Extent of Food Processing and Risk of Prostate Cancer: The PROtEuS Study in Montreal, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
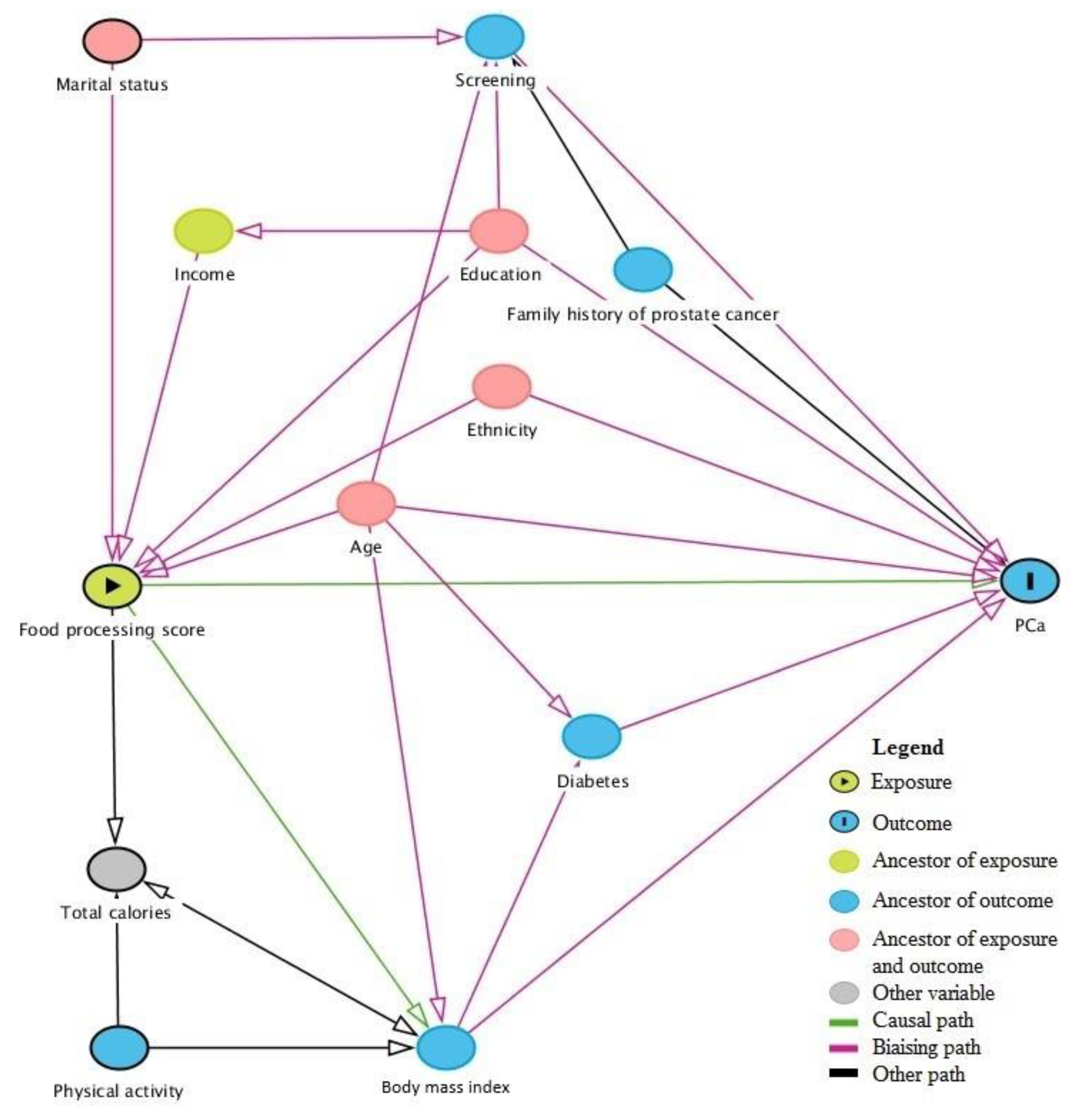
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Association between Food Classification and Prostate Cancer Risk
4. Discussion
4.1. Potential Mechanisms
4.2. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Société Canadienne du Cancer. Comité consultatif de la Société canadienne du cancer. In Statistiques Canadiennes sur le Cancer 2019; Société Canadienne du Cancer: Toronto, ON, Canada, 2019. [Google Scholar]
- Poirier, A.E.; Ruan, Y.; Walter, S.D.; Franco, E.L.; Villeneuve, P.J.; King, W.D.; Volesky, K.D.; O’Sullivan, D.E.; Friedenreich, C.M.; Brenner, D.R. The future burden of cancer in Canada: Long-term cancer incidence projections 2013–2042. Cancer Epidemiol. 2019, 59, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Chokkalingam, A.P. Prostate cancer epidemiology. Front. Biosci. 2006, 11, 1388–1413. [Google Scholar] [CrossRef] [PubMed]
- Schottenfeld, D.; Fraumeni, J.F. Cancer Epidemiology and Prevention, 3rd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- World Cancer Research Fund International/American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Prostate Cancer; Continuous Update Project Report; World Cancer Research Fund International: London, UK, 2014. [Google Scholar]
- Wilson, K.M.; Giovannucci, E.L.; Mucci, L.A. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J. Androl. 2012, 14, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Masko, E.M.; Allott, E.H.; Freedland, S.J. The Relationship between Nutrition and Prostate Cancer: Is More Always Better? Eur. Urol. 2013, 63, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A. Energy imbalance and prostate cancer. J. Nutr. 2002, 132 (Suppl. 11), 3471S–3481S. [Google Scholar] [CrossRef]
- Deschasaux, M.; Huybrechts, I.; Murphy, N.; Julia, C.; Hercberg, S.; Srour, B.; Kesse-Guyot, E.; Latino-Martel, P.; Biessy, C.; Casagrande, C.; et al. Nutritional quality of food as represented by the FSAm-NPS nutrient profiling system underlying the Nutri-Score label and cancer risk in Europe: Results from the EPIC prospective cohort study. PLoS Med. 2018, 15, e1002651. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.L.; Parra, D.; Ricardo, C.; et al. NOVA. The star shines bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Sante prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Martins, A.P.; Claro, R.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. Consumption of ultra-processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. 2013, 16, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Lajous, M. Ultra-processed foods and cancer. BMJ 2018, 360, k599. