Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic Hydrolysis of Sargassum maclurei Protein
2.3. ACE Inhibition Activity and Inhibition Kinetics
2.4. Purification by Gel Chromatography and RP-HPLC
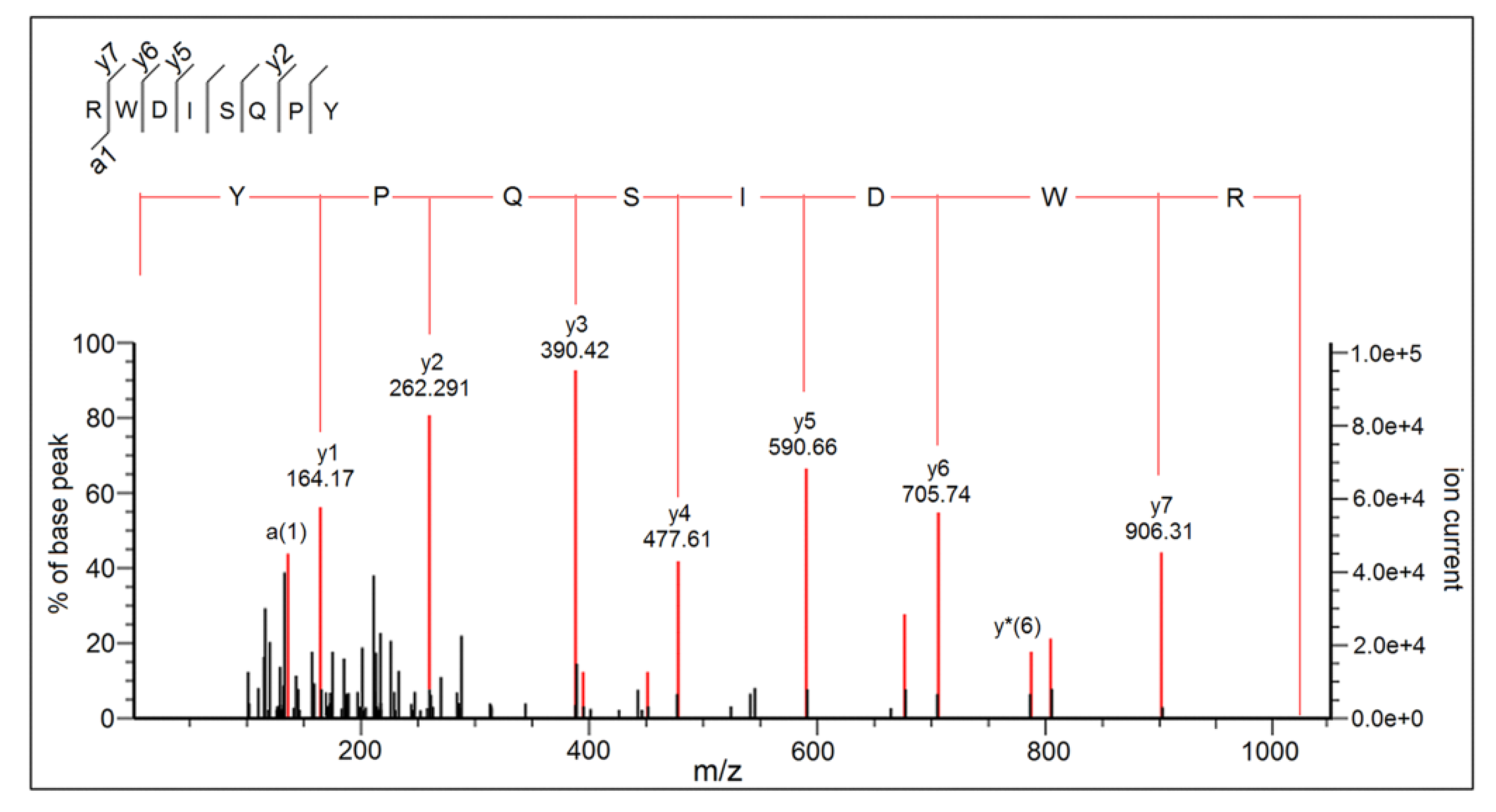
2.5. Peptide Sequence Identification
2.6. In silico Screening of Peptides and Chemical Synthesis
2.7. Molecular Docking
2.8. Antihypertensive Effect In Vivo
2.9. Effects on Intracellular Endothelin-1 (ET-1)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of SMPH
3.2. Purification of the ACE-Inhibitory Peptides
3.3. In Silico Screening and Chemical Synthesis of ACE-Inhibitory Peptides
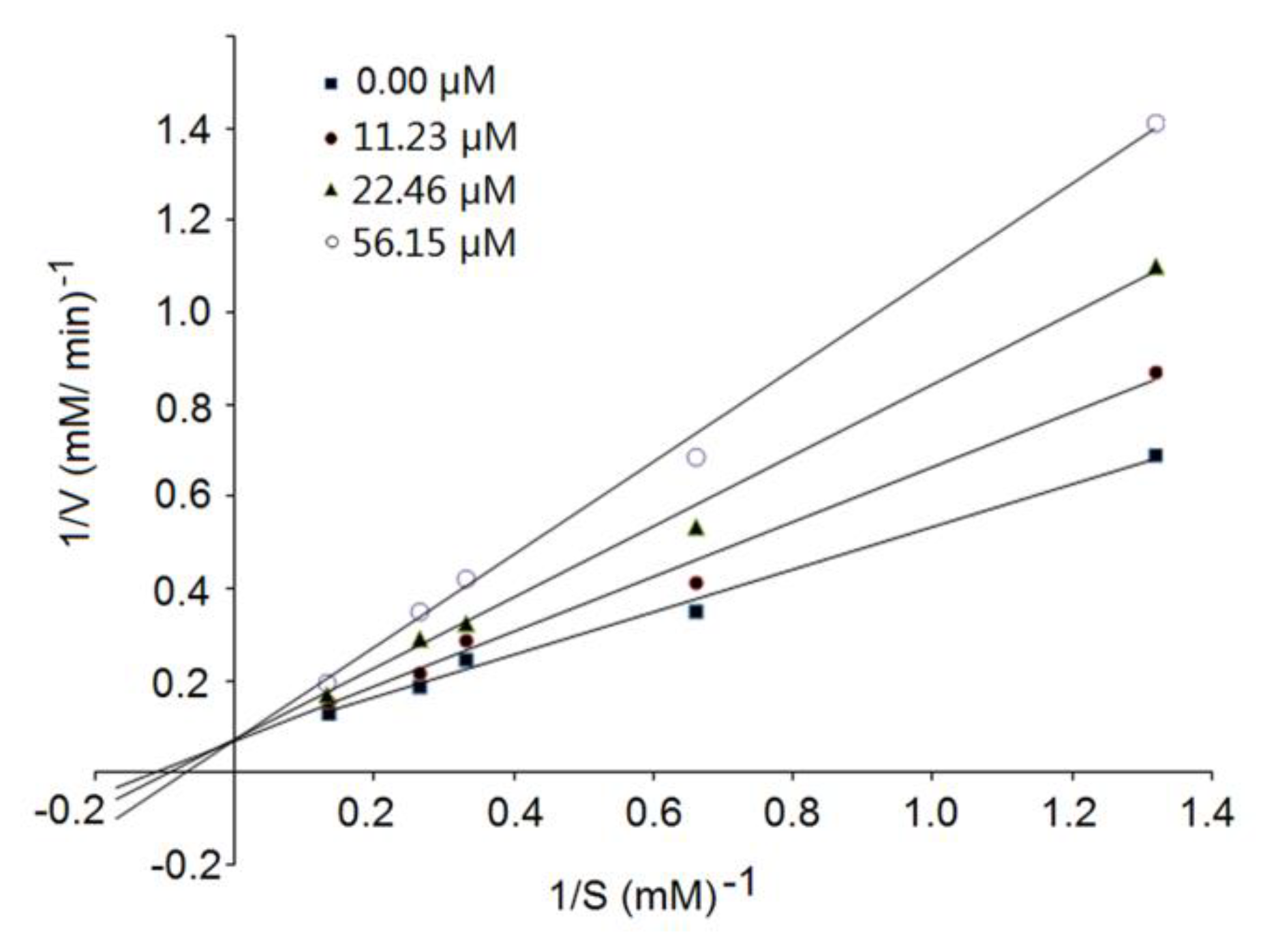
3.4. Inhibition Kinetics of Synthetic Peptides
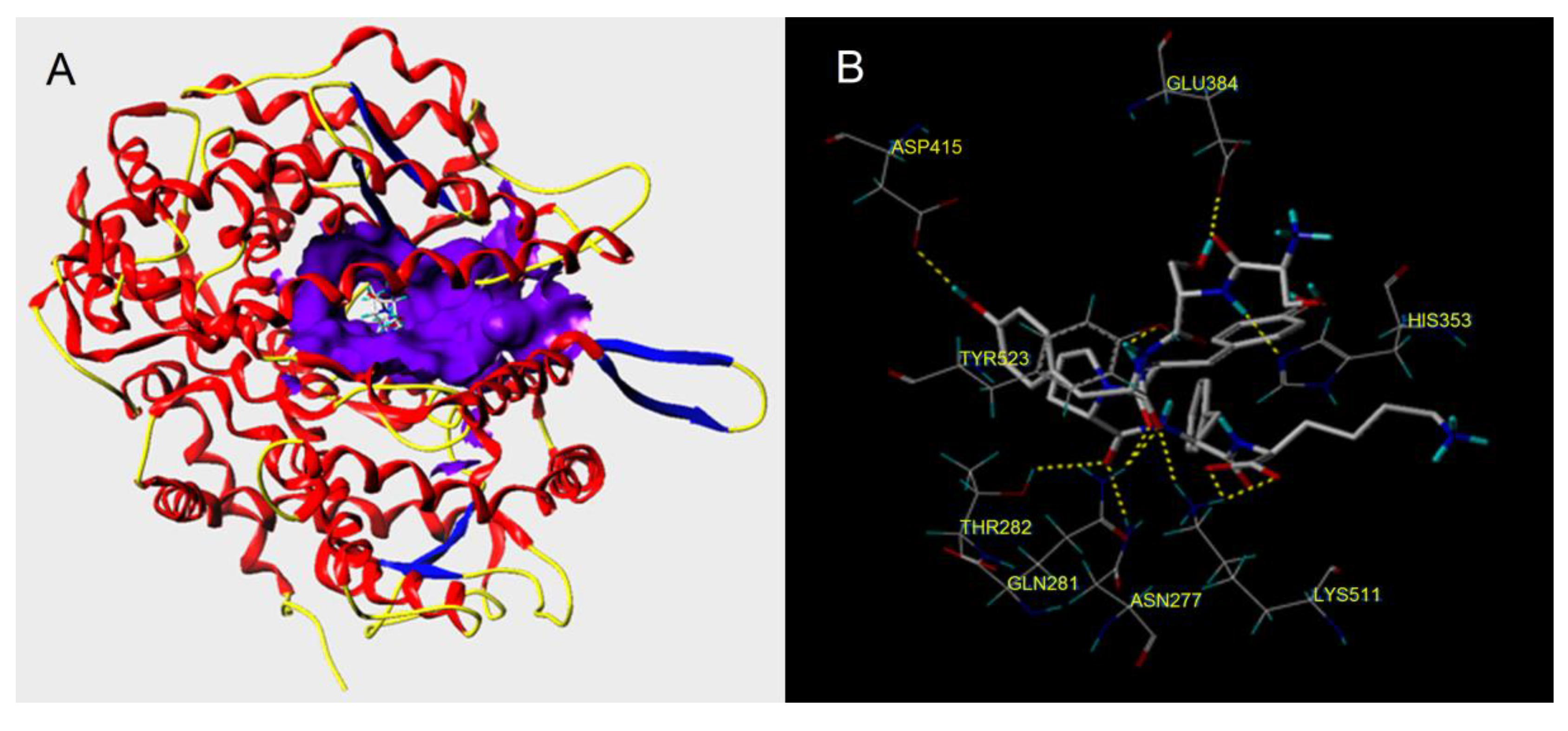
3.5. Molecular Docking Simulation
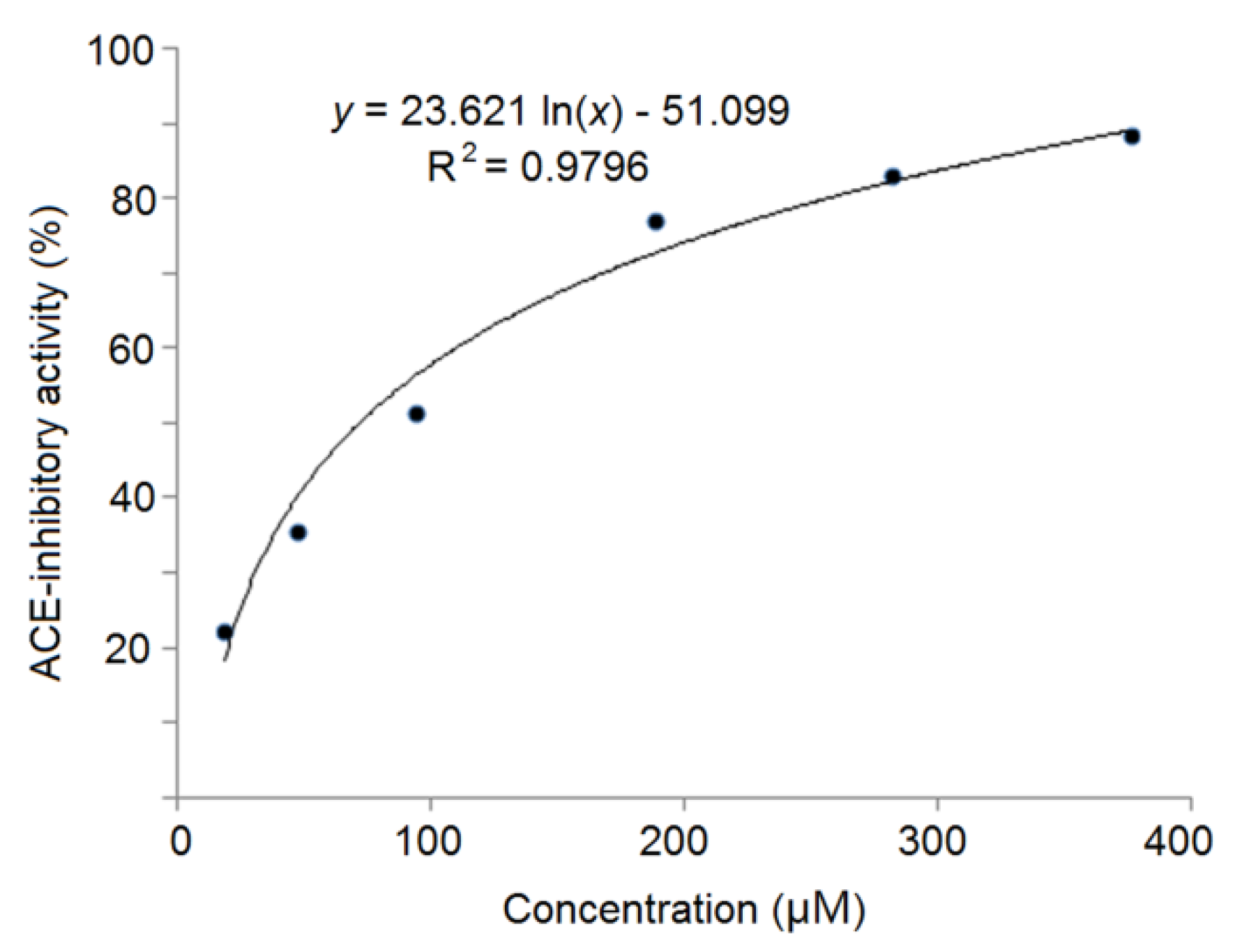
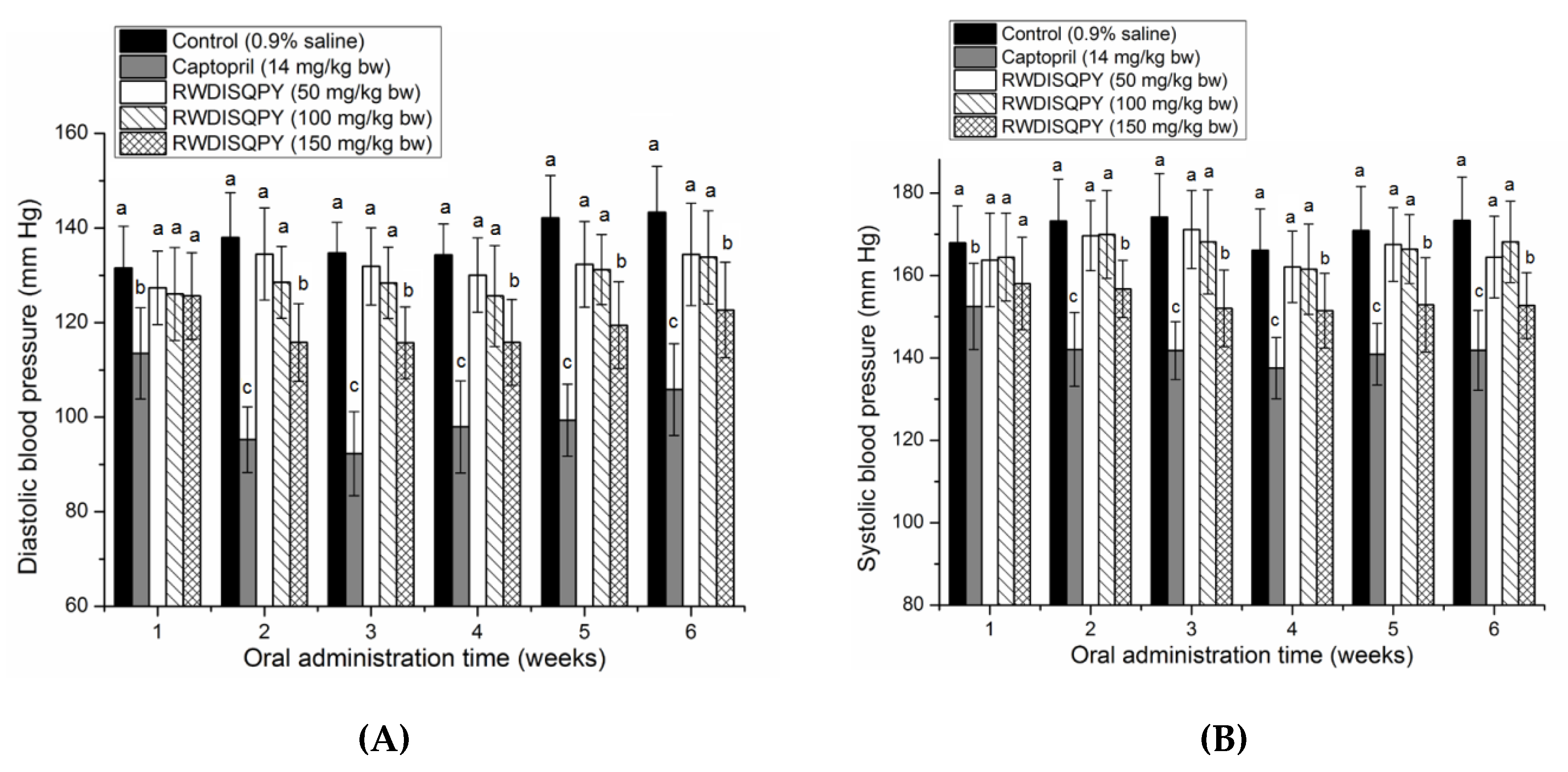
3.6. Antihypertensive Effect of Peptides
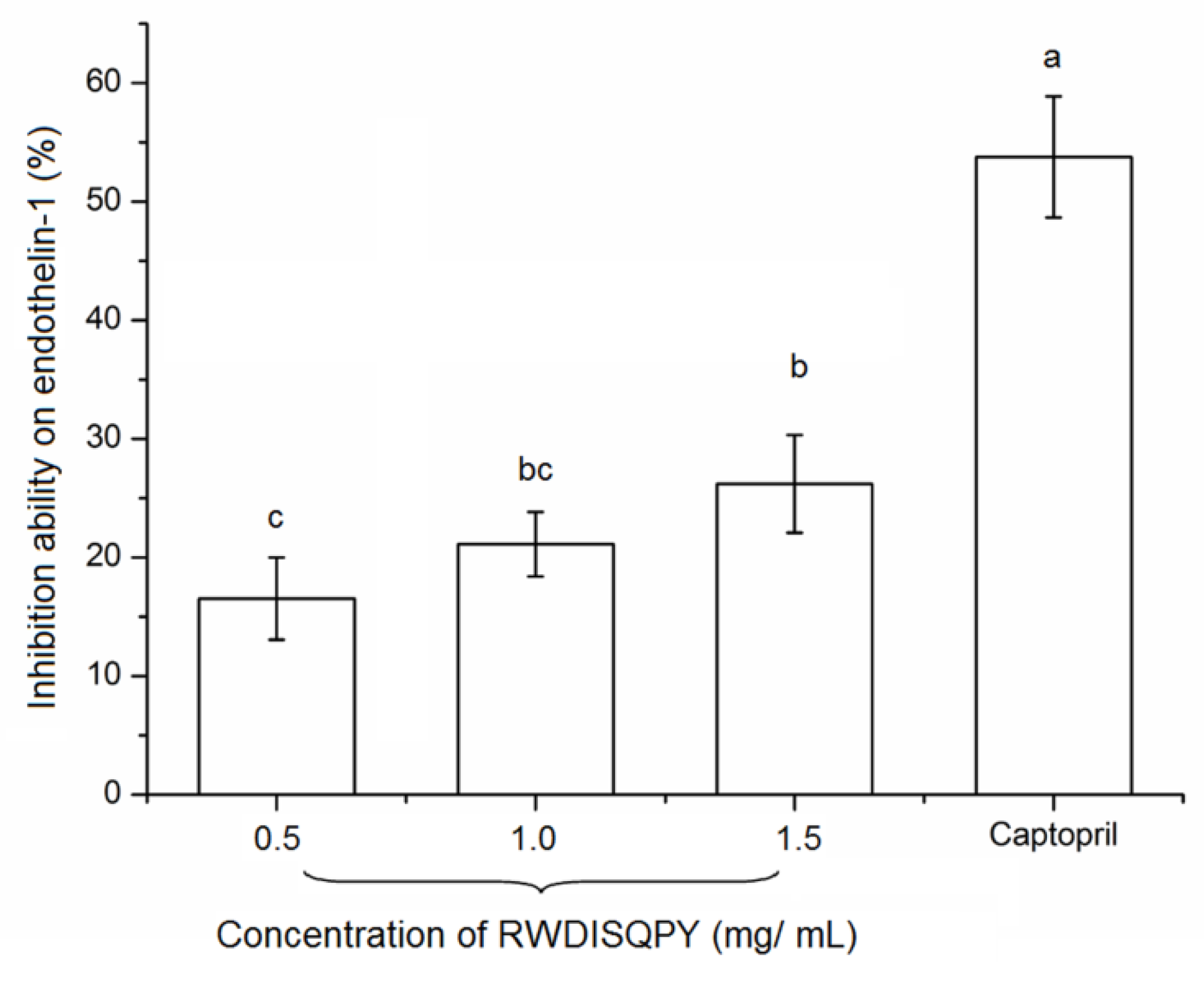
