Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Baseline Clinical, Anthropometric, Biochemical, and Lifestyle Variables
2.3. Serum Fatty Acid Determinations
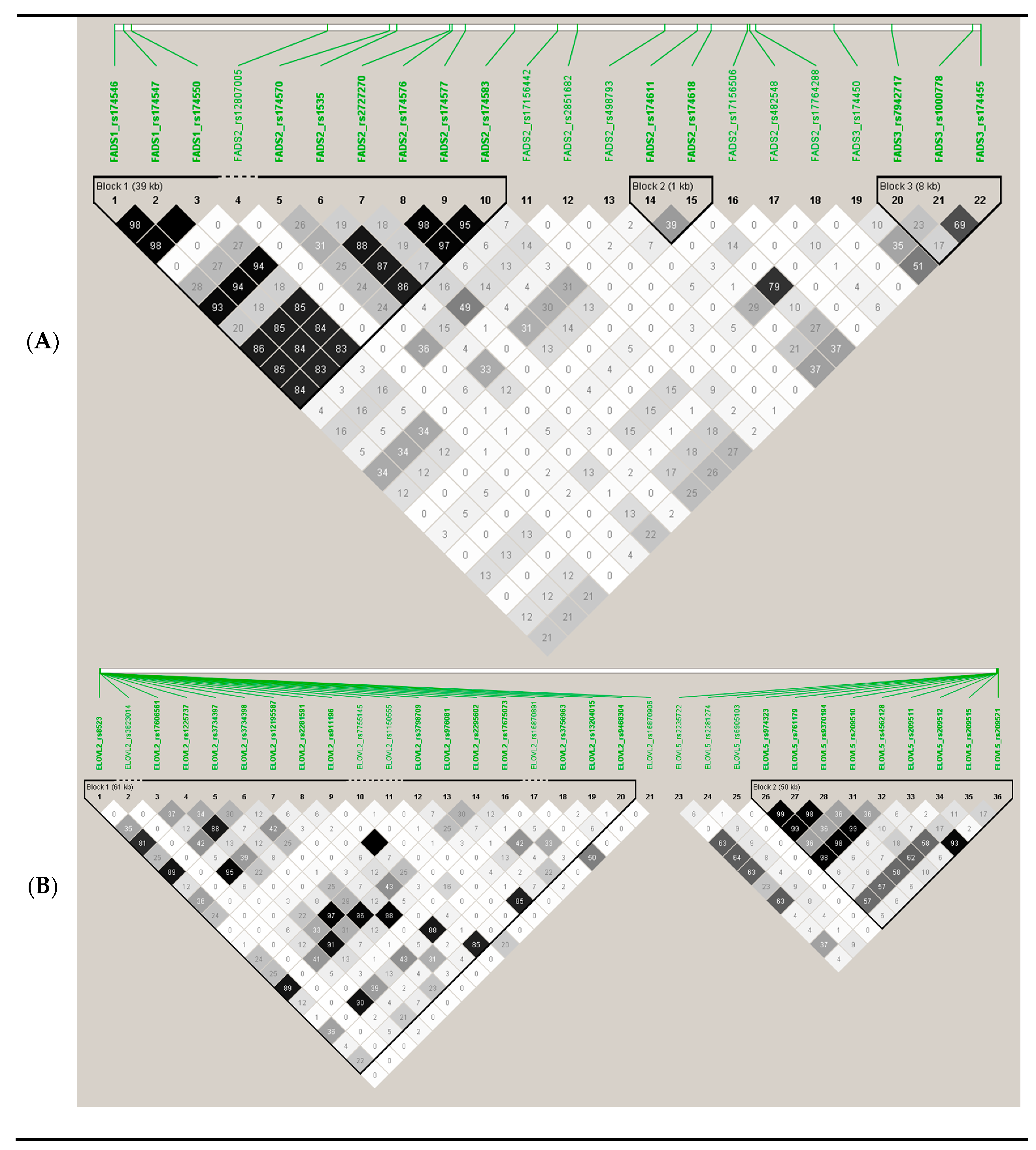
2.4. Genome-Wide Genotyping
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
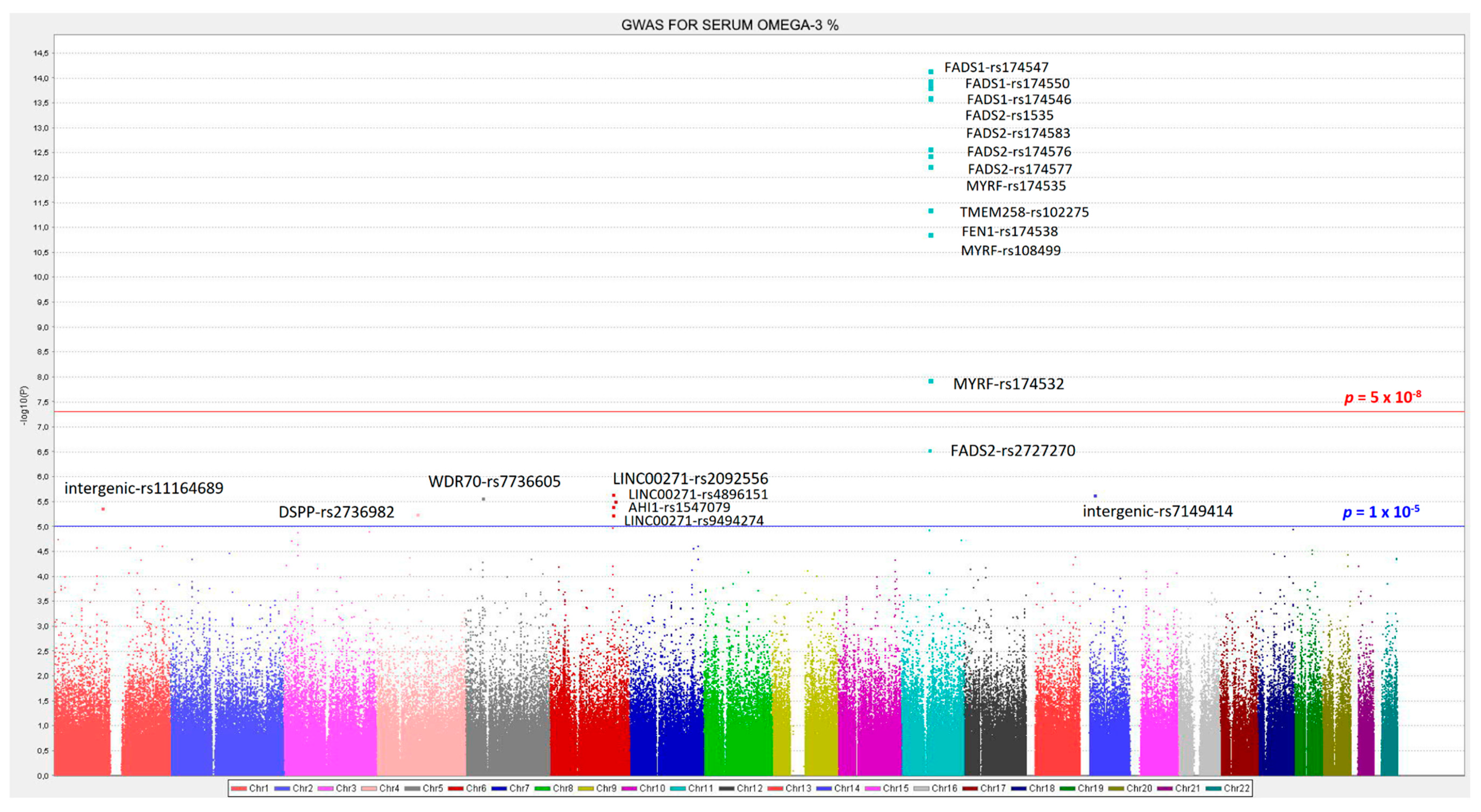
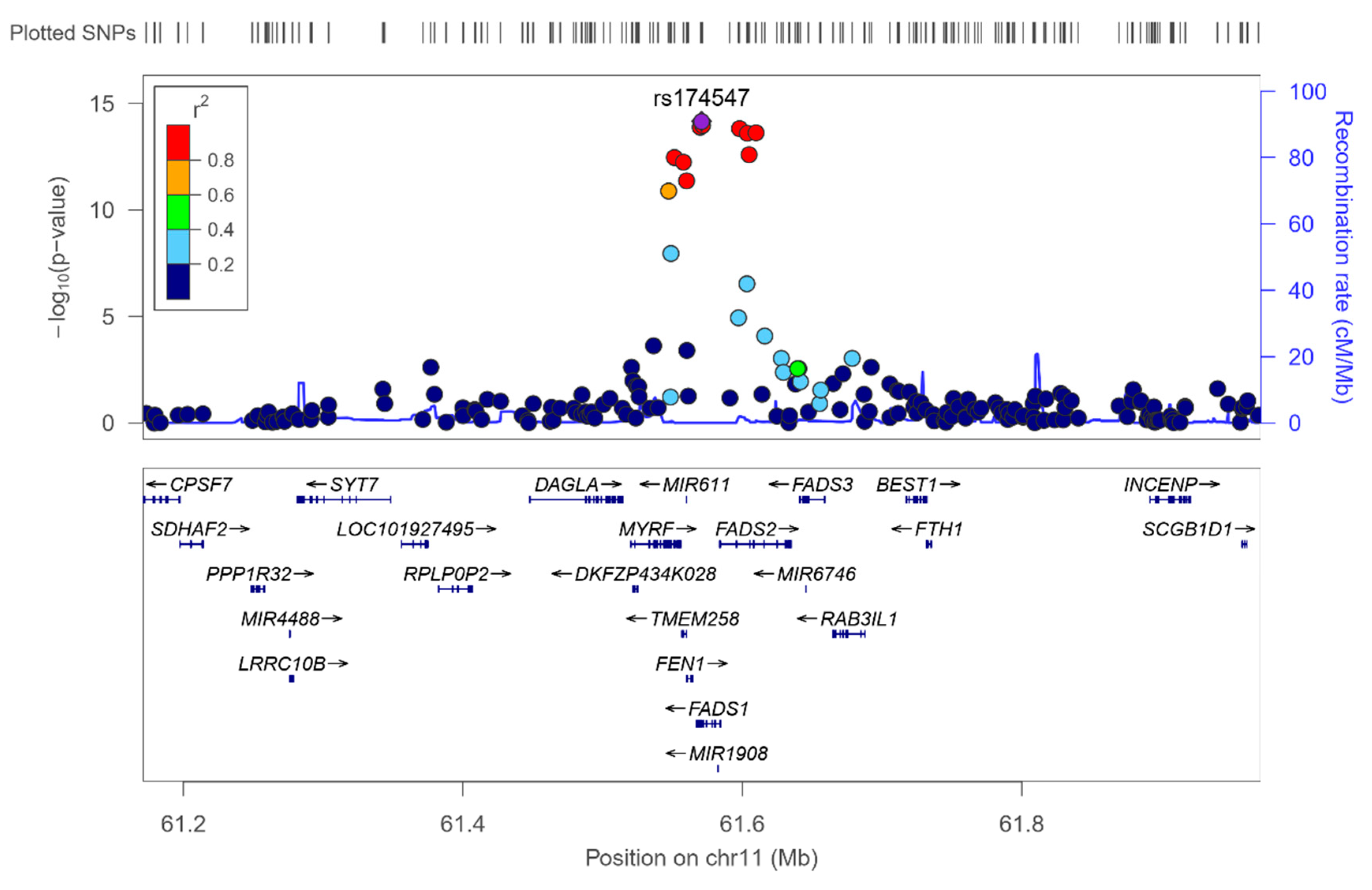
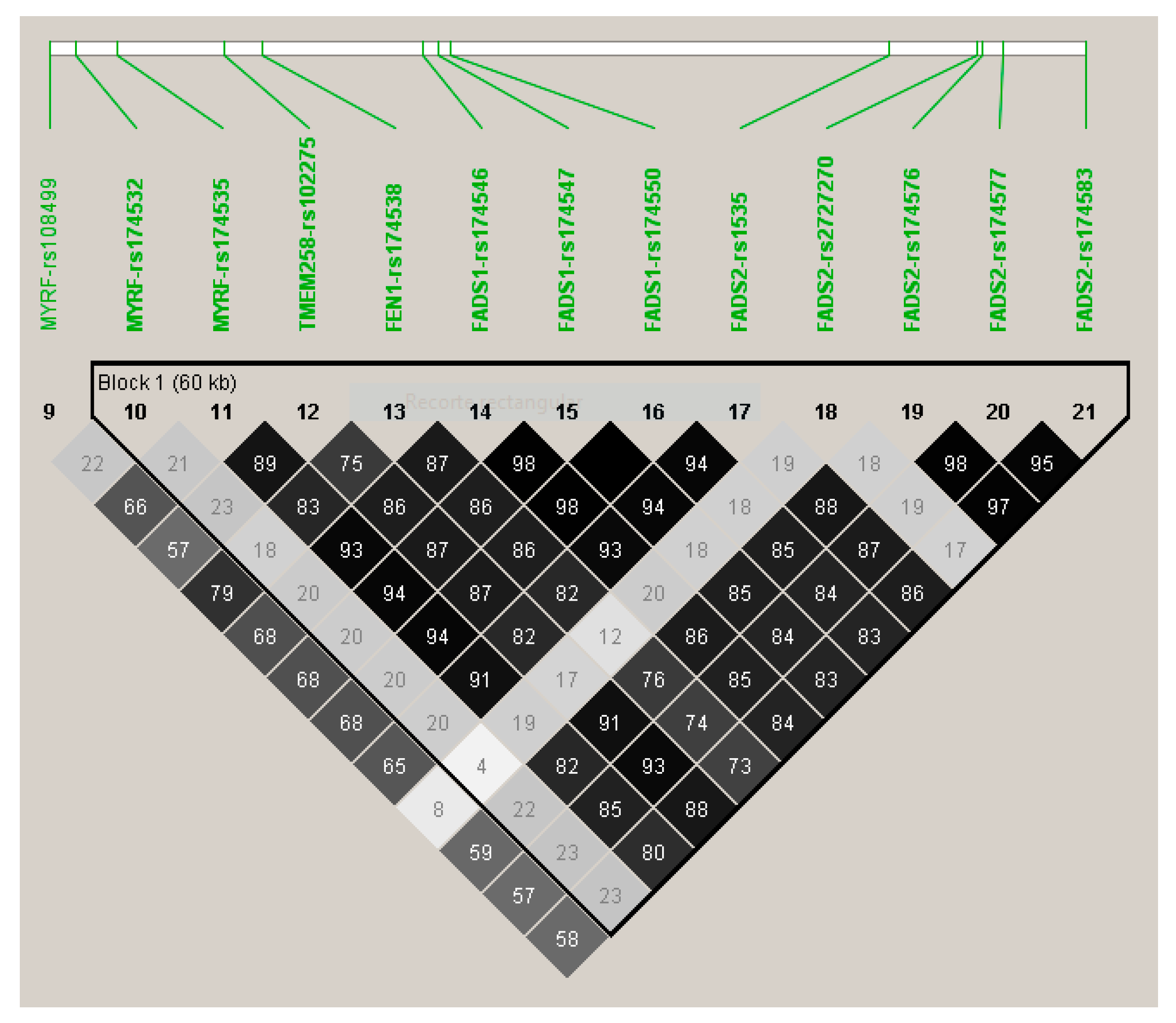
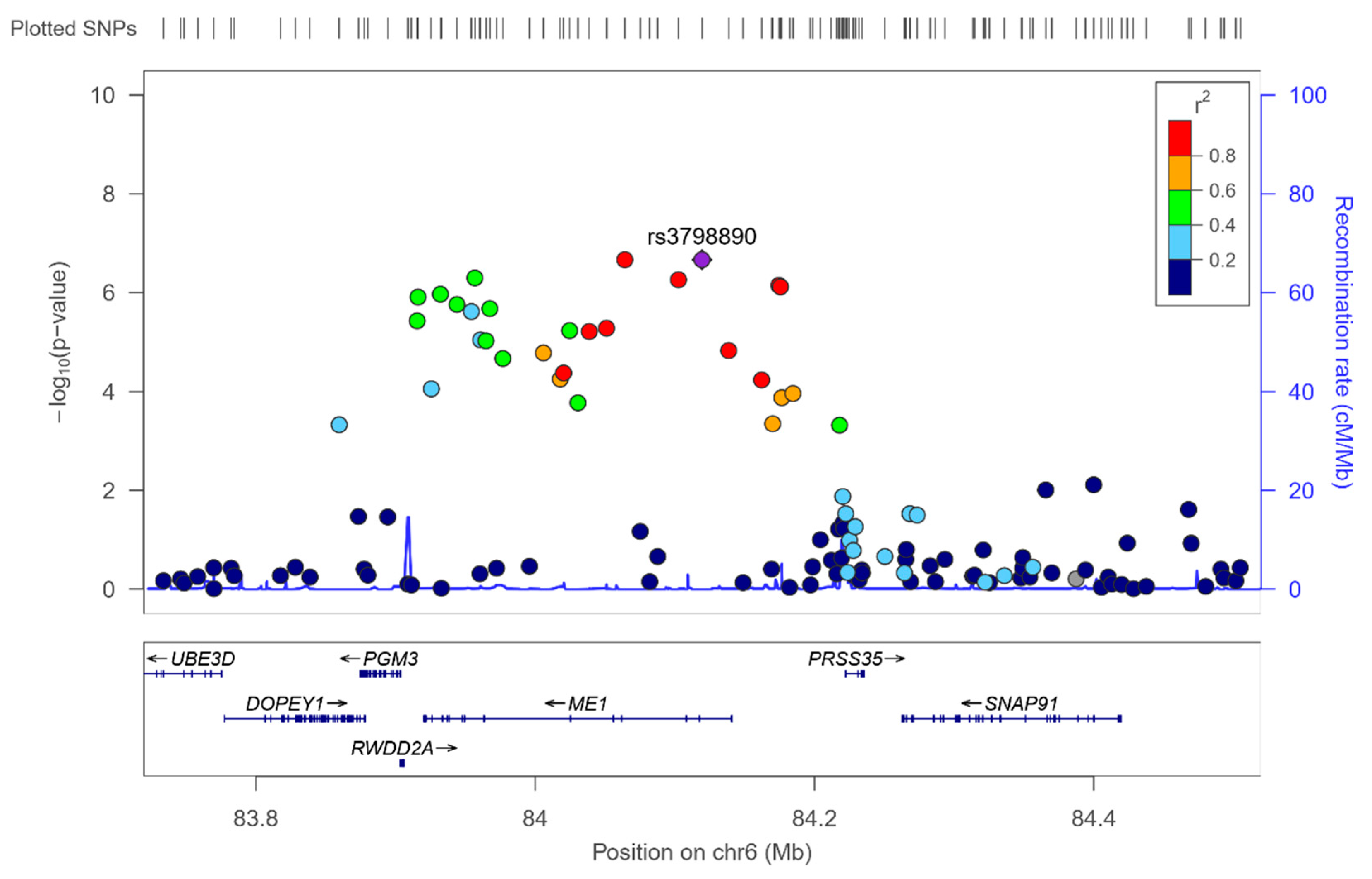
3.2. GWAS for Serum Omega-3 PUFA (%) in this Population
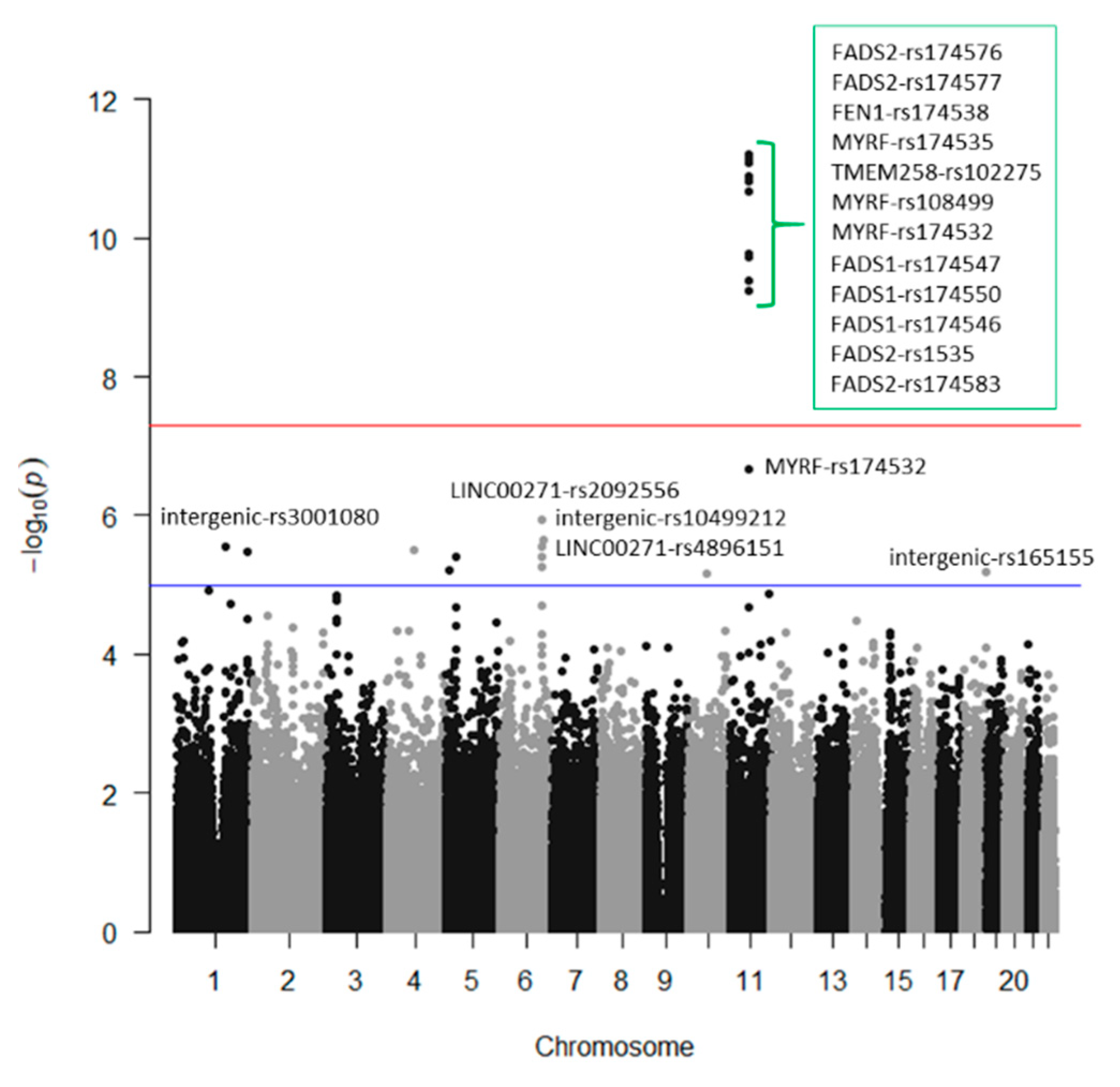
3.3. GWAS for Serum DHA Concentrations (%) in this Population
3.4. GWAS for Serum Omega-6 Concentrations (%) in this Population
3.5. GWAS for Serum LA Concentrations (%) in this Population
3.6. GWAS for Serum Total PUFA Concentrations (%) in this Population
3.7. GWAS for the Omega-6 to Omega 3 Ratio
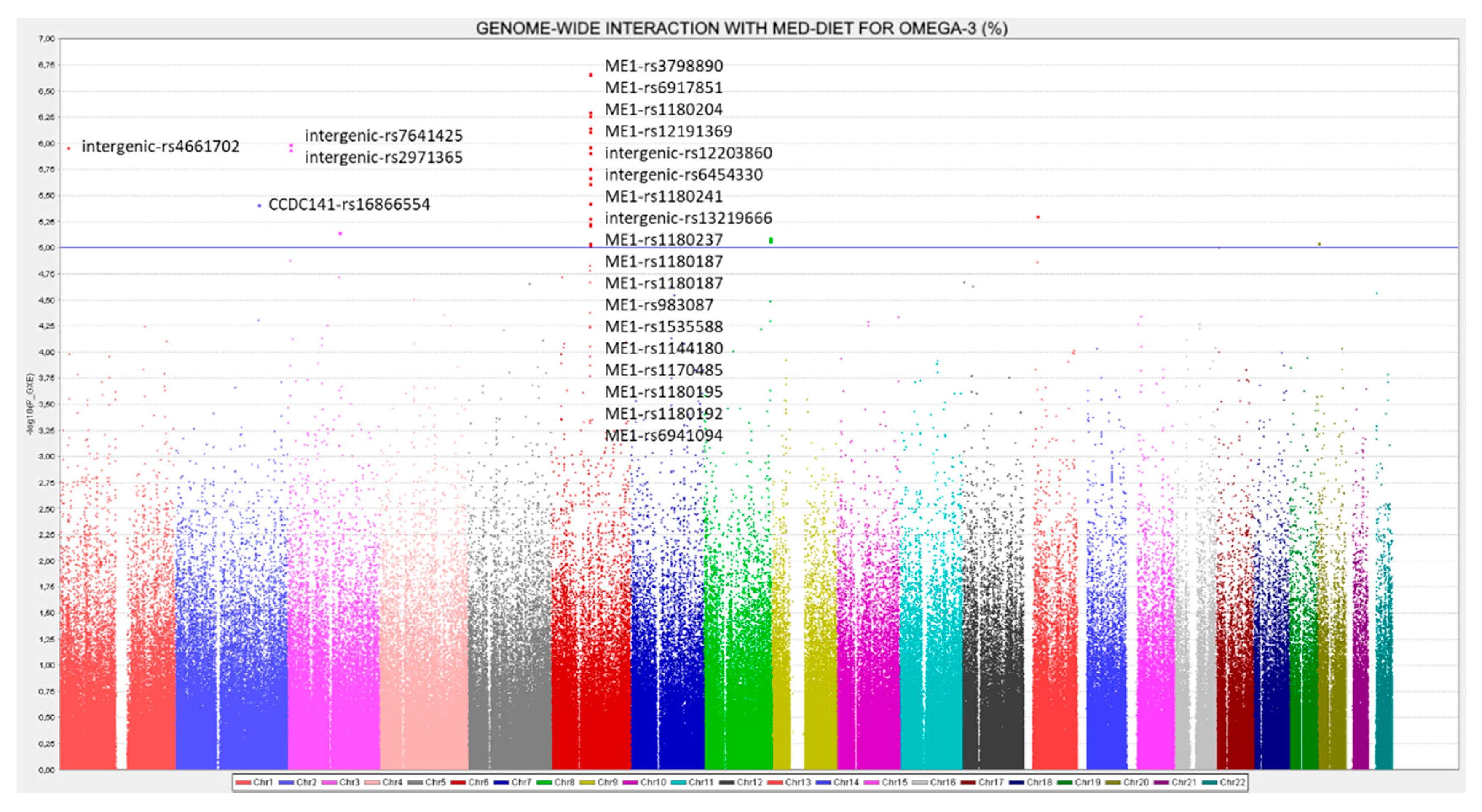
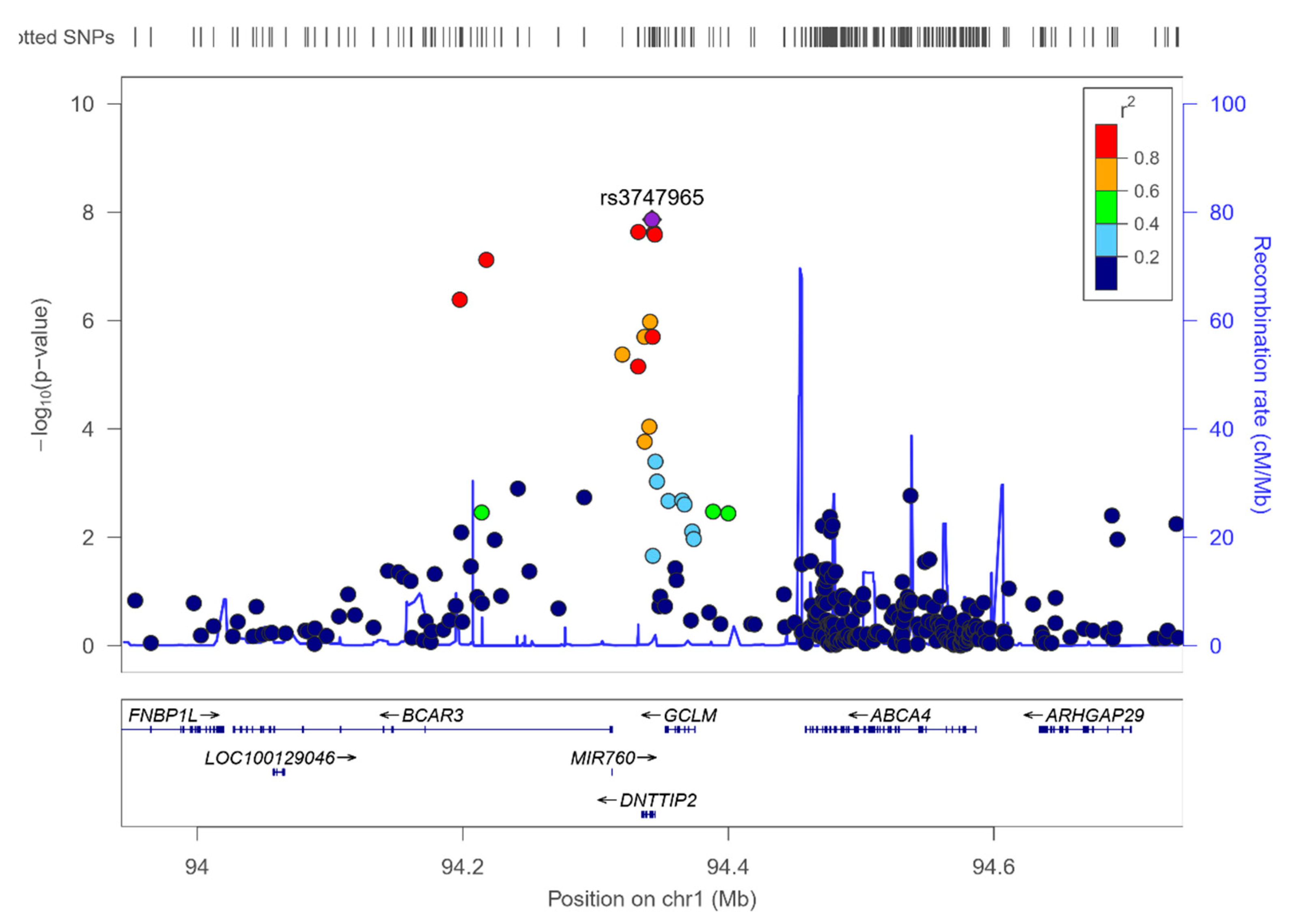
3.8. RWASs for Serum PUFA Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chilton, F.; Dutta, R.; Reynolds, L.; Sergeant, S.; Mathias, R.; Seeds, M. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Park, W.J.; Kothapalli, K.S.D.; Brenna, J.T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Δ4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015, 29, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Lee, S.; An, W. Impact of Blood or Erythrocyte Membrane Fatty Acids for Disease Risk Prediction: Focusing on Cardiovascular Disease and Chronic Kidney Disease. Nutrients 2018, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Kothapalli, K.S.D.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Astorg, P.; Bertrais, S.; Laporte, F.; Arnault, N.; Estaquio, C.; Galan, P.; Favier, A.; Hercberg, S. Plasma n-6 and n-3 polyunsaturated fatty acids as biomarkers of their dietary intakes: a cross-sectional study within a cohort of middle-aged French men and women. Eur. J. Clin. Nutr. 2008, 62, 1155–1161. [Google Scholar] [CrossRef]
- Li, D.; Wahlqvist, M.L.; Sinclair, A.J. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac. J. Clin. Nutr. 2019, 28, 1–5. [Google Scholar]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef]
- Ortega Anta, R.M.; González Rodríguez, L.G.; Villalobos Cruz, T.K.; Perea Sánchez, J.M.; Aparicio Vizuete, A.; López Sobaler, A.M. Food sources and adequacy of intake of omega 3 and omega-6 fatty acids in a representative sample of Spanish adults. Nutr. Hosp. 2013, 28, 2236–2245. [Google Scholar]
- González-Rodríguez, L.G.; Aparicio, A.; López-Sobaler, A.M.; Ortega, R.M. Omega 3 and omega 6 fatty acids intake and dietary sources in a representative sample of Spanish adults. Int. J. Vitam. Nutr. Res. 2013, 83, 36–47. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Demaison, L.; Moreau, D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: A possible mechanism of action. Cell. Mol. Life Sci. 2002, 59, 463–477. [Google Scholar] [CrossRef]
- Harbige, L.S. Fatty acids, the immune response, and autoimmunity: A question of n-6 essentiality and the balance between n-6 and n-3. Lipids 2003, 38, 323–341. [Google Scholar] [CrossRef]
