Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants
Abstract
1. Introduction
2. Methods
2.1. Bacterial Strain and Culture Conditions
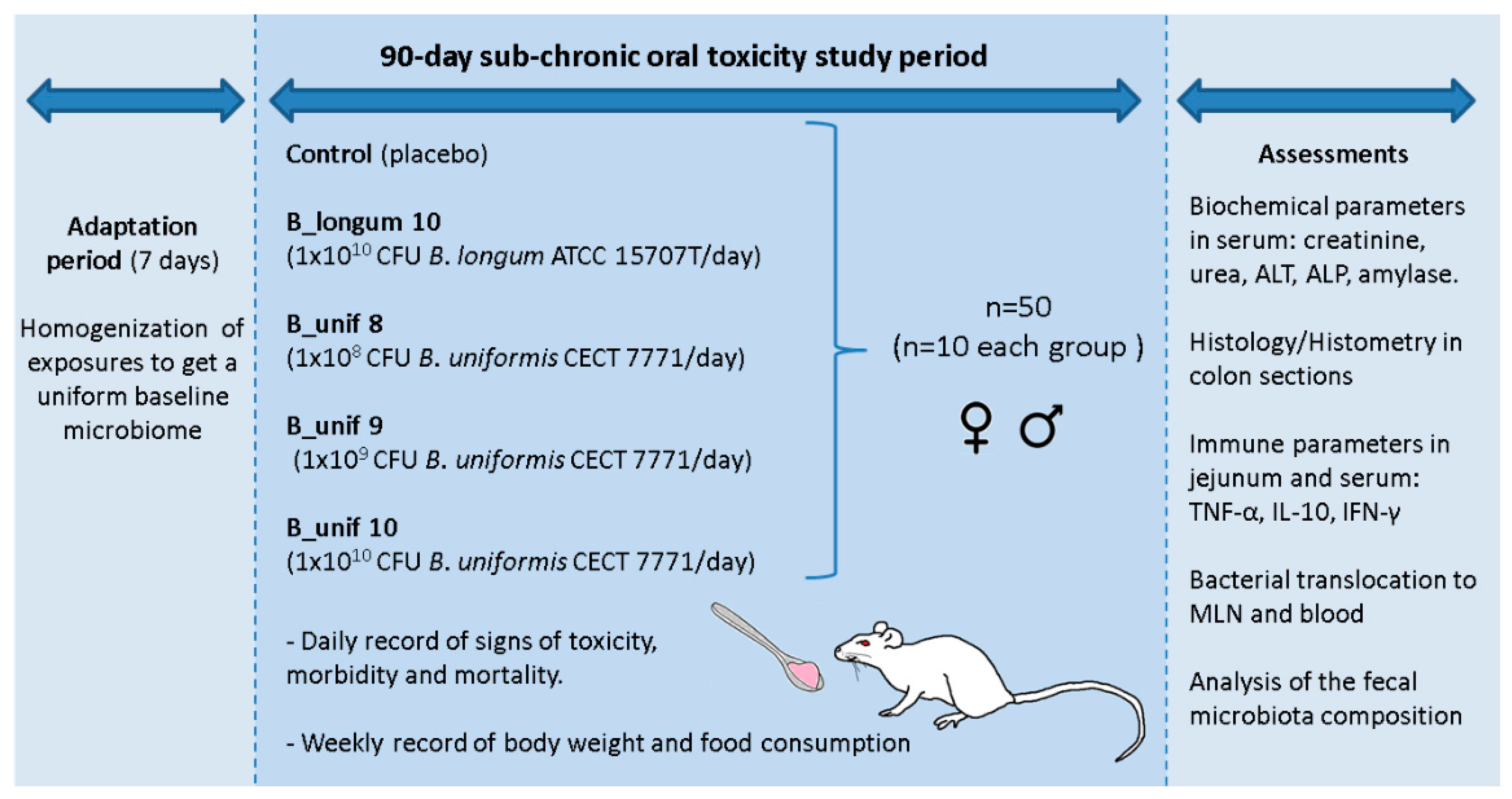
2.2. Sub-Chronic (90-Day) Oral Toxicity Study in Rats
2.3. Bacterial Translocation
2.4. Determination of Cytokine Concentrations
2.5. Biochemical Parameter Analysis
2.6. Histology and Histometry
2.7. Statistical Analysis
2.8. Fecal Microbiota
2.9. Next-Generation Sequencing Data Analysis
3. Results
3.1. General Health, Food Intake, and Weight of the Animals
3.2. Bacterial Translocation
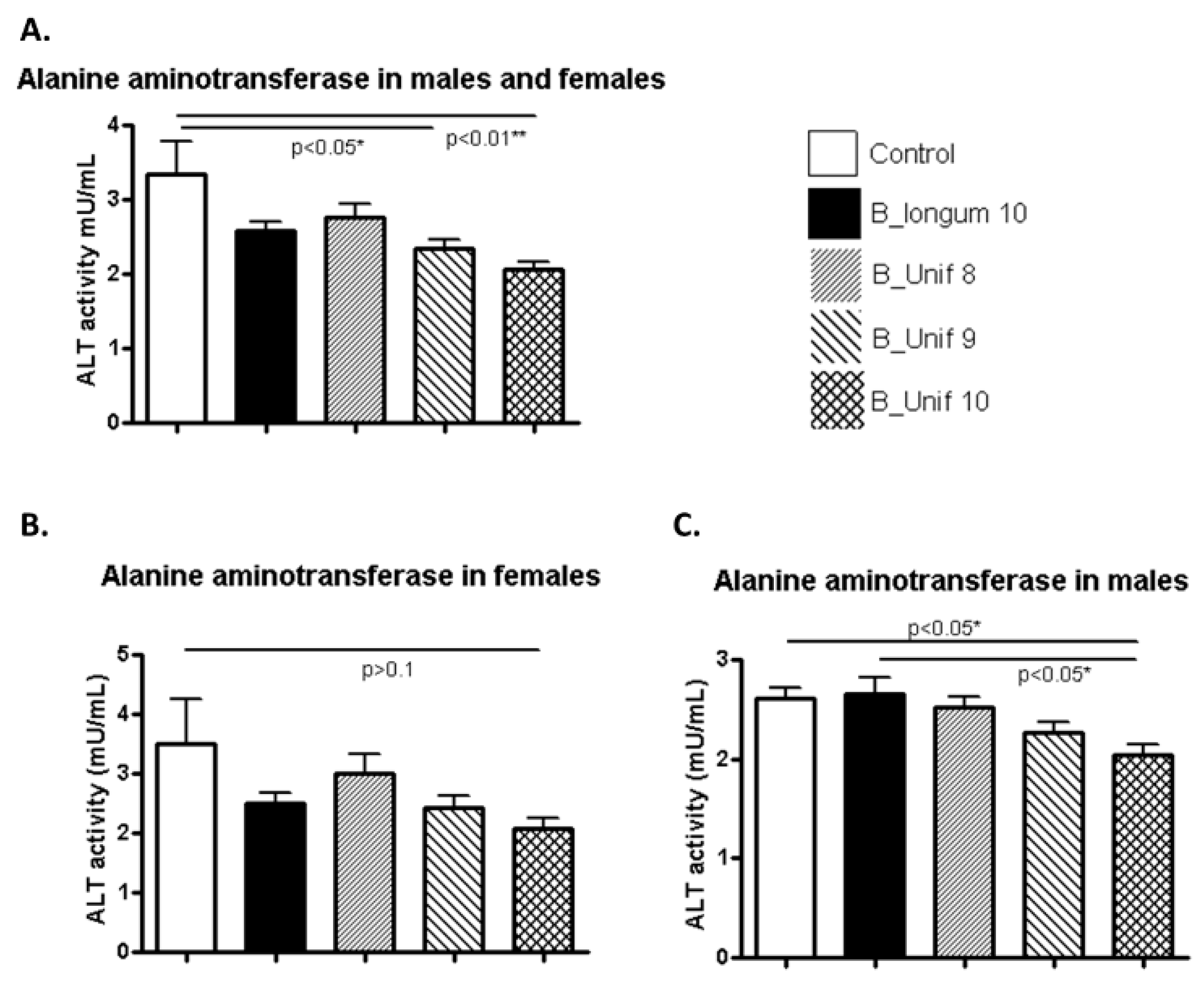
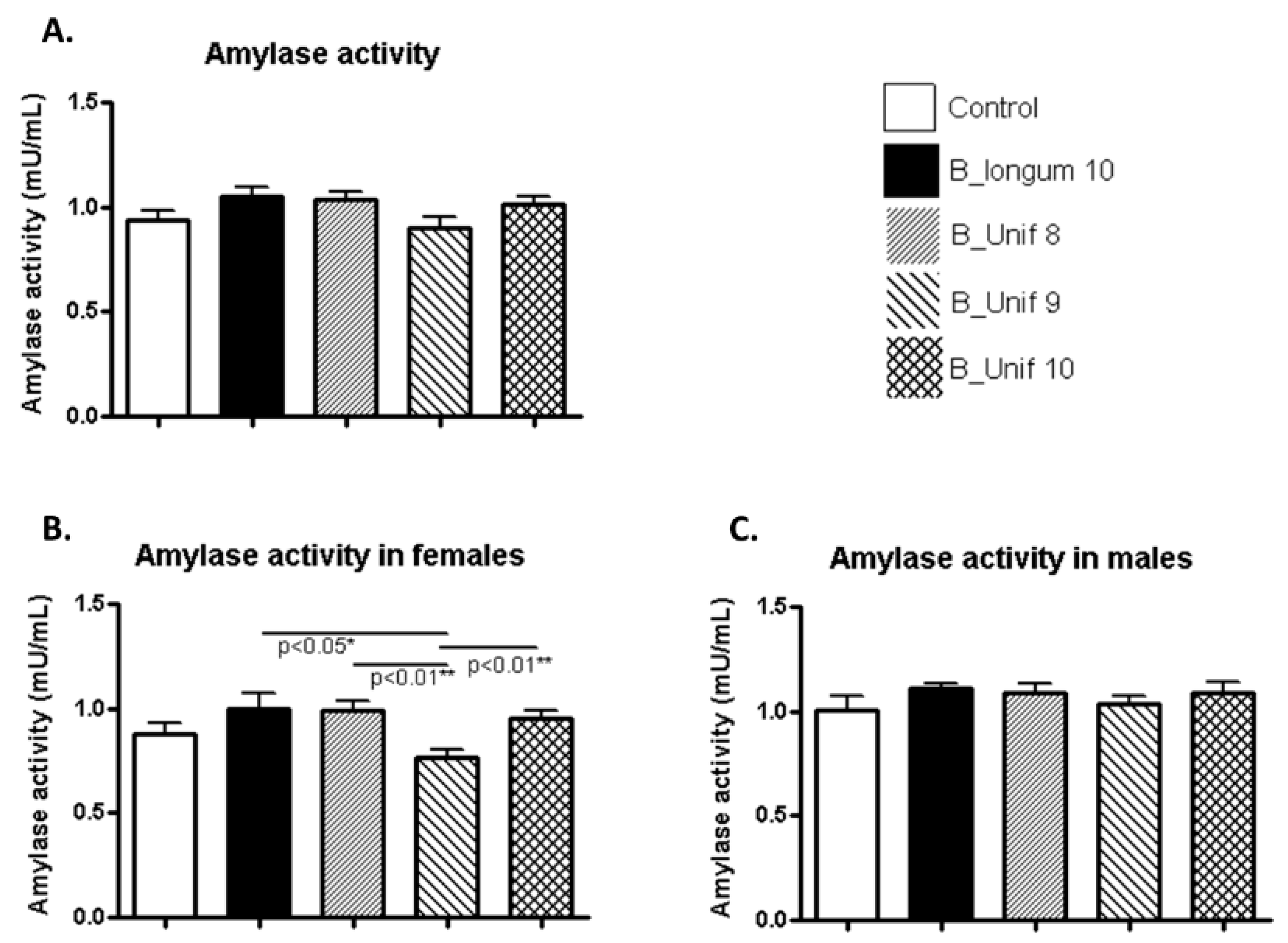
3.3. Biochemical Parameters
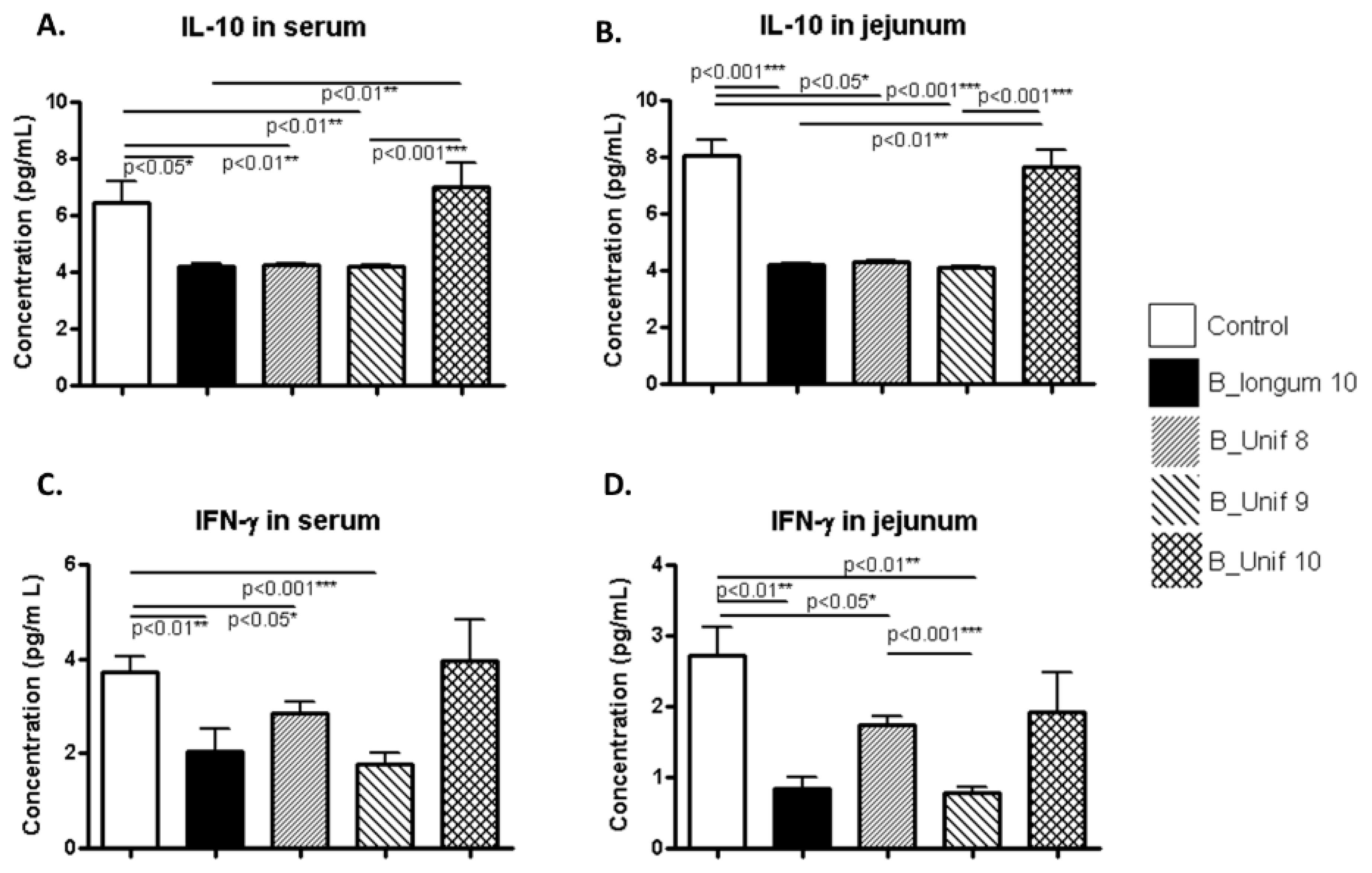
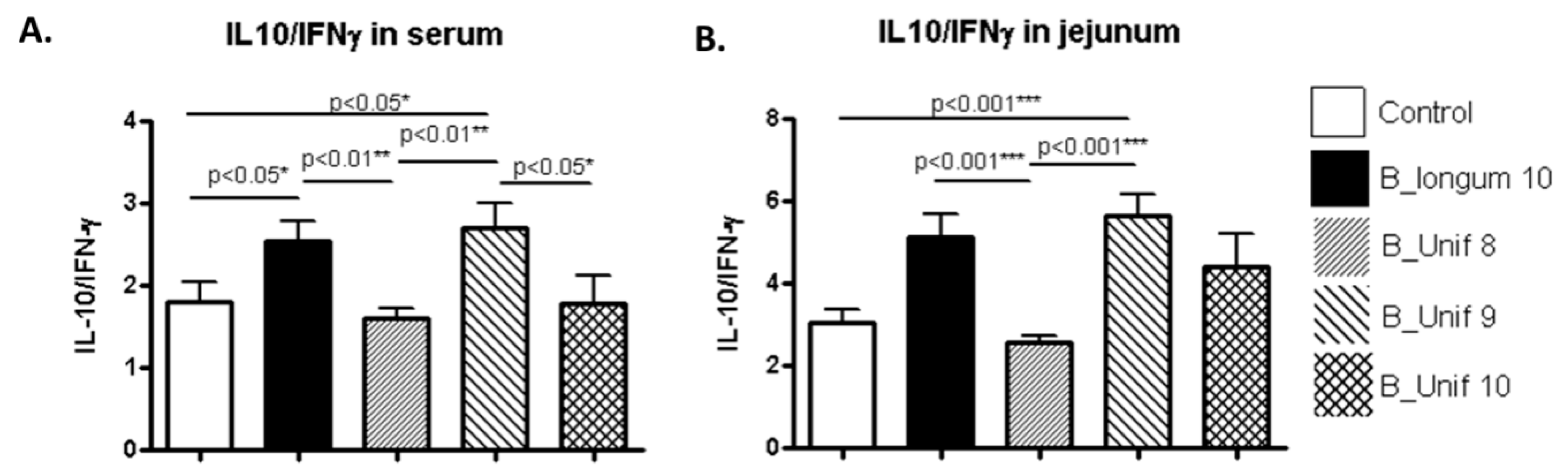
3.4. Cytokine Concentrations in Serum and Jejunum
3.5. Colon Mucosal Histology
3.6. Effect of the Intervention on Fecal Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neef, A.; Sanz, Y. Future for probiotic science in functional food and dietary supplement development. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Ther. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Committee EFSAS. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA-Opinion of the Scientific Committee (Question No EFSA-Q-2005-293. EFSA J. 2007, 587, 1–16. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2007.587 (accessed on 20 February 2020).
