 2539–2552. [Google Scholar] [CrossRef]
- Mirzaei, H.; Masoudifar, A.; Sahebkar, A.; Zare, N.; Sadri Nahand, J.; Rashidi, B.; Mehrabian, E.; Mohammadi, M.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: A novel target of curcumin in cancer therapy. J. Cell. Physiol. 2018, 233, 3004–3015. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Nath, N.C.D.; Kim, E.-K.; Lee, K.-G. Role of phytochemicals in the modulation of miRNA expression in cancer. Food Funct. 2017, 8, 3432–3442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, S.; Shen, H.; Chen, W.; Xu, H.; Chen, X.; Sun, D.; Zhong, S.; Zhao, J.; Tang, J. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biol. 2017, 39, 1010428317691680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Rosenberg, A.Z.; Choi, S.M.; Fox-Talbot, K.; De Marzo, A.M.; Nonn, L.; Brennen, W.N.; Marchionni, L.; Halushka, M.K.; Lupold, S.E. Cell-type specific expression of oncogenic and tumor suppressive microRNAs in the human prostate and prostate cancer. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Cao, H.; Yu, H.; Feng, Y.; Chen, L.; Liang, F. Curcumin inhibits prostate cancer by targeting PGK1 in the FOXD3/miR-143 axis. Cancer Chemother. Pharmacol. 2017, 79, 985–994. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Naoe, T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol. Rep. 2006, 16, 845–850. [Google Scholar] [CrossRef]
- Lin, T.; Dong, W.; Huang, J.; Pan, Q.; Fan, X.; Zhang, C.; Huang, L. MicroRNA-143 as a tumor suppressor for bladder cancer. J. Urol. 2009, 181, 1372–1380. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wang, Y.; Luo, J. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR-143 and miR-143 mediated autophagy inhibition. J. Drug Target. 2017, 25, 645–652. [Google Scholar] [CrossRef]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New Insights into an Ancient Ingredient against Cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Comparison of Duration of Treatment Interruption with or Without Curcumin During the Off Treatment Periods in Patients With Prostate Cancer Undergoing Intermittent Androgen Deprivation Therapy. Available online: https://ClinicalTrials.gov/show/NCT03211104 (accessed on 2 January 2020).
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Mahammedi, H.; Planchat, E.; Pouget, M.; Durando, X.; Curé, H.; Guy, L.; Van-Praagh, I.; Savareux, L.; Atger, M.; Bayet-Robert, M. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: A pilot phase II study. Oncology 2016, 90, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Radiosensitizing and Radioprotectve Effects of Curcumin in Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT01917890 (accessed on 2 January 2020).
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.; Molana, S.; Ehtejab, G. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Cancer Sci. 2013, 5, 320–324. [Google Scholar]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Adjuvant Curcumin to Assess Recurrence Free Survival in Patients Who Have Had a Radical Prostatectomy. Available online: https://ClinicalTrials.gov/show/NCT02064673 (accessed on 2 January 2020).
- Trial of Curcumin to Prevent Progression of Low-risk Prostate Cancer Under Active Surveillance. Available online: https://ClinicalTrials.gov/show/NCT03769766 (accessed on 2 January 2020).