- Nageswari, K.; Banerjee, R.; Menon, V.P. Effect of saturated, omega-3 and omega-6 polyunsaturated fatty acids on myocardial infarction. J. Nutr. Biochem. 1999, 10, 338–344. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Salonen, R.; Penttilä, I.; Herranen, J.; Jauhiainen, M.; Kantola, M.; Lappeteläinen, R.; Mäenpää, P.H.; Alfthan, G.; Puska, P. Serum fatty acids, apolipoproteins, selenium and vitamin antioxidants and the risk of death from coronary artery disease. Am. J. Cardiol. 1985, 56, 226–231. [Google Scholar] [CrossRef]
- Kang, J.X.; Leaf, A. The cardiac antiarrhythmic effects of polyunsaturated fatty acid. Lipids 1996, 31, S41–S44. [Google Scholar] [CrossRef]
- Nordøy, A.; Marchioli, R.; Arnesen, H.; Videbaek, J. N-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids 2001, 36, S127–S129. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Mori, T.A. Omega-3 fatty acids and hypertension in humans. Clin. Exp. Pharmacol. Physiol. 2006, 33, 842–846. [Google Scholar] [CrossRef]
- Rupp, H. Omacor (prescription omega-3-acid ethyl esters 90): From severe rhythm disorders to hypertriglyceridemia. Adv. Ther. 2009, 26, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Emma-Okon, B.; Remaley, A.T. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: A mini review. Lipids Health Dis. 2016, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Schunck, W.-H.; Konkel, A.; Fischer, R.; Weylandt, K.-H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, A.M.; Sosnowski, D.K.; Lee, T.Y.; Keshavarz-Bahaghighat, H.; Seubert, J.M. Insights into the cardioprotective properties of n-3 PUFAs against ischemic heart disease via modulation of the innate immune system. Chem. Biol. Interact. 2019, 308, 20–44. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58, 25145. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Maki, K.; Dicklin, M. Omega-3 Fatty Acid Supplementation and Cardiovascular Disease Risk: Glass Half Full or Time to Nail the Coffin Shut? Nutrients 2018, 10, 864. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Are n-3 fatty acids still cardioprotective? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. On behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD012345. [Google Scholar]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD011094. [Google Scholar]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.; Halladay, C.; Chung, M.; Lin, L.; Robertson, S.; Yip, A.; Steele, D.; Smith, B.T.; et al. Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016. [Google Scholar]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, l4697. [Google Scholar] [CrossRef]
- Jeffery, N.M.; Newsholme, E.A.; Calder, P.C. Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot. Essent. Fatty Acids 1997, 57, 149–160. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Andrioli, G.; Carletto, A.; Guarini, P.; Galvani, S.; Biasi, D.; Bellavite, P.; Corrocher, R. Differential effects of dietary supplementation with fish oil or soy lecithin on human platelet adhesion. Thromb. Haemost. 1999, 82, 1522–1527. [Google Scholar] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Eaton, S.B.; Eaton, S.B.; Konner, M.J.; Shostak, M. An evolutionary perspective enhances understanding of human nutritional requirements. J. Nutr. 1996, 126, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Sanders, T.A.B.; Davies, I.G.; Morgan, L.M.; Millward, D.J.; Lewis, F.; Slaughter, S.; Cooper, J.A.; Miller, G.J.; Griffin, B.A. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am. J. Clin. Nutr. 2006, 84, 1290–1298. [Google Scholar] [CrossRef]