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Gauffin-Cano, P.; Santacruz, A.; Moya, Á.; Sanz, Y. Bacteroides uniformis CECT 7771 Ameliorates Metabolic and Immunological Dysfunction in Mice with High-Fat-Diet Induced Obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Ulmer, J.; Kechaou, N.; Langella, P.; Bermúdez-Humarán, L.G. Role of commensal and probiotic bacteria in human health: A focus on inflammatory bowel disease. Microb. Cell Factories 2013, 12, 71. [Google Scholar] [CrossRef]
- Muñoz, J.A.M.; Chenoll, E.; Casinos, B.; Bataller, E.; Ramón, D.; Genovés, S.; Montava, R.; Ribes, J.M.; Buesa, J.; Fàbrega, J.; et al. Novel Probiotic Bifidobacterium longum subsp. infantis CECT 7210 Strain Active against Rotavirus Infections. Appl. Environ. Microbiol. 2011, 77, 8775–8783. [Google Scholar] [CrossRef]
- Collins, J.; Thornton, G.; Sullivan, G. Selection of Probiotic Strains for Human Applications. Int. Dairy J. 1998, 8, 487–490. [Google Scholar] [CrossRef]
- Kumar, H.; Salminen, S.; Verhagen, H.; Rowland, I.; Heimbach, J.; Bañares, S.; Young, T.; Nomoto, K.; LaLonde, M. Novel probiotics and prebiotics: Road to the market. Curr. Opin. Biotechnol. 2015, 32, 99–103. [Google Scholar] [CrossRef]
- Biohaz, E.P.O.B.H.; Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 7: Suitability of taxonomic units notified to EFSA until September 2017. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Lepaslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.D.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Wu, J.-H.; Liu, W.-T. Relative Abundance of Bacteroides spp. in Stools and Wastewaters as Determined by Hierarchical Oligonucleotide Primer Extension. Appl. Environ. Microbiol. 2008, 74, 2882–2893. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Schofield, W.B.; Barry, N.A.; Putnam, E.E.; Rundell, E.A.; Trent, M.S.; Degnan, P.H.; Booth, C.J.; Yu, H.; Goodman, A.L. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 2015, 347, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Telesford, K.M.; Ochoa-Re, J.; Haque-Begum, S.; Christy, M.; Kasper, E.J.; Wang, L.; Wu, Y.; Robson, S.C.; Kasper, D.L.; et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 2014, 5, 4432. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Takeda, K. Polysaccharide A of Bacteroides fragilis: Actions on dendritic cells and T cells. Mol. Cell 2014, 54, 206–207. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Del Pulgar, E.M.G.; Sanz, Y. The Glycolytic Versatility of Bacteroides uniformis CECT 7771 and Its Genome Response to Oligo and Polysaccharides. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Sánchez, E.; De Palma, G.; Capilla, A.; Nova, E.; Pozo, T.; Castillejo, G.; Varea, V.; Marcos, A.; Garrote, J.A.; Polanco, I.; et al. Influence of Environmental and Genetic Factors Linked to Celiac Disease Risk on Infant Gut Colonization by Bacteroides Species. Appl. Environ. Microbiol. 2011, 77, 5316–5323. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Sanz, Y. Safety Assessment of Bacteroides uniformis CECT 7771 Isolated from Stools of Healthy Breast-Fed Infants. PLoS ONE 2016, 11, e0145503. [Google Scholar] [CrossRef]
- OECD. Guideline for the Testing of Chemicals: Repeating Dose 90-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 1998. [Google Scholar]