3.7. Effects on Intracellular Endothelin-1 (ET-1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hippauf, C.; Huettner, F.; Lunow, D.; Borchardt, L.; Henle, T.; Kaskel, S. Towards a continuous adsorption process for the enrichment of ACE inhibiting peptides from food protein hydrolysates. Carbon 2016, 107, 116–123. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.H.; Ryu, B.; Kim, S.K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Global Burden of Disease (GBD) 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Tao, J.; Chi, C.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Liu, X. Transport of a novel angiotensin-I-Converting enzyme inhibitory peptide Ala-His-Leu-Leu across Human intestinal epithelial Caco-2 Cells. J. Med. Food. 2017, 20, 243–250. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Ling, Y.; Sun, L.P.; Zhuang, Y.L. Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food Funct. 2018, 9, 5251–5259. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Brayden, D.J.; Ryan, S.M. Evaluation of Pep T1 transport of food-derived antihypertensive peptides, Ile-Pro-Pro and Leu-Lys-Pro using in vitro, ex vivo, and in vivo, transport models. Eur. J. Pharm. Biopharm. 2017, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, J.; Lee, Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr. Res. Pract. 2016, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Husni, A.; Lailatussifa, R.; Isnansetyo, A. Sargassum hystrix as a source of functional food to improve blood biochemistry profiles of rats under stress. Prev. Nutr. Food Sci. 2019, 24, 150–158. [Google Scholar] [CrossRef]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef]
- Yinting, L.; Bingjie, C.; Weidu, W.; Ge, K.; Wei, X.Y.; Kong, L.M.; Xie, Y.Y.; Gu, J.P.; Zhang, J.C.; Zhou, T. Antioxidant and antimicrobial evaluation of carboxymethylated and hydroxamated degraded polysaccharides from Sargassum fusiforme. Int. J. Biol. Macromol. 2018, 118, 1550–1557. [Google Scholar]