| Molecular Target | Cell Lines/In-Vivo | Molecular Mechanism Modulated by Curcumin | Reference |
|---|---|---|---|
| Androgen receptor (AR) | LNCaP | Downregulated AR expression via limiting the binding activity to the ARE of the PSA gene | [100,101] |
| LNCaP | Inhibited cell proliferation and growth via modulation of AR and its signalling pathway | [102] | |
| LNCaP | Inhibited tumour growth and suppressed the PSA level by the activation of AR and interleukin-6 | [100] | |
| LNCaP & PC-3 | Downregulated AR expression and transcriptional activity | [103] | |
| LNCaP & TRAMP model | Decreased intracellular prostate testosterone level | [102] | |
| PC-3 | Reduced AR availability by altering the over-expressed heat shock protein (Hsp90) | [104] | |
| LNCaP xenograft | Delayed the tumour growth and suppressed AR expression | [105] | |
| LNCaP xenografts | Inhibited AR through the modulation of Wnt/ß-catenin signalling | [106] | |
| LNCaP & PC-3 | Downregulated the activation of AR-related cofactors | [100,103] | |
| LNCaP | Initiated apoptosis and downregulated the AR activity | [93] | |
| LNCaP | Reduced NKX3.1 and AR expression | [107] | |
| NF-κB | LNCaP & DU145 | Suppress NF-κB expression thus abrogates their survival mechanisms | [92] |
| PC-3 | Inhibited cell proliferation and induced apoptosis via suppressed NF-κB expression | [108] | |
| LNCaP | Suppressed cell proliferation through downregulation of cyclin D1 by inhibiting NF-κB | [92] | |
| PC-3 | Enhanced cytotoxicity by suppressed constitutional and TNF-α-induced NF-κB activation | [109] | |
| PC-3 mouse model | Prevented metastasis by downregulating CXCL-1 and -2 by targeting NF-κB signalling | [110] | |
| LNCaP, PC-3 & DU145 | Sensitised PCa cells towards TRAIL-induced apoptosis | [111,112] | |
| LNCaP | Initiated apoptosis by effecting intrinsic and extrinsic pathways | [111] | |
| LNCaP | Induced cytotoxicity by inhibiting phosphorylation and degradation of IκBα | [111] | |
| LNCaP & PC-3 | Combination of TRAIL inhibits Akt-regulated NF-κB and NF-κB-dependent anti-apoptotic proteins | [113] | |
| LNCaP & PC-3 | Chemosensitization to TRAIL therapy inhibited a constitutively active NF-κB, AP-1 and active anti-apoptotic Akt (p-Akt) | [112,113,114] | |
| PC-3 xenograft model | Combination with TRAIL inhibition the growth indicated by NF-κB and AP-1 inhibition | [115] | |
| Activating protein-1 (AP-1) | PC-3 & LNCaP | Suppressed tumour progression of AP-1, which indicated by the reduced colony forming ability in soft agar | [92,103] |
| PC-3 | Exhibited anti-cancer effects by impeding AP-1 protein | [108] | |
| LNCaP | Promoted cell cycle arrest and apoptosis by regulating the level of c-Jun proteins, which is activated via phosphorylation by the c-Jun amino terminal kinase (JNK) | [116,117] | |
| LNCaP | Reduced cell proliferation and migration by suppressing the activation of AP-1 which stimulated by hydrogen peroxide | [118] | |
| DU145 | Disruption of the survival pathways by sensitising the cells, thus potentiating TNF-induced apoptosis | [92] | |
| PI3K/Akt | LNCaP | Apoptosis and cell cycle arrest by downregulating PI3K/Akt/mTOR pathway | [119] |
| LNCaP, DU145 & PC-3 | Apoptosis by downregulating PI3K p110 and p85 subunits, and phosphorylation of Ser 473 Akt. | [120] | |
| PC-3 | Decreased PI3K activity mediated by changes in the phosphorylation status of Akt | [96] | |
| PC-3 | Inhibited the phosphorylation of Akt, mTOR, and their downstream substrates which directly affect the downstream of PI3K and PDK1 activities | [121] | |
| DU145 | Suppressed cell proliferation by inhibiting Akt/mTOR signalling | [121,122] | |
| Bcl-2 family | LNCaP | Induced apoptosis in concentration-dependent manner | [120] |
| LNCaP | Initiated apoptosis by translocation of Bax and p53 to mitochondria, the production of ROS, the release of mitochondrial proteins, and activation of caspase-3 | [120,123] | |
| LNCaP implanted nude mice | Induced apoptosis | [94] | |
| PC-3 & DU145 | Apoptosis and autophagy, mediated by cell cycle arrest at G2/M phase | [124] | |
