Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens
Abstract
1. Introduction
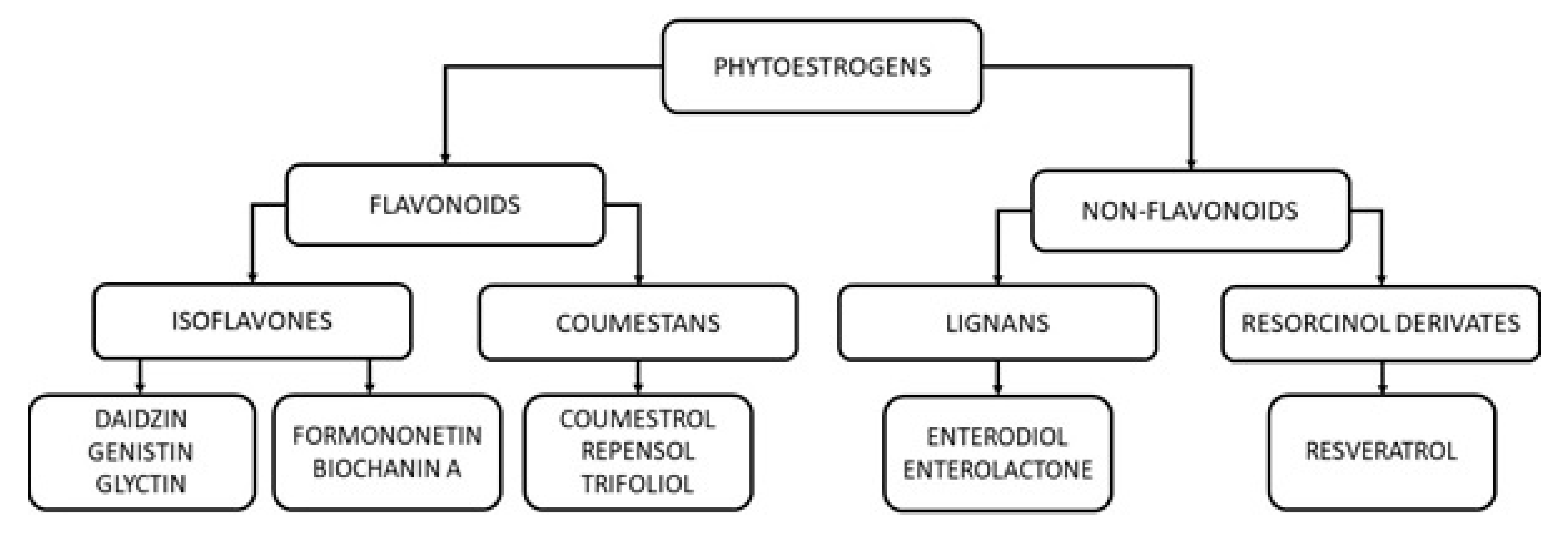
2. Groups, Classes and Natural Sources of Phytoestrogens
3. Modes of Phytoestrogen Action
3.1. Modulation of Transcriptional Activity of Nuclear Receptors
3.2. Nuclear Receptor-Independent Mechanisms of Phytoestrogen Action
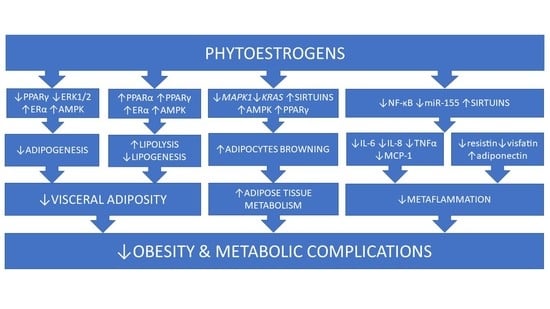
4. Role of Phytoestrogens in Adipose Tissue Development and Distribution
4.1. Adipogenesis
4.2. Adipose Tissue Browning
5. Role of Phytoestrogens in Adipose Tissue Metabolism
5.1. Lipogenesis and Lipolysis
5.2. Adipose Tissue Inflammation and Secretory Activity
6. Role of Phytoestrogens in the Management of Obesity and Related Complications
6.1. Influence of Phytoestrogens on Body Weight and Composition
6.2. Influence of Phytoestrogens on Hyperlipidemia and Liver Steatosis
6.3. Influence of Phytoestrogens on Glucose Metabolism and Diabetes
7. Final Remarks and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO report 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 February 2020).