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Alles, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International/American Institute for Cancer Research. Wholegrains, Vegetables and Fruit and the Risk of Cancer; Continuous Update Project Expert Report; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Blanc-Lapierre, A.; Spence, A.; Karakiewicz, P.I.; Aprikian, A.; Saad, F.; Parent, M.E. Metabolic syndrome and prostate cancer risk in a population-based case-control study in Montreal, Canada. BMC Public Health 2015, 15, 913. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Ugnat, A.M.; Mao, Y.; Wen, S.W.; Johnson, K.C. Canadian Cancer Registries Epidemiology Research G: A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1521–1527. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Chan, T.Y.; Partin, A.W.; Walsh, P.C.; Epstein, J.I. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology 2000, 56, 823–827. [Google Scholar] [CrossRef]
- Mamede, A.C.; Tavares, S.D.; Abrantes, A.M.; Trindade, J.; Maia, J.M.; Botelho, M.F. The role of vitamins in cancer: A review. Nutr. Cancer 2011, 63, 479–494. [Google Scholar] [CrossRef]
- Lotan, R. Retinoids and apoptosis: Implications for cancer chemoprevention and therapy. J. Natl. Cancer Inst. 1995, 87, 1655–1657. [Google Scholar] [CrossRef]
- Fontana, J.A.; Rishi, A.K. Classical and novel retinoids: Their targets in cancer therapy. Leukemia 2002, 16, 463–472. [Google Scholar] [CrossRef]
- Heaney, M.L.; Gardner, J.R.; Karasavvas, N.; Golde, D.W.; Scheinberg, D.A.; Smith, E.A.; O’Connor, O.A. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008, 68, 8031–8038. [Google Scholar] [CrossRef]
- Parent, M.-E.; Richard, H.; Rousseau, M.-C.; Trudeau, K. Vitamin C intake and risk of prostate cancer: The Montreal PROtEuS Study. Front. Physiol. 2018, 9, 1218. [Google Scholar] [CrossRef]
- Cantoni, O.; Fiorani, M.; Cattabeni, F.; Bellomo, G. DNA breakage caused by hydrogen peroxide produced during the metabolism of 2-methyl-1,4-naphthoquinone (menadione) does not contribute to the cytotoxic action of the quinone. Biochem. Pharmacol. 1991, 42 (Suppl. 1), S220–S222. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Munoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. Health effects of resveratrol: Results from human intervention trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar]
- Xu, M.; Richardson, L.; Campbell, S.; Pintos, J.; Siemiatycki, J. Response rates in case-control studies of cancer by era of fieldwork and by characteristics of study design. Ann. Epidemiol. 2018, 28, 385–391. [Google Scholar] [CrossRef]
- Willet, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
| NOVA Food Group 1: Unprocessed or Minimally Processed Foods | |
| Examples of foods in NOVA group | Fresh fruits and vegetables, herbs, legumes, meat, poultry, fish, eggs, pasteurized milk, plain yogurt, tea, coffee, oats |