- Miyoshi, J.; Leone, V.; Nobutani, K.; Musch, M.W.; Martinez, K.; Wang, Y.; Miyoshi, S.; Bobe, A.M.; Eren, A.M.; Chang, E.B. Minimizing confounders and increasing data quality in murine models for studies of the gut microbiome. PeerJ 2018, 6, e5166. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Hauck, L.; Jeffrey, B.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; I Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Schloot, N.C.; Hanifi-Moghaddam, P.; Goebel, C.; Shatavi, S.V.; Flohé, S.; Kolb, H.; Rothe, H. Serum IFN-gamma and IL-10 levels are associated with disease progression in non-obese diabetic mice. Metab. Res. Rev. 2002, 18, 64–70. [Google Scholar]
- Jamil, B.; Shahid, F.; Hasan, Z.; Nasir, N.; Razzaki, T.; Dawood, G.; Hussain, R. Interferonγ/IL10 ratio defines the disease severity in pulmonary and extra pulmonary tuberculosis. Tuberculosis 2007, 87, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ala, Y.; Pasha, M.K.; Rao, R.N.; Komaravalli, P.L.; Jahan, P. Association of IFN-γ: IL-10 Cytokine Ratio with Nonsegmental Vitiligo Pathogenesis. Autoimmune Dis. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2010, 37, 91–98. [Google Scholar] [CrossRef]
- Panel, E.B. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015, 13, 4138. [Google Scholar] [CrossRef][Green Version]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Gérard, P.; Lepercq, P.; Leclerc, M.; Gavini, F.; Raibaud, P.; Juste, C. Bacteroides sp. Strain D8, the First Cholesterol-Reducing Bacterium Isolated from Human Feces. Appl. Environ. Microbiol. 2007, 73, 5742–5749. [Google Scholar] [CrossRef]