- Gaggini, M.; Saponaro, C.; Gastaldelli, A. Not all fats are created equal: adipose vs. ectopic fat, implication in cardiometabolic diseases. Horm. Mol. Biol. Clin. Invest. 2015, 22, 7–18. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 levels are higher in subcutaneous adipose tissue, but obesity is associated with their increased content in visceral fat depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Han, T.S.; Seidell, J.C. Impairment of health and quality of life in people with large waist circumference. Lancet. 1998, 351, 853–856. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Moore, S.C.; Jacobs, E.J.; Kitahara, C.M.; Rosenberg, P.S.; Adami, H.O.; Ebbert, J.O.; English, D.R.; Gapstur, S.M.; Giles, G.G.; et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo. Clin. Proc. 2014, 89, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [PubMed]
- White, U.A.; Tchoukalova, Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.; Wing, A.; Holtrup, B.; Sebo, Z.; Kaplan, J.L.; Saavedra-Peña, R.; Church, C.D.; Colman, L.; Berry, R.; Rodeheffer, M.S. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016, 24, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef]
- Riggs, B.L.; Hartmann, L.C. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003, 348, 618–629. [Google Scholar] [CrossRef]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef]
- Hasenbrink, G.; Sievernich, A.; Wildt, L.; Ludwig, J.; Lichtenberg-Fraté, H. Estrogenic effects of natural and synthetic compounds, including tibolone assessed in Saccharomyces cerevisiae expressing the human estrogen alpha and beta receptors. FASEB. J. 2006, 20, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J., Jr.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachan, J.A.; et al. Minireview: endocrine disruptors: past lessons and future directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Shahnazaryan, U.; Wójcik, M.; Bednarczuk, T.; Kuryłowicz, A. Role of obesogens in the pathogenesis of obesity. Medicina (Kaunas) 2019, 55, 515. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Kirichenko, T.V.; Melnichenko, A.A.; Orekhova, V.A.; Ravani, A.; Poggio, P.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Anti-atherosclerotic effects of a phytoestrogen-rich herbal preparation in postmenopausal women. Int. J. Mol. Sci. 2016, 17, 1318. [Google Scholar] [CrossRef]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity o phytoestrogens: a review. Adv. Technol. 2017, 6, 96–106. [Google Scholar]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Piskula, M.K. Factors affecting flavonoids absorption. Biofactors 2000, 12, 175–180. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytoestrogens. Ann. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef]
- Duncan, A.M.; Phipps, W.R.; Kurzer, M.S. Phyto-oestrogens. Best Prac.t Res. Clin. Endocrinol. Metab. 2003, 17, 253–271. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: a review of the present state of research. Phytother Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and its human metabolites – effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuznicka, M.; Pawlik-Pachucka, E.; Owczarz, M.; Budzinska, M.; Polosak, J. Small-molecule Hormones: Molecular Mechanisms of Action. Int. J. Endocrinol. 2013, 601246. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Rowland, I.R. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br. J. Nutr. 2004, 91, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Löwik, C.W. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J. Bone Miner. Res. 2004, 19, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Le Maire, A.; Grimaldi, M.; Roecklin, D.; Dagnino, S.; Vivat-Hannah, V.; Balaguer, P.; Bourguet, W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar]