- Griffin, B.A. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr. Opin. Lipidol. 2008, 19, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, B.S.; Bosire, R.V.; Deckelbaum, R.J. Omega 3 and omega 6 fatty acids in human and animal health: An African perspective. Mol. Cell. Endocrinol. 2014, 398, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.; Holland, O.J.; Perkins, A.V.; Yu Yau, S.; McAinch, A.J.; Hryciw, D.H. Role Of Omega-6 and Omega-3 fatty acids in fetal programming. Clin. Exp. Pharmacol. Physiol. 2019. [Google Scholar] [CrossRef]
- Kaliannan, K.; Li, X.-Y.; Wang, B.; Pan, Q.; Chen, C.-Y.; Hao, L.; Xie, S.; Kang, J.X. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun. Biol. 2019, 2, 276. [Google Scholar] [CrossRef]
- Steffen, B.T.; Guan, W.; Stein, J.H.; Tattersall, M.C.; Kaufman, J.D.; Sandfort, V.; Szklo, M.; Tsai, M.Y. Plasma n-3 and n-6 Fatty Acids Are Differentially Related to Carotid Plaque and Its Progression: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 653–659. [Google Scholar] [CrossRef]
- Virtanen, J.K. Randomized trials of replacing saturated fatty acids with n-6 polyunsaturated fatty acids in coronary heart disease prevention: Not the gold standard? Prostaglandins Leukot. Essent. Fatty Acids 2018, 133, 8–15. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar] [CrossRef]
- Dumont, J.; Huybrechts, I.; Spinneker, A.; Gottrand, F.; Grammatikaki, E.; Bevilacqua, N.; Vyncke, K.; Widhalm, K.; Kafatos, A.; Molnar, D.; et al. FADS1 genetic variability interacts with dietary α-linolenic acid intake to affect serum non-HDL-cholesterol concentrations in European adolescents. J. Nutr. 2011, 141, 1247–1253. [Google Scholar] [CrossRef]
- Cormier, H.; Rudkowska, I.; Paradis, A.-M.; Thifault, E.; Garneau, V.; Lemieux, S.; Couture, P.; Vohl, M.-C. Association between polymorphisms in the fatty acid desaturase gene cluster and the plasma triacylglycerol response to an n-3 PUFA supplementation. Nutrients 2012, 4, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, S.; Sonestedt, E.; Ericson, U.; Gullberg, B.; Wirfält, E.; Hedblad, B.; Orho-Melander, M. Intake levels of dietary long-chain PUFAs modify the association between genetic variation in FADS and LDL-C. J. Lipid Res. 2012, 53, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Brayner, B.; Kaur, G.; Keske, M.A.; Livingstone, K.M. FADS Polymorphism, Omega-3 Fatty Acids and Diabetes Risk: A Systematic Review. Nutrients 2018, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Heydari, B.; Ge, Y.; Abdullah, S.; Fujikura, K.; Kaneko, K.; Harris, W.S.; Jerosch-Herold, M.; Antman, E.M.; Seidman, J.G.; et al. Genetic profiling of fatty acid desaturase polymorphisms identifies patients who may benefit from high-dose omega-3 fatty acids in cardiac remodeling after acute myocardial infarction-Post-hoc analysis from the OMEGA-REMODEL randomized controlled trial. PLoS ONE 2019, 14, e0222061. [Google Scholar] [CrossRef]