- Lim, S.J.; Choi, A.H.; Kwon, M.; Joung, E.J.; Shin, T.; Lee, S.G.; Kim, N.G.; Kim, H.R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falque, E.; Dominguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.-M.; Kang, B.-K.; Kim, K.B.W.R.; Ahn, D. Anti-inflammatory effects of grasshopperketone from Sargassum fulvellum ethanol extract on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Microbiol. Biotechnol. 2019, 29, 820–826. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P.P. Macroalgal-derived protein hydrolysates and bioactive peptides: Enzymatic release and potential health enhancing properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Mæhre, H.; Jensen, I.J.; Eilertsen, K.E. Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs 2016, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.G.; Lin, L.D.; Xu, T.; Zhang, X.; Wang, L.H.; Zou, H.X.; Wu, M.J.; Yan, X.F. Parallel analysis of proteins in brown seaweed Sargassum fusiforme responding to hyposalinity stress. Aquaculture 2016, 465, 189–197. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Cheng, D.Y. Novel angiotensin I-converting enzyme inhibitory peptides from protease hydrolysates of Qula casein: Quantitative structure-activity relationship modeling and molecular docking study. J. Funct. Foods 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, G.W.; Yeh, C.H.; Song, H.; Tsai, J.S. Purification and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides and the Antihypertensive Effect of Chlorella sorokiniana Protein Hydrolysates. Nutrient 2018, 10, 1397. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, Y.; Zhang, Y.L.; Ruan, X.H.; Zhang, R.G.; Zhang, Y.F.; Zhao, S.L. Purification, characterization, synthesis, in vivo ACE inhibition and in vitro antihypertensive activity of bioactive peptides derived from palm kernel expeller glutelin-2 hydrolysates. J. Funct. Foods 2017, 28, 48–58. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Montone, R.Z.; Laganà, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef] [PubMed]
- Tondo, A.R.; Caputo, L.; Mangiatordi, G.F.; Monaci, L.; Lentini, G.; Logrieco, A.F.; Montaruli, M.; Nicolotti, O.; Quintieri, L. Structure-Based Identification and Design of Angiotensin Converting Enzyme-Inhibitory Peptides from Whey Proteins. J. Agric. Food Chem. 2020, 68, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Wang, J.L.; He, G.Q.; Wu, J. Purification, identification, and in vivo activity of angiotensin I-converting enzyme inhibitory peptide, from ribbon fish (Trichiurus haumela) backbone. J. Food Sci. 2014, 79, C1–C7. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Protein quality evaluation. In FAO/WHO Nutrition Meetings, Report Series 51; Food and Agricultural Organization/World Health Organization: Rome, Italy, 1990.