- Shen, P.; Liu, M.H.; Ng, T.Y.; Chan, Y.H.; Yong, E.L. Differential effects of isoflavones, from Astragalus membranaceus and Pueraria thomsonii, on the activation of PPARα, PPARγ, and adipocyte differentiation in vitro. J. Nutr. 2006, 136, 899–905. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C. Targeting the redox-sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R.; Levin, E.R. Extranuclear steroid receptors: nature and actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kühne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Phytoestrogens and the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2014, 139, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Yun, H.; Lee, E.B.; Min, B.I.; Bae, H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Khedgikar, V.; Kushwaha, P.; Choudhary, D.; Nagar, G.K.; Dev, K.; Dixit, P.; Singh, D.; Maurya, R.; Trivedi, R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017, 117, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.C.; Li, Y.L.; Qin, Y.F.; Quarles, L.D.; Xu, K.K.; Li, R.; Zhou, H.H.; Xiao, Z.S. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J. Cell. Biochem. 2008, 104, 1853–1864. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Role of sirtuins in adipose tissue development and metabolism. In Adipose Tissue - An Update, 3rd ed.; Szablewski, L., Ed.; IntechOpen: London, UK, 2019; Volume 4, pp. 13–28. [Google Scholar]
- Setchell, K.D.; Brown, N.M.; Desai, P.B.; Zimmer-Nechimias, L.; Wolfe, B.; Jakate, A.S.; Creutzinger, V.; Heubi, J.E. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003, 133, 1027–1035. [Google Scholar] [CrossRef]
- Blomquist, C.H.; Lima, P.H.; Hotchkiss, J.R. Inhibition of 3α-hydroxysteroid dehydrogenase (3α-HSD) activity of human lung microsomes by genistein, daidzein, coumestrol and C(18)-, C(19)- and C(21)-hydroxysteroids and ketosteroids. Steroids 2005, 70, 507–514. [Google Scholar] [CrossRef]
- Wang, C.; Mäkelä, T.; Hase, T.; Adlercreutz, H.; Kurzer, M.S. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1994, 50, 205–212. [Google Scholar] [CrossRef]
- Zhang, A.J.; Rimando, A.M.; Mizuno, C.S.; Mathews, S.T. α-Glucosidase inhibitory effect of resveratrol and piceatannol. J. Nutr. Biochem. 2017, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. (Lond) 2016, 130, 943–986. [Google Scholar] [CrossRef] [PubMed]
- Pudenz, M.; Roth, K.; Gerhauser, C. Impact of soy isoflavones on the epigenome in cancer prevention. Nutrients 2014, 6, 4218–4272. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sharma, P.; Capalash, N. DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer. Curr. Cancer Drug Targets 2013, 13, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Shi, H.; Hewett, J.E.; Ruhlen, R.L.; MacDonald, R.S.; Rottinghaus, G.E.; Chen, Y.C.; Sauter, E.R. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef]
- Howard, T.D.; Ho, S.M.; Zhang, L.; Chen, J.; Cui, W.; Slager, R.; Gray, S.; Hawkins, G.A.; Medvedovic, M.; Wagner, J.D. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS ONE 2011, 6, e26791. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19, 2118. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuznicka, M. NGS reveals molecular pathways affected by obesity and weight loss-related changes in miRNA levels in adipose tissue. Int. J. Mol. Sci. 2017, 19, 66. [Google Scholar]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. Genistein protects against ox-LDL-induced inflammation through microRNA-155/SOCS1-mediated repression of NF-ĸB signaling pathway in HUVECs. Inflammation 2017, 40, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Pochetti, G.; Dossou, K.S.S.; Laghezza, A.; Montanari, R.; Capelli, D.; Prada, E.; Loiodice, F.; Massolini, G.; Bernier, M.; et al. Resveratrol and Its Metabolites Bind to PPARs. Chembiochem. 