- Deng, H.; Li, Z.; Tan, Y.; Guo, Z.; Liu, Y.; Wang, Y.; Yuan, Y.; Yang, R.; Bi, Y.; Bai, Y.; et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016, 6, 29401. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Rastmanesh, R.; Agostoni, C. Understanding the role of gut microbes and probiotics in obesity: How far are we? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Reiss, J.G.; Gibson, R.W.; Walker, L.R. Effect of Infant Feeding on the Risk of Obesity Across the Life Course: A Quantitative Review of Published Evidence. Pediatr. 2005, 115, 1367–1377. [Google Scholar] [CrossRef]
- Ford, H.R.; Avanoğlu, A.; Boechat, P.R.; Melgoza, D.; LumCheong, R.S.; Boyle, P.; Garrett, M.; Rowe, M.I. The microenvironment influences the pattern of bacterial translocation in formula-fed neonates. J. Pediatr. Surg. 1996, 31, 486–489. [Google Scholar] [CrossRef]
- Ma, L.; Deitch, E.; Specian, R.; Steffen, E.; Berg, R.; Berg, R. Translocation ofLactobacillus murinus from the gastrointestinal tract. Curr. Microbiol. 1990, 20, 177–184. [Google Scholar] [CrossRef]
- Santiago, A.; Sánchez, E.; Clark, A.; Pozuelo, M.; Calvo, M.; Yañez, F.; Sarrabayrouse, G.; Perea, L.; Vidal, S.; Gallardo, A.; et al. Sequential Changes in the Mesenteric Lymph Node Microbiome and Immune Response during Cirrhosis Induction in Rats. mSystems 2019, 4. [Google Scholar] [CrossRef]
- MacPherson, A.J.; Smith, K. Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 2006, 203, 497–500. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef]
- Houtmeyers, A.; Duchateau, L.; Grünewald, B.; Hermans, K. Reference intervals for biochemical blood variables, packed cell volume, and body temperature in pet rats (Rattus norvegicus) using point-of-care testing. Vet. Clin. Pathol. 2016, 45, 669–679. [Google Scholar] [CrossRef] [PubMed]