| DU145 | Induced apoptosis by suppressing the Bcl-2 expression, while activating procaspase-3 simultaneously | [125] | |
| PC-3 nude mice model | Apoptosis by upregulating Bax and downregulating Bcl-2, and regulating the mitochondrial outer membrane permeability | [126] | |
| PC-3 | Apoptosis by mitochondria damage and cell ceramide accumulation | [127] | |
| PC-3 | Increased apoptotic cell death mediated by caspase activation and the loss of mitochondrial membrane integrity | [128] | |
| PC-3 | Induced the apoptosis proteins by inhibition of NF-κB and NF-κB-regulated anti-apoptotic genes products through suppression of Akt | [113] | |
| Cyclin D1 | LNCaP | Inhibited growth through cell cycle arrest indicated by downregulation of cyclin D1 expression via inhibition of CDK4-mediated phosphorylation of Rb protein | [92] |
| LNCaP & PC-3 | Induced cell cycle arrest at G1/S, followed by apoptosis | [97] | |
| LNCaP & PC-3 | Induced cell cycle arrest at G2/M phase | [129] | |
| DU145 | Induced G0/G1 arrest by suppression of cyclin D1 and CDK2 expression, while upregulating p21 and p27 | [125] | |
| LNCaP xenograft model | Suppressed cell proliferation by downregulating cyclin D1 and upregulating TRAIL-R1/DR4, TRAIL-R2/DR5, Bax, Bak, p21 and p27 proteins | [130]. | |
| LNCaP & LNCaP xenograft model | Downregulated cyclin D1 expression through inhibition of ß-catenin accumulation | [102,106]. | |
| LNCaP | Inhibiting ligand-induced activation for EGFR and its intrinsic tyrosine kinase activity associated with cyclin D1 downregulation | [131] | |
| PC-3 | Inhibited the EGFR phosphorylation | [132] | |
| Wnt/ß -catenin | LNCaP | Inhibited cell growth by reducing the level TCF-4, CBP, and p300 proteins that leads to the decrease of ß-catenin/TCF-4 transcriptional activity thus decreased β-catenin expression | [70,133] |
| LNCaP | Inhibited cancer growth by suppressing the Wnt/ß-catenin signalling pathway | [102,106] | |
| LNCaP | Inhibited cell proliferation by suppressing the GSK-3β phosphorylation thus inducing the degradation of β-catenin | [102] | |
| MiRNA | DU145 | Inhibited cancer growth and migration by upregulating the expression of miR-143 | [34,134] |
| LNCaP, PC-3 & DU145 | Inhibited cell proliferation and migration by restoring miR-143/miR-145 cluster expression | [135,136] |
| Intervention | Study | Status | Identifier Number/ Reference |
|---|---|---|---|
| Curcumin | Effects on PCa patients that undergo intermittent androgen deprivation (IAD) | Completed | NCT03211104/ [66] |
| Curcumin, Docetaxel & Prednisone | Combination with standard chemotherapy agents, docetaxel and prednisone in patients with castration-resistance PCa | Completed | *[286] |
| Curcumin | Radiosensitizing and radioprotective effects in PCa patients | Completed | NCT01917890/ [287] |
| Curcumin & Isoflavones | Combination with isoflavones who had prostate biopsy due to elevated PSA levels but do not have PCa | Completed | * [290] |
| Curcumin | Adjuvant use of curcumin after prostatectomy in improving recurrence-free survival for PCa patients | Recruiting | NCT02064673/ [291] |
| Curcumin | Effects on prevention progression of low-risk PCa under active surveillance | Recruiting | NCT03769766/ [292] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd. Wahab, N.A.; H. Lajis, N.; Abas, F.; Othman, I.; Naidu, R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679. https://doi.org/10.3390/nu12030679
Abd. Wahab NA, H. Lajis N, Abas F, Othman I, Naidu R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients. 2020; 12(3):679. https://doi.org/10.3390/nu12030679
Chicago/Turabian StyleAbd. Wahab, Nurul Azwa, Nordin H. Lajis, Faridah Abas, Iekhsan Othman, and Rakesh Naidu. 2020. "Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer" Nutrients 12, no. 3: 679. https://doi.org/10.3390/nu12030679
APA StyleAbd. Wahab, N. A., H. Lajis, N., Abas, F., Othman, I., & Naidu, R. (2020). Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients, 12(3), 679. https://doi.org/10.3390/nu12030679