2014, 15, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Floyd, Z.E.; Wang, Z.Q.; Kilroy, G.; Cefalu, W.T. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism 2008, 57, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Huang, B.; Choi, S.K.; Seo, J.S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, N.K.; Thungapathra, M. Resveratrol regulates body weight in healthy and ovariectomized rats. Nutr. Metab. (Lond) 2017, 14, 30. [Google Scholar] [CrossRef]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomiyama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) α or β. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [CrossRef]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Pampaloni, B.; Tanini, A.; Brandi, M.L.; Alonso-Magdalena, P.; Nadal, A.; Ottobrini, L.; et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006, 147, 5740–5751. [Google Scholar] [CrossRef]
- Zanella, I.; Marrazzo, E.; Biasiotto, G.; Penza, M.; Romani, A.; Vignolini, P.; Caimi, L.; Di Lorenzo, D. Soy and the soy isoflavone genistein promote adipose tissue development in male mice on a low-fat diet. Eur. J. Nutr. 2015, 54, 1095–1107. [Google Scholar] [CrossRef]
- Park, H.J.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Ambati, S.; Baile, C.A. Genistein inhibits differentiation of primary human adipocytes. J. Nutr. Biochem. 2009, 20, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.; Frank, O.; Kampmann, G.; Sochocky, N.; Pennimpede, T.; Fuchs, P.; Hunziker, W.; Weber, P.; Martin, I.; Bendik, I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004, 145, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Relic, B.; Zeddou, M.; Desoroux, A.; Beguin, Y.; de Seny, D.; Malaise, M.G. Genistein induces adipogenesis but inhibits leptin induction in human synovial fibroblasts. Lab. Invest. 2009, 89, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.H.; Ji, H.; Kim, J.E.; Yoo, R.; Kim, J.H.; Suk, S.; Huh, C.S.; Park, J.H.Y.; Heo, Y.S.; et al. Orobol, an enzyme-convertible product of genistein, exerts anti-obesity effects by targeting casein kinase 1 epsilon. Sci. Rep. 2019, 9, 8942. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; Powell, H.A.; Rajic, L.; Korach, K.S. The role of dietary phytoestrogens and the nuclear receptor PPARγ in adipogenesis: An in vitro study. Environ. Health Perspect. 2019, 127, 37007. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yoon, M. 17β-Estradiol inhibition of PPARγ-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol. Sin. 2011, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic activation of estrogen receptor β increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J. 2017, 31, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Su, S.J.; Yeh, Y.T.; Su, S.H.; Chang, K.L.; Shyu, H.W.; Chen, K.M.; Yeh, H. Biochanin a promotes osteogenic but inhibits adipogenic differentiation: evidence with primary adipose-derived stem cells. Evid. Based Complement Alternat. Med. 2013, 2013, 846039. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, J.; Xiang, H.; Zeng, Y.Y.; Li, X.B.; Xiao, H.; Chen, D.Y.; Ma, R.L. Synthesis and biological evaluation of new flavonoid fatty acid esters with anti-adipogenic and enhancing glucose consumption activities. Bioorg. Med. Chem. 2011, 19, 3192–3203. [Google Scholar] [CrossRef]