- De Goede, J.; Verschuren, W.M.M.; Boer, J.M.A.; Kromhout, D.; Geleijnse, J.M. Gender-specific associations of marine n-3 fatty acids and fish consumption with 10-year incidence of stroke. PLoS ONE 2012, 7, e33866. [Google Scholar] [CrossRef]
- Ferdouse, A.; Leng, S.; Winter, T.; Aukema, H.M. Dietary n-6 and n-3 PUFA alter the free oxylipin profile differently in male and female rat hearts. Br. J. Nutr. 2019, 122, 252–261. [Google Scholar] [CrossRef]
- Lauritzen, L.; Sørensen, L.B.; Harsløf, L.B.; Ritz, C.; Stark, K.D.; Astrup, A.; Dyssegaard, C.B.; Egelund, N.; Michaelsen, K.F.; Damsgaard, C.T. Mendelian randomization shows sex-specific associations between long-chain PUFA-related genotypes and cognitive performance in Danish schoolchildren. Am. J. Clin. Nutr. 2017, 106, 88–95. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Ip, W.T.K.; McAlindon, A.; Miller, S.E.; Bell, J.R.; Curl, C.L.; Huggins, C.E.; Mellor, K.M.; Raaijmakers, A.J.A.; Bienvenu, L.A.; McLennan, P.L.; et al. Dietary omega-6 fatty acid replacement selectively impairs cardiac functional recovery after ischemia in female (but not male) rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H768–H780. [Google Scholar] [CrossRef]
- Balogun, K.A.; Cheema, S.K. Dietary Omega-3 Fatty Acids Prevented Adipocyte Hypertrophy by Downregulating DGAT-2 and FABP-4 in a Sex-Dependent Fashion. Lipids 2016, 51, 25–38. [Google Scholar] [CrossRef]
- Ghasemifard, S.; Hermon, K.; Turchini, G.M.; Sinclair, A.J. Metabolic fate (absorption, β-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. Br. J. Nutr. 2015, 114, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Kew, S.; Finnegan, Y.E.; Minihane, A.M.; Leigh-Firbank, E.C.; Williams, C.M.; Calder, P.C. Increased dietary α-linolenic acid has sex-specific effects upon eicosapentaenoic acid status in humans: Re-examination of data from a randomised, placebo-controlled, parallel study. Nutr. J. 2014, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Nicodemus-Johnson, J. Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients 2018, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-G.; Huang, Y.-H.; Li, H.-Y.; Xu, T.-C.; Fan, Y.-W.; Li, J.; Deng, Z.-Y. Effects of Chinese Dietary Pattern of Fat Content, n-6/n-3 Polyunsaturated Fatty Acid Ratio, and Cholesterol Content on Lipid Profile in Rats. Biomed. Res. Int. 2018, 2018, 4398086. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Adamsson, V.; Cederholm, T.; Vessby, B.; Risérus, U. Influence of a healthy Nordic diet on serum fatty acid composition and associations with blood lipoproteins—Results from the NORDIET study. Food Nutr. Res. 2014, 58, 24114. [Google Scholar] [CrossRef][Green Version]
- Muzsik, A.; Bajerska, J.; Jeleń, H.H.; Walkowiak, J.; Krzyżanowska-Jankowska, P.; Chmurzynska, A. FADS1 and FADS2 polymorphism are associated with changes in fatty acid concentrations after calorie-restricted Central European and Mediterranean diets. Menopause 2019, 26, 1415–1424. [Google Scholar] [CrossRef]