- An, H.-M.; Park, S.Y.; Lee, D.-K.; Kim, J.R.; Cha, M.-K.; Lee, S.-W.; Lim, H.; Kim, K.; Ha, N.-J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Bejar, W.; Hamden, K.; Ben Salah, R.; Chouayekh, H. Lactobacillus plantarum TN627 significantly reduces complications of alloxan-induced diabetes in rats. Anaerobe 2013, 24, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Erkens, T.; Goeminne, N.; Kegels, A.; Byloos, M.; Vinken, P. Analytical performance of a commercial multiplex Luminex-based cytokine panel in the rat. J. Pharmacol. Toxicol. Methods 2018, 91, 43–49. [Google Scholar] [CrossRef]
- Staples, E.; Ingram, R.J.M.; Atherton, J.C.; Robinson, K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. J. Immunol. Methods 2013, 394, 1–9. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Le Chatelier, E.; MetaHIT Consortium; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphilaand improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2015, 65, 426–436. [Google Scholar] [CrossRef]
| B_longum10 | B_unif8 | B_unif9 | B_unif10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | Both | ♀ | ♂ | Both | ♀ | ♂ | Both | ♀ | ♂ | Both | |
| Physiology | ||||||||||||
| Weight gain | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Liver weight | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Food intake | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Liver function | ||||||||||||
| Serum ALT | ns | ns | ns | ns | ns | ns | ns | ns | YES↓ | ns | YES↓ | YES↓ |
| Serum ALP | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Kidney function | ||||||||||||