- Jang, M.K.; Yun, Y.R.; Kim, J.H.; Park, M.H.; Jung, M.H. Gomisin N inhibits adipogenesis and prevents high-fat diet-induced obesity. Sci. Rep. 2017, 7, 40345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, H.; Jin, Q.; You, W.; Cheng, H.; Liu, Y.; Song, E.; Liu, G.; Tan, X.; Zhang, X.; et al. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signaling pathway in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2018, 439, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-induced white adipose tissue browning in obese mice by remodeling fecal microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Porter, J.P.; Lund, T.D.; Bu, L.; Setchell, K.D.; Ramoz, G.; Crowley, W.R. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr. Metab. (Lond) 2004, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Zhao, S.; Mao, L.; Yang, Y.; Sun, W.; Lin, X.; Liu, S.; Li, K.; Sun, Y.; Li, P.; et al. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARγ activity. Br. J. Pharmacol. 2018, 175, 1439–1450. [Google Scholar] [CrossRef]
- Kim, S.N.; Ahn, S.Y.; Song, H.D.; Kwon, H.J.; Saha, A.; Son, Y.; Cho, Y.K.; Jung, Y.S.; Jeong, H.W.; Lee, Y.H. Antiobesity effects of coumestrol through expansion and activation of brown adipose tissue metabolism. J. Nutr. Biochem. 2019, 76, 108300. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef]
- Lundholm, L.; Putnik, M.; Otsuki, M.; Andersson, S.; Ohlsson, C.; Gustafsson, J.A.; Dahlman-Wright, K. Effects of estrogen on gene expression profiles in mouse hypothalamus and white adipose tissue: target genes include glutathione peroxidase 3 and cell death-inducing DNA fragmentation factor, α-subunit-like effector A. J. Endocrinol. 2008, 196, 547–557. [Google Scholar] [CrossRef]
- van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; van ’t Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: a double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Igarashi, K.; Yu, C. Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nogowski, L.; Maćkowiak, P.; Kandulska, K.; Szkudelski, T.; Nowak, K.W. Genistein-induced changes in lipid metabolism of ovariectomized rats. Ann. Nutr. Metab. 1998, 42, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Jeong, T.S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Son, H.J.; Ahn, J.; Jung, C.H.; Ha, T. Coumestrol modulates Akt and Wnt/β-catenin signaling during the attenuation of adipogenesis. Food Funct. 2016, 7, 4984–4991. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345. [Google Scholar] [CrossRef]
- Kurylowicz, A. In search of new therapeutic targets in obesity treatment: Sirtuins. Int. J. Mol. Sci. 2016, 17, 572. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kanatsu, J.; Toh, M.; Naka, A.; Kondo, K.; Iida, K. The dietary isoflavone daidzein reduces expression of pro-inflammatory genes through PPARα/γ and JNK pathways in adipocyte and macrophage co-cultures. PLoS One 2016, 11, e0149676. [Google Scholar] [CrossRef] [PubMed]
- Gillum, M.P.; Kotas, M.E.; Erion, D.M.; Kursawe, R.; Chatterjee, P.; Nead, K.T.; Muise, E.S.; Hsiao, J.J.; Frederick, D.W.; Yonemitsu, S.; et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011, 60, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Genistein, a plant-derived isoflavone, counteracts the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 109, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Piaggesi, L.; De Simone, L.; Orlandi, R.; Genazzani, A.R. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J. Clin. Endocrinol. Metab. 1997, 82, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Roccasino, D.; Henneberg, M. Soy consumption and obesity. Food. Nutr. Sci. 2012, 3, 2. [Google Scholar]