- Tanaka, T.; Shen, J.; Abecasis, G.R.; Kisialiou, A.; Ordovas, J.M.; Guralnik, J.M.; Singleton, A.; Bandinelli, S.; Cherubini, A.; Arnett, D.; et al. Genome-Wide Association Study of Plasma Polyunsaturated Fatty Acids in the InCHIANTI Study. PLoS Genet. 2009, 5, e1000338. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.-C.; Bhattacharya, S.; et al. Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-Wide Association Study of Plasma N6 Polyunsaturated Fatty Acids Within the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef]
- Tintle, N.L.; Pottala, J.V.; Lacey, S.; Ramachandran, V.; Westra, J.; Rogers, A.; Clark, J.; Olthoff, B.; Larson, M.; Harris, W.; et al. A genome-wide association study of saturated, mono- and polyunsaturated red blood cell fatty acids in the Framingham Heart Offspring Study. Prostaglandins Leukot. Essent. Fatty Acids 2015, 94, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Lemaitre, R.N.; Manichaikul, A.; Guan, W.; Tanaka, T.; Foy, M.; Kabagambe, E.K.; Djousse, L.; Siscovick, D.; Fretts, A.M.; et al. Genome-Wide Association Study Identifies Novel Loci Associated with Concentrations of Four Plasma Phospholipid Fatty Acids in the De Novo Lipogenesis Pathway: Results From the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Circ. Cardiovasc. Genet. 2013, 6, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Dorajoo, R.; Sun, Y.; Han, Y.; Ke, T.; Burger, A.; Chang, X.; Low, H.Q.; Guan, W.; Lemaitre, R.N.; Khor, C.-C.; et al. A genome-wide association study of n-3 and n-6 plasma fatty acids in a Singaporean Chinese population. Genes. Nutr. 2015, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Moltke, I.; Grarup, N.; Racimo, F.; Bjerregaard, P.; Jorgensen, M.E.; Korneliussen, T.S.; Gerbault, P.; Skotte, L.; Linneberg, A.; et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 2015, 349, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, H.; Lu, L.; Manichaikul, A.; Zhu, J.; Chen, Y.-D.I.; Sun, L.; Liang, S.; Siscovick, D.S.; Steffen, L.M.; et al. Genome-wide meta-analyses identify novel loci associated with n-3 and n-6 polyunsaturated fatty acid levels in Chinese and European-ancestry populations. Hum. Mol. Genet. 2016, 25, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- PAGE Consortium; Yoneyama, S.; Yao, J.; Guo, X.; Fernandez-Rhodes, L.; Lim, U.; Boston, J.; Buzková, P.; Carlson, C.S.; Cheng, I.; et al. Generalization and fine mapping of European ancestry-based central adiposity variants in African ancestry populations. Int. J. Obes. 2017, 41, 324–331. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef]
- Duncan, L.; Shen, H.; Gelaye, B.; Meijsen, J.; Ressler, K.; Feldman, M.; Peterson, R.; Domingue, B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 2019, 10, 3328. [Google Scholar] [CrossRef]
- Ortega-Azorín, C.; Coltell, O.; Asensio, E.M.; Sorlí, J.V.; González, J.I.; Portolés, O.; Saiz, C.; Estruch, R.; Ramírez-Sabio, J.B.; Pérez-Fidalgo, A.; et al. Candidate Gene and Genome-Wide Association Studies for Circulating Leptin Levels Reveal Population and Sex-Specific Associations in High Cardiovascular Risk Mediterranean Subjects. Nutrients 2019, 11, 2751. [Google Scholar] [CrossRef]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef]