| Foods from the FFQ 2 | Banana, apple, orange, peaches, apricots, cantaloupe, watermelon, berries, potatoes, sweet potatoes, legumes, broccoli, carrots, spinach, cabbage, cauliflower, dark lettuce, tomatoes, sweet red pepper, beef, pork, chicken, veal, lamb, liver, fish, eggs, rice, oatmeal, milk, cream, tea, coffee |
| NOVA Food Group 2: Processed Culinary Ingredients | |
| Examples of foods in NOVA group | Salt, sugar, maple syrup, vegetable oils, butter |
| Foods from the FFQ | Butter |
| NOVA Food Group 3: Processed Foods | |
| Examples of foods in NOVA group | Cheese, fruits in syrup, beer, cider, wine |
| Foods from the FFQ | Tomato soup, vegetable soup, tofu, meat grilled on the barbeque, spaghetti, cheese, nuts, beer, wine |
| NOVA Food Group 4: Ultra-Processed Foods | |
| Examples of foods in NOVA group | Breakfast cereals, soft drinks, spirits, fruit yogurt, ice cream, chocolate, candies, pasta dishes, pizza, sausages, pastries, cakes, pre-prepared pasta |
| Foods from the FFQ | Fried potatoes, ketchup, salsa, salad dressing, mayonnaise, hot-dog, cheese macaroni, pizza, cookies, muffins, white bread, brown bread, breakfast cereal, real juice, tomato juice, dark carbonated soft, drinks, other carbonated soft drinks, margarine, fried food, chips, chocolate, yogurt, ice cream |
| Characteristics | Cases (n = 1919) | Controls (n = 1991) | ||
|---|---|---|---|---|
| Age in years, mean (SD) | 64 | (7) | 65 | (7) |
| Ethnicity, n (%) | ||||
| French | 1316 | (69) | 1154 | (58) |
| Black | 126 | (7) | 86 | (4) |
| Asian | 22 | (1) | 67 | (3) |
| Other European | 371 | (19) | 530 | (27) |
| Greater Middle Eastern | 42 | (2) | 100 | (5) |
| Latino | 33 | (2) | 31 | (2) |
| Family income in $ CAD, n (%) | ||||
| <20,000 | 223 | (12) | 245 | (12) |
| 20,000–29,999 | 262 | (14) | 252 | (13) |
| 30,000–49,999 | 445 | (23) | 462 | (23) |
| 50,000–79,999 | 422 | (22) | 410 | (21) |
| >80,000 | 425 | (22) | 428 | (22) |
| Unknown | 142 | (7) | 194 | (10) |
| Education, n (%) | ||||
| Primary school of less | 443 | (23) | 426 | (21) |
| High school | 572 | (30) | 578 | (29) |
| College | 313 | (16) | 375 | (19) |
| University | 588 | (31) | 610 | (31) |
| Other | 3 | (0.2) | 2 | (0.1) |
| Body mass index 2 years ago (kg/m2), mean (SD) | 26.8 | (4) | 27.1 | (4) |
| Ever smoked, n (%) | ||||
| No | 514 | (27) | 514 | (26) |
| Yes | 1404 | (73) | 1477 | (74) |
| Overall physical activity, n (%) | ||||
| Not very active | 431 | (23) | 488 | (25) |
| Moderately active | 522 | (27) | 558 | (28) |
| Very active | 965 | (50) | 945 | (47) |
| Timing of last prostate screening test by PSA 1 or DRE 2, n (%) | ||||
| <2 years before index date | 1903 | (99) | 1510 | (76) |
| 2–5 years before index date | 1 | (0.1) | 154 | (8) |
| >5 years before index date | 0 | (0) | 81 | (4) |