- Lin, Y.; Wolk, A.; Håkansson, N.; Peñalvo, J.L.; Lagergren, J.; Adlercreutz, H.; Lu, Y. Validation of FFQ-based assessment of dietary lignans compared with serum enterolactone in Swedish women. Br. J. Nutr. 2013, 109, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Relationship of obesity and high urinary enterolignan concentrations in 6806 children and adults: analysis of National Health and Nutrition Examination Survey data. Eur. J. Clin. Nut.r 2013, 67, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Q.; Zhang, Q.; Gu, A.; Jiang, Z.Y. Urinary enterolactone is associated with obesity and metabolic alteration in men in the US National Health and Nutrition Examination Survey 2001-10. Br. J. Nutr. 2015, 113, 683–690. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Cardiometabolic risk factors are associated with high urinary enterolactone concentration, independent of urinary enterodiol concentration and dietary fiber intake in adults. J. Nutr. 2014, 144, 1445–1453. [Google Scholar] [CrossRef]
- de Kleijn, M.J.; van der Schouw, Y.T.; Wilson, P.W.; Grobbee, D.E.; Jacques, P.F. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. women: The Framingham study. J. Nutr. 2002, 132, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Kastrati, N.; Musa, J.; Milic, J.; Asllanaj, E.; Portilla Fernandez, E.; Nano, J.; Ochoa Rosales, C.; Amiri, M.; Kraja, B.; et al. Phytoestrogen supplementation and body composition in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2018, 115, 74–83. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediators Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. (Oxf) 2013, 78, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. (Lond) 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Smith, J.J.; Kenney, R.D.; Gagne, D.J.; Frushour, B.P.; Ladd, W.; Galonek, H.L.; Israelian, K.; Song, J.; Razvadauskaite, G.; Lynch, A.V.; et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst. Biol. 2009, 3, 31. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. (Phila) 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Chen, W.H.; Guo, J.J.; Fu, Z.H.; Yi, C.; Zhang, M.; Na, X.L. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women -- a meta-analysis. Nutrition 2013, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; López-Romero, P. Urinary enterolignan concentrations are positively associated with serum HDL cholesterol and negatively associated with serum triglycerides in U.S. adults. J. Nutr. 2012, 142, 751–756. [Google Scholar] [CrossRef]
- Demonty, I.; Lamarche, B.; Deshaies, Y.; Jacques, H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J. Nutr. Biochem. 2002, 13, 671–677. [Google Scholar] [CrossRef]
- Uesugi, T.; Toda, T.; Tsuji, K.; Ishida, H. Comparative study on reduction of bone loss and lipid metabolism abnormality in ovariectomized rats by soy isoflavones, daidzin, genistin, and glycitin. Biol. Pharm. Bull. 2001, 24, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Lee, S.O.; Murphy, P.A.; Hendrich, S. Soy protein with or without isoflavones, soy germ and soy germ extract, and daidzein lessen plasma cholesterol levels in golden Syrian hamsters. Exp. Biol. Med. (Maywood) 2003, 228, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005, 16, 693–699. [Google Scholar] [CrossRef]
- Venkatasubramanian, S.; Noh, R.M.; Daga, S.; Langrish, J.P.; Joshi, N.V.; Mills, N.L.; Hoffmann, E.; Jacobson, E.W.; Vlasuk, G.P.; Waterhouse, B.R.; et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J. Am. Heart Assoc. 2013, 2, e000042. [Google Scholar] [CrossRef]