- Malerba, G.; Schaeffer, L.; Xumerle, L.; Klopp, N.; Trabetti, E.; Biscuola, M.; Cavallari, U.; Galavotti, R.; Martinelli, N.; Guarini, P.; et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 2008, 43, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Heinrich, J.; Klopp, N.; Schaeffer, L.; Hoff, S.; Wolfram, G.; Illig, T.; Linseisen, J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 2008, 101, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Gao, F.; Wang, D.; Bar-Yosef, O.; Keinan, A. Dietary adaptation of FADS genes in Europe varied across time and geography. Nat. Ecol. Evol. 2017, 1, 0167. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.N.; Ruczinski, I.; Yanek, L.R.; Becker, L.C.; Becker, D.M.; Guio, H.; Cui, T.; Chilton, F.H.; Mathias, R.A.; O’Connor, T.D. Evolution of Hominin Polyunsaturated Fatty Acid Metabolism: From Africa to the New World. Genome Biol. Evol. 2019, 11, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.T.; Racimo, F.; Allentoft, M.E.; Jensen, M.K.; Jonsson, A.; Huang, H.; Hormozdiari, F.; Sikora, M.; Marnetto, D.; Eskin, E.; et al. Selection in Europeans on Fatty Acid Desaturases Associated with Dietary Changes. Mol. Biol. Evol. 2017, 34, 1307–1318. [Google Scholar] [CrossRef]
- Ameur, A.; Enroth, S.; Johansson, Å.; Zaboli, G.; Igl, W.; Johansson, A.C.V.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic Adaptation of Fatty-Acid Metabolism: A Human-Specific Haplotype Increasing the Biosynthesis of Long-Chain Omega-3 and Omega-6 Fatty Acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Macian, F.; Ordovás, J.M. Advances in Understanding the Molecular Basis of the Mediterranean Diet Effect. Annu. Rev. Food Sci. Technol. 2018, 9, 227–249. [Google Scholar] [CrossRef]
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72, 83–91. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Aspects Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- De la Garza Puentes, A.; Montes Goyanes, R.; Chisaguano Tonato, A.M.; Torres-Espínola, F.J.; Arias García, M.; de Almeida, L.; Bonilla Aguirre, M.; Guerendiain, M.; Castellote Bargalló, A.I.; Segura Moreno, M.; et al. Association of maternal weight with FADS and ELOVL genetic variants and fatty acid levels- The PREOBE follow-up. PLoS ONE 2017, 12, e0179135. [Google Scholar] [CrossRef] [PubMed]
- Salas Lorenzo, I.; Chisaguano Tonato, A.M.; de la Garza Puentes, A.; Nieto, A.; Herrmann, F.; Dieguez, E.; Castellote, A.I.; López-Sabater, M.C.; Rodríguez-Palmero, M.; Campoy, C. The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients 2019, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Chisaguano, A.M.; Montes, R.; Pérez-Berezo, T.; Castellote, A.I.; Guerendiain, M.; Bustamante, M.; Morales, E.; García-Esteban, R.; Sunyer, J.; Franch, À.; et al. Gene Expression of Desaturase (FADS1 and FADS2) and Elongase (ELOVL5) Enzymes in Peripheral Blood: Association with Polyunsaturated Fatty Acid Levels and Atopic Eczema in 4-Year-Old Children. PLoS ONE 2013, 8, e78245. [Google Scholar] [CrossRef] [PubMed]