- Lammi, C.; Zanoni, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Hypocholesterolaemic Activity of Lupin Peptides: Investigation on the Crosstalk between Human Enterocytes and Hepatocytes Using a Co-Culture System Including Caco-2 and HepG2 Cells. Nutrient 2016, 8, 437. [Google Scholar] [CrossRef]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-Huertas, E. Antihypertensive Effects of Virgin Olive Oil (Unfiltered) Low Molecular Weight Peptides with ACE Inhibitory Activity in Spontaneously Hypertensive Rats. Nutrient 2020, 12, 271. [Google Scholar] [CrossRef]
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Abdul-Hamid, A.; Saari, N. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: Stability study against the ACE and inhibition kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Purification of novel angiotensin converting enzyme inhibitory peptides from beef myofibrillar proteins and analysis of their effect in spontaneously hypertensive rat model. Biomed Pharmacother. 2019, 116, 109046. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jing, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Bhaskar, B.; Ananthanarayana, L.; Jamdar, S. Purification, identification, and characterization of novel angiotensin I converting enzyme (ACE) inhibitory peptides from alcalase digested horse gram flour. LWT Food Sci. Technol. 2019, 103, 155–161. [Google Scholar] [CrossRef]
- Rohit, A.C.; Sathisha, K.; Aparna, H.S. A variant peptide of buffalo colostrum β-lactoglobulin inhibits angiotensin I-converting enzyme activity. Eur. J. Med. Chem. 2012, 53, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Rij, A.V.; Solomon, C.; Thomson, I.A.; Packer, S.G.K. Endothelin-1 is increased overlying atherosclerotic plaques in human arteries. Atherosclerosis 1996, 124, 25–35. [Google Scholar] [CrossRef]
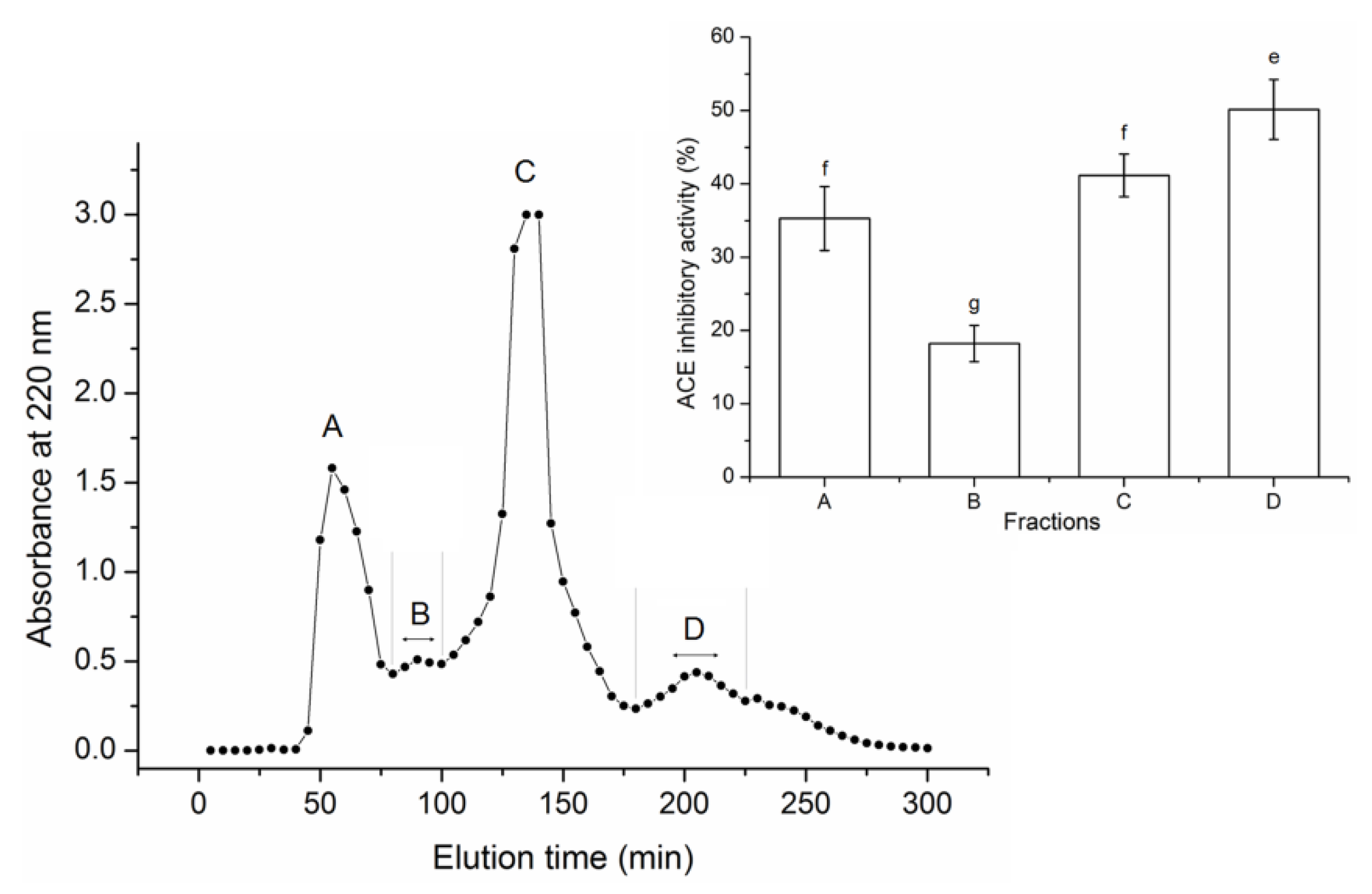

| Amino Acid | Thr | Leu | Ile | Val | Phe | Lys | Met | Tyr | Trp |
| Content | 3.62 ± 0.16 | 6.20 ± 0.38 | 3.75 ± 0.23 | 4.67 ± 0.22 | 4.05 ± 0.14 | 4.11 ± 0.12 | 1.29 ± 0.09 | 2.13 ± 0.17 | 2.33 ± 0.22 |
| Amino Acid | Asp | Glu | Cys | Ser | His | Gly | Pro | Ala | Arg |
|---|---|---|---|---|---|---|---|---|---|
| Content | 8.27 ± 0.06 | 29.77 ± 2.23 | 3.50 ± 0.43 | 3.32 ± 0.12 | 1.35 ± 0.08 | 4.26 ± 0.20 | 3.93 ± 0.62 | 7.92 ± 0.63 | 3.87 ± 0.12 |
| Peptide | Theo. MH+ (Da) | B (μM) | ALC (%) | SVMS | Prediction | Hydrophobicity | Net Hydrogen | Calculated pI | Solvation | IC50 (μM) on ACE-Inhibitory Activity |