- Shi, L.; Ryan, H.H.; Jones, E.; Simas, T.A.; Lichtenstein, A.H.; Sun, Q.; Hayman, L.L. Urinary isoflavone concentrations are inversely associated with cardiometabolic risk markers in pregnant U.S. women. J. Nutr. 2014, 144, 344–351. [Google Scholar] [CrossRef]
- Bell, A.; Korourian, S.; Zeng, H.; Phelps, J.; Hakkak, R. A diet containing a high- versus low-daidzein level does not protect against liver steatosis in the obese Zucker rat model. Food Funct. 2017, 8, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, H.; Jiang, Z. Genistein ameliorates fat accumulation through AMPK activation in fatty acid-induced BRL cells. J. Food Sci. 2017, 82, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hur, H.J.; Kim, S.H.; Park, S.J.; Hong, M.J.; Sung, M.J.; Kwon, D.Y.; Kim, M.S. Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Li, X.; Wang, Q.; Yan, M.; Zhang, H.; Zhao, T.; Zhang, N.; Zhang, P.; Peng, L.; et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J. Nutr. Biochem. 2019, 73, 108214. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Schjoldager, J.G.; Tortzen, C.G.; Vegge, A.; Hufeldt, M.R.; Skaanild, M.T.; Vogensen, F.K.; Kristiansen, K.; Hansen, A.K.; Nielsen, J. 2-heptyl-formononetin increases cholesterol and induces hepatic steatosis in mice. Biomed. Res. Int. 2013, 2013, 926942. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl. Physiol. Nutr. Metab. 2012, 37, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef]
- van der Schouw, Y.T.; Sampson, L.; Willett, W.C.; Rimm, E.B. The usual intake of lignans but not that of isoflavones may be related to cardiovascular risk factors in U.S. men. J. Nutr. 2005, 135, 260–266. [Google Scholar] [CrossRef]
- Talaei, M.; Lee, B.L.; Ong, C.N.; van Dam, R.M.; Yuan, J.M.; Koh, W.P.; Pan, A. Urine phyto-oestrogen metabolites are not significantly associated with risk of type 2 diabetes: the Singapore Chinese health study. Br. J. Nutr. 2016, 115, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shu, X.O.; Jin, F.; Elasy, T.; Li, H.L.; Li, Q.; Huang, F.; Zhang, X.L.; Gao, Y.T.; Zheng, W. Soyfood consumption and risk of glycosuria: a cross-sectional study within the Shanghai Women’s Health Study. Eur. J. Clin. Nutr. 2004, 58, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Franco, O.H.; Ye, J.; Demark-Wahnefried, W.; Ye, X.; Yu, Z.; Li, H.; Lin, X. Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J. Nutr. 2008, 138, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Jayagopal, V.; Kilpatrick, E.S.; Chapman, T.; Atkin, S.L. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007, 30, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Cipriani, S.; Chiaffarino, F.; Malvezzi, M.; Parazzini, F. Soy isoflavones and bone mineral density in perimenopausal and postmenopausal Western women: a systematic review and meta-analysis of randomized controlled trials. J. Womens Health (Larchmt) 2010, 19, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Pan, A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 271–283. [Google Scholar] [CrossRef] [PubMed]
| Influence on | Experimental Model | ||||||
|---|---|---|---|---|---|---|---|
| Group of Phytoestrogens | Mechanism of Action | Adipogenesis | Lipolysis/Lipogenesis | Inflammation | References | ||
| Isoflavones | daidzein | ↑PPARγ | ↑ adipocytes differentiation ↑ adipocytes browning | ↓lipids synthesis | KS483 cells 3LT3 cells rats on an HFD C57BL/6 mice human AT | [60] [76] [77] [83] [84] | |
| ↑PPARγ ↑PPARα ↓JNK pathway ↓MAPK1 ↓KRAS | ↓MCP-1 ↓ IL-6 ↑adiponectin ↑IL10RA | 3LT3 cells and RAW264 macrophages | [94] | ||||
| ↑ERα | ↓ adipocytes differentiation | 3LT3 cells | [30] | ||||