- Coltell, O.; Asensio, E.M.; Sorlí, J.V.; Barragán, R.; Fernández-Carrión, R.; Portolés, O.; Ortega-Azorín, C.; Martínez-LaCruz, R.; González, J.I.; Zanón-Moreno, V.; et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients 2019, 11, 90. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2018, 42, 777–788. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef] [PubMed]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.-R.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef]
- Tukiainen, T.; Kettunen, J.; Kangas, A.J.; Lyytikäinen, L.-P.; Soininen, P.; Sarin, A.-P.; Tikkanen, E.; O’Reilly, P.F.; Savolainen, M.J.; Kaski, K.; et al. Detailed metabolic and genetic characterization reveals new associations for 30 known lipid loci. Hum. Mol. Genet. 2012, 21, 1444–1455. [Google Scholar] [CrossRef]
- Würtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Jelenkovic, A.; Bogl, L.H.; Rose, R.J.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Kaprio, J.; Silventoinen, K. Association between serum fatty acids and lipoprotein subclass profile in healthy young adults: Exploring common genetic and environmental factors. Atherosclerosis 2014, 233, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Deelen, J.; Kettunen, J.; Fischer, K.; van der Spek, A.; Trompet, S.; Kastenmüller, G.; Boyd, A.; Zierer, J.; van den Akker, E.B.; Ala-Korpela, M.; et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019, 10, 3346. [Google Scholar] [CrossRef]
- Jääskeläinen, O.; Solje, E.; Hall, A.; Katisko, K.; Korhonen, V.; Tiainen, M.; Kangas, A.J.; Helisalmi, S.; Pikkarainen, M.; Koivisto, A.; et al. Low Serum High-Density Lipoprotein Cholesterol Levels Associate with the C9orf72 Repeat Expansion in Frontotemporal Lobar Degeneration Patients. J. Alzheimers Dis. 2019, 72, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Kettunen, J.; Soininen, P.; Silander, K.; Ripatti, S.; Kumpula, L.S.; Hämäläinen, E.; Jousilahti, P.; Kangas, A.J.; Männistö, S.; et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol. Syst. Biol. 2010, 6, 441. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Ruczinski, I.; Ivester, P.; Lee, T.C.; Morgan, T.M.; Nicklas, B.J.; Mathias, R.A.; Chilton, F.H. Impact of methods used to express levels of circulating fatty acids on the degree and direction of associations with blood lipids in humans. Br. J. Nutr. 2016, 115, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Turner, S.D. Qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv 2014. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Veenstra, J.; Westra, J.; Disselkoen, C.; Koch, K.; McKenzie, K.A.; O’Bott, J.; Vander Woude, J.; Fischer, K.; Shearer, G.C.; et al. A genome-wide association study of red-blood cell fatty acids and ratios incorporating dietary covariates: Framingham Heart Study Offspring Cohort. PLoS ONE 2018, 13, e0194882. [Google Scholar] [CrossRef] [PubMed]