| Never screened | 2 | (0.1) | 190 | (10) |
| Unknown | 13 | (0.7) | 56 | (3) |
| First-degree relative with prostate cancer, n (%) | ||||
| No | 1409 | (73) | 1736 | (87) |
| Yes | 447 | (23) | 199 | (10) |
| Total calories/day 2 years before index date, mean | ||||
| (SD) | 1989 | (663) | 1917 | (646) |
| History of type 2 diabetes, n (%) | ||||
| No | 1627 | (85) | 1593 | (80) |
| Yes | 289 | (15) | 395 | (20) |
| Consumption Score by Category of Food Processing 2 | 1991 Controls n | All Prostate Cancers 1917 Cases 5 n Cases OR (95% CI) | Low-Grade Prostate Cancers 3 1386 Cases n Cases OR (95% CI) | High-Grade Prostate Cancers 4 530 Cases n Cases OR (95% CI) |
|---|---|---|---|---|
| Unprocessed/minimally processed foods | ||||
| Q1 (lower score) | 498 | 527, 1.00 (reference) | 376, 1.00 (reference) | 150, 1.00 (reference) |
| Q2 | 497 | 509, 0.98 (0.81–1.19) | 362, 0.97 (0.78–1.20) | 146, 1.01 (0.77–1.32) |
| Q3 | 498 | 497, 0.95 (0.78–1.15) | 366, 0.98 (0.79–1.21) | 130, 0.88 (0.67–1.17) |
| Q4 (higher score) | 498 | 386, 0.86 (0.70–1.07) | 282, 0.90 (0.71–1.12) | 104, 0.80 (0.59–1.08) |
| Processed foods | ||||
| Q1 (lower score) | 498 | 527, 1.00 (reference) | 376, 1.00 (reference) | 150, 1.00 (reference) |
| Q2 | 497 | 486, 1.18 (0.96–1.45) | 346, 1.18 (0.94–1.47) | 140, 1.20 (0.90–1.61) |
| Q3 | 498 | 484, 1.09 (0.89–1.34) | 361, 1.13 (0.90–1.41) | 121, 1.13 (0.90–1.41) |
| Q4 (higher score) | 498 | 533, 1.29 (1.05–1.59) | 381, 1.27 (1.02–1.60) | 151, 1.33 (0.99–1.78) |
| Ultra-processed foods | ||||
| Q1 (lower score) | 498 | 527, 1.00 (reference) | 376, 1.00 (reference) | 150, 1.00 (reference) |
| Q2 | 497 | 495, 0.86 (0.66–1.11) | 367, 0.85 (0.64–1.13) | 128, 0.87 (0.59–1.27) |
| Q3 | 499 | 479, 0.95 (0.74–1.22) | 342, 0.98 (0.75–1.29) | 135, 0.87 (0.61–1.26) |
| Q4 (higher score) | 497 | 516, 0.92 (0.72–1.17) | 364, 0.89 (0.68–1.16) | 151, 0.97 (0.68–1.38) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trudeau, K.; Rousseau, M.-C.; Parent, M.-É. Extent of Food Processing and Risk of Prostate Cancer: The PROtEuS Study in Montreal, Canada. Nutrients 2020, 12, 637. https://doi.org/10.3390/nu12030637
Trudeau K, Rousseau M-C, Parent M-É. Extent of Food Processing and Risk of Prostate Cancer: The PROtEuS Study in Montreal, Canada. Nutrients. 2020; 12(3):637. https://doi.org/10.3390/nu12030637
Chicago/Turabian StyleTrudeau, Karine, Marie-Claude Rousseau, and Marie-Élise Parent. 2020. "Extent of Food Processing and Risk of Prostate Cancer: The PROtEuS Study in Montreal, Canada" Nutrients 12, no. 3: 637. https://doi.org/10.3390/nu12030637
APA StyleTrudeau, K., Rousseau, M.-C., & Parent, M.-É. (2020). Extent of Food Processing and Risk of Prostate Cancer: The PROtEuS Study in Montreal, Canada. Nutrients, 12(3), 637. https://doi.org/10.3390/nu12030637