|---|---|---|---|---|---|---|---|---|---|---|
| RVLSAAFNTR | 1134.42 | ND | 93 | −1.19 | Non-AHT | −0.24 | 1.20 | 12.01 | 0.28 | ND |
| IMNILEK | 860.16 | 0.0012 | 77 | −0.46 | Non-AHT | −0.02 | 0.71 | 6.35 | 0.88 | ND |
| GGVQAIR | 699.90 | 0.0333 | 71 | −0.86 | Non-AHT | −0.09 | 0.86 | 10.11 | 0.25 | ND |
| KAALMEK | 790.07 | ND | 75 | −1.20 | Non-AHT | −0.22 | 0.71 | 8.94 | 0.53 | ND |
| GVFDGPCGT | 852.05 | ND | 80 | −0.43 | Non-AHT | 0.08 | 0.22 | 3.80 | 0.52 | ND |
| SGVFDGPCGT | 939.14 | ND | 81 | −0.35 | Non-AHT | 0.04 | 0.30 | 3.80 | 0.47 | ND |
| QNIGDPR | 798.94 | 0.0016 | 77 | −0.27 | Non-AHT | −0.43 | 1.29 | 6.19 | −0.15 | ND |
| AYSSGVSFK | 945.15 | 0.0044 | 73 | −0.80 | Non-AHT | −0.03 | 0.67 | 8.94 | 0.61 | ND |
| RWDISQPY | 1063.47 | 0.0016 | 91 | 0.34 | AHT | −0.30 | 1.25 | 6.19 | 0.47 | 72.24 |
| LVYIVQGR | 947.25 | 0.0012 | 78 | −0.74 | Non-AHT | 0.01 | 0.88 | 9.10 | 0.76 | ND |
| KPGGSGR | 657.82 | 0.0733 | 70 | −0.10 | Non-AHT | −0.39 | 1.00 | 11.01 | −0.21 | ND |
| LGLSAKNYGR | 1078.36 | 0.0008 | 77 | −0.68 | Non-AHT | −0.21 | 1.00 | 10.01 | 0.28 | ND |
| KEAWLIEK | 1016.31 | 0.0411 | 90 | −0.75 | Non-AHT | −0.20 | 0.88 | 6.49 | 0.55 | ND |
| REVADDK | 831.96 | 0.0007 | 74 | −0.02 | Non-AHT | −0.59 | 1.29 | 4.56 | −0.52 | ND |
| ENFFFAGIDK | 1187.44 | ND | 87 | −0.69 | Non-AHT | −0.01 | 0.60 | 4.38 | 0.63 | ND |
| QEMVDK | 748.93 | ND | 73 | −0.10 | Non-AHT | −0.39 | 1.00 | 4.38 | 0.19 | ND |
| EEEEEEQQQ | 1177.23 | 0.0372 | 79 | −0.30 | Non-AHT | −0.64 | 1.33 | 3.46 | −0.58 | ND |
| Ligand | T-Score | Hydrogen Bonds Number | Distance (Å) |
|---|---|---|---|
| RWDISQPY | 10.70 | 10 | Glu384: 1.99; ASP415:2.08; His353:2.72; Tyr523:2.52; Thr282:1.82; 2.04; Gln281:2.04; Asn277:2.73; Lys511:2.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhang, Y.; San, S. Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1. Nutrients 2020, 12, 653. https://doi.org/10.3390/nu12030653
Zheng Y, Zhang Y, San S. Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1. Nutrients. 2020; 12(3):653. https://doi.org/10.3390/nu12030653
Chicago/Turabian StyleZheng, Yajun, Yufeng Zhang, and Sang San. 2020. "Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1" Nutrients 12, no. 3: 653. https://doi.org/10.3390/nu12030653
APA StyleZheng, Y., Zhang, Y., & San, S. (2020). Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1. Nutrients, 12(3), 653. https://doi.org/10.3390/nu12030653