- Koronowicz, A.A.; Banks, P.; Szymczyk, B.; Leszczyńska, T.; Master, A.; Piasna, E.; Szczepański, W.; Domagała, D.; Kopeć, A.; Piątkowska, E.; et al. Dietary conjugated linoleic acid affects blood parameters, liver morphology and expression of selected hepatic genes in laying hens. Br. Poult. Sci. 2016, 57, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, A.; Brown, A.R.; Pabona, J.M.P.; Van, T.H.; Hamdan, H.; Mercado, C.P.; Quick, C.M.; Wight, P.A.; Simmen, R.C.M.; Simmen, F.A. Enhanced Gastrointestinal Expression of Cytosolic Malic Enzyme (ME1) Induces Intestinal and Liver Lipogenic Gene Expression and Intestinal Cell Proliferation in Mice. PLoS ONE 2014, 9, e113058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Ye, T.; Liu, C.; Fang, F.; Chen, Y.; Dong, Y. Maternal high-fat diet during pregnancy and lactation affects hepatic lipid metabolism in early life of offspring rat. J. Biosci. 2017, 42, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Cop, F.; Llaó-Cid, C.; Härtl, R.; Damm, M.; Bethani, I.; Parrilla, M.; Husainie, D.; Schänzer, A.; et al. Endothelial Dab1 signaling orchestrates neuro-glia-vessel communication in the central nervous system. Science 2018, 361, eaao2861. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.-Y.; Petersen, A.-K.; Mohney, R.P.; Meredith, D.; Wägele, B.; Altmaier, E.; CARDIoGRAM; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Sasaki, H.; Sueyasu, T.; Tokuda, H.; Ito, M.; Kaneda, Y.; Rogi, T.; Kawashima, H.; Horiguchi, S.; Kawabata, T.; Shibata, H. Aging and FADS1 polymorphisms decrease the biosynthetic capacity of long-chain PUFAs: A human trial using [U-13C] linoleic acid. Prostaglandins Leukot. Essent. Fatty Acids 2019, 148, 1–8. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Lv, X.; Ma, J. Dietary n-3 Polyunsaturated Fatty Acid Intakes Modify the Effect of Genetic Variation in Fatty Acid Desaturase 1 on Coronary Artery Disease. PLoS ONE 2015, 10, e0121255. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; de Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Nyalala, J.O.; Wang, J.; Dang, A.; Faas, F.H.; Smith, W.G. Hypertriglyceridemia and hypercholesterolemia: Effects of drug treatment on fatty acid composition of plasma lipids and membranes. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 271–280. [Google Scholar] [CrossRef]
- Risé, P.; Marangoni, F.; Galli, C. Regulation of PUFA metabolism: Pharmacological and toxicological aspects. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Huang, H.; Jiang, X.; Ardisson Korat, A.V.; Song, M.; Sun, Q.; Willett, W.C.; Jensen, M.K.; Kraft, P. Joint effects of fatty acid desaturase 1 polymorphisms and dietary polyunsaturated fatty acid intake on circulating fatty acid proportions. Am. J. Clin. Nutr. 2018, 107, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harding, S.V.; Rideout, T.C.; Yurkova, N.; Cunnane, S.C.; Eck, P.K.; Jones, P.J. Dietary oils and FADS1-FADS2 genetic variants modulate [13C] α-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 2013, 97, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Kolossa, S.; Gedrich, K.; Celis-Morales, C.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Marsaux, C.F.M.; et al. Predicting fatty acid profiles in blood based on food intake and the FADS1 rs174546 SNP. Mol. Nutr. Food. Res. 2015, 59, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-H.; Zhang, R.-H.; Lv, N.-N.; Yang, G.-P.; Wang, Y.-S.; Pan