| Serum creatinine | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Serum urea | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Pancreas function | ||||||||||||
| Serum amylase | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Cytokine production | ||||||||||||
| Serum IL-10 | ns | YES↓ | YES↓ | ns | YES↓ | YES↓ | ns | YES↓ | YES↓ | ns | ns | ns |
| Jejunum IL-10 | ns | YES↓ | YES↓ | ns | YES↓ | YES↓ | ns | YES↓ | YES↓ | ns | ns | ns |
| Serum IFN-γ | ns | YES↓ | YES↓ | ns | ns | YES↓ | ns | YES↓ | YES↓ | ns | ns | ns |
| Jejunum IFN-γ | YES↓ | ns | YES↓ | YES↓ | ns | YES↓ | YES↓ | ns | YES↓ | ns | ns | ns |
| Serum IL-10/IFNγ | ns | YES↓ | YES↓ | ns | ns | ns | ns | YES↓ | YES↓ | ns | ns | ns |
| Jejunum IL-10/IFNγ | ns | YES↓ | YES↓ | ns | ns | ns | YES↓ | ns | YES↓ | ns | ns | ns |
| Colon mucosa architecture | ||||||||||||
| Lieberkhün crypt depth | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Goblet cells/crypt | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez del Pulgar, E.M.; Benítez-Páez, A.; Sanz, Y. Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients 2020, 12, 551. https://doi.org/10.3390/nu12020551
Gómez del Pulgar EM, Benítez-Páez A, Sanz Y. Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients. 2020; 12(2):551. https://doi.org/10.3390/nu12020551
Chicago/Turabian StyleGómez del Pulgar, Eva M., Alfonso Benítez-Páez, and Yolanda Sanz. 2020. "Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants" Nutrients 12, no. 2: 551. https://doi.org/10.3390/nu12020551
APA StyleGómez del Pulgar, E. M., Benítez-Páez, A., & Sanz, Y. (2020). Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients, 12(2), 551. https://doi.org/10.3390/nu12020551