| ↓cAMP PDE | ↑lipolysis | rat adipocytes | [89] | ||||
| genistein | ↑AMPK | ↓ adipocytes differentiation | CD1-mice | [37] | |||
| ↑PPARγ | ↑ adipocytes differentiation | ↓lipids synthesis | C57BL/6 mice C57BL/6 mice ovariectomized rats | [61] [85] [86] | |||
| ↓ERK1/2 | ↓ adipocytes differentiation | mice on LFD human adipocytes human PBMSC | [62] [63] [64] | ||||
| ↑sirtuin 1 | ↑ adipocytes browning | 3LT3 cells | [76] | ||||
| ↓cAMP PDE | ↑lipolysis | rat adipocytes | [96] | ||||
| ↓NF-κB | ↓IL-6 ↓IL-8 ↓leptin ↑ IFNβ | human fibroblasts and 3LT3 cells | [65] | ||||
| ↓miR-155 ↓miR-34a | ↓ E-selectin ↓ P-selectin ↓VACM-1 ↓ICAM-1 ↓MCP-1 ↓IL-8 | HUVECs | [53] [54] | ||||
| biochanin A | ↓PPARγ | ↓ adipocytes differentiation | ↓leptin, ↓TNFα ↓IL-6 | yeast cells rat adipocytes | [31] [37] | ||
| formononetin | ↑AMPK | ↓ adipocytes differentiation | C57BL/6 mice | [38] | |||
| ↑ PPARγ | ↑ adipocytes browning | C57BL/6 mice | [79] | ||||
| ↑lipolysis | 3LT3 cells | [72] | |||||
| formononetin esters | ↑AMPK | ↓ adipocytes differentiation ↓ adipocytes proliferation | 3LT3 cells | [72] | |||
| Coumestans | coumestrol | ↑AMPK ↑sirtuin 1 | ↑ adipocytes browning | ↑lipolysis | mice on an HFD 3LT3 cells | [80] [87] | |
| ↓lipids synthesis | rat adipocytes | [88] | |||||
| Lignans | gomisin N | ↑AMPK ↓PPARγ | ↓ adipocytes differentiation | 3LT3 cells | [73] | ||
| Resorcinol derivates | resveratrol | ↑AMPK ↓PPARγ | ↓ adipocytes differentiation ↓ adipocytes proliferation | 3LT3 cells 3LT3 cells mice on an HFD ovariectomized rats | [56] [57] [58] [59] | ||
| ↑sirtuins | ↑ adipocytes browning | ↑lipolysis | ↑adiponectin ↑omentin ↓ resistin ↓ visfatin | mice on an HFD | [75] | ||
| Influence on | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phytoestrogen | BMI | References | Visceral Obesity | References | Glucose Metabolism | References | Serum Lipids | References | Liver Steatosis | References |
| daidzein | ↔ | [101] [123] | ↓glucose ↓insulin | [104] | ↑HDL-C ↓TG ↓TC | [104] [123] [119] | ↔ | [123] | ||
| ↑ | [106] | ↑HDL-C ↓LDL-C ↓TG ↓TC | [118] | |||||||
| genistein | ↔ | [101] | ↓WC | [104] | ↓DM progression ↓glucose ↓insulin | [104] [130] | ↓TG | [38] | ↓ | [86] [125] |
| ↓ | [104] | |||||||||
| formononetin | ↓ | [38] | ↓VAT | [38] | ↓IR | [125] | ↓TG ↑HDL-C | [38] | ↓ | [38] [125,126] |
| ↑ | [127] | |||||||||
| soy isoflavones | ↓ | [115] [137] | ↓↑↔ glucose ↓↔ insulin ↓IR ↓glycosuria | [104] [122] [133,134,135,136,137] | ↑HDL-C ↓LDL-C ↓TG ↓TC | [124] | ||||
| soy isoflavones and lignans | ↔ | [106] | ↔ WC ↓ WHR | [106] | ↓glucose ↓insulin ↓IR | [119,120] [128] [85] | ↓TG | [103] | ||
| lignans | ↓insulin ↓C-peptide | [131] | ||||||||
| enterolactone* | ↓ | [100,101] | ↓ WC | [102] | ↓glucose ↓insulin | [101] | ↓TG ↑HDL-C | [101] [102] | ||
| ↓ WHR | [103] | |||||||||
| enterodiol* | ↓ | [100] | ↓ WC | [100] | ||||||
| ↓ WHR | [103] | |||||||||
| resveratrol | ↔ | [109] [110,111,112,113,114] | ↓TG | [109] | ↓ | [109] | ||||
| ↓TC | [121] | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. https://doi.org/10.3390/nu12020582
Kuryłowicz A, Cąkała-Jakimowicz M, Puzianowska-Kuźnicka M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients. 2020; 12(2):582. https://doi.org/10.3390/nu12020582
Chicago/Turabian StyleKuryłowicz, Alina, Marta Cąkała-Jakimowicz, and Monika Puzianowska-Kuźnicka. 2020. "Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens" Nutrients 12, no. 2: 582. https://doi.org/10.3390/nu12020582
APA StyleKuryłowicz, A., Cąkała-Jakimowicz, M., & Puzianowska-Kuźnicka, M. (2020). Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients, 12(2), 582. https://doi.org/10.3390/nu12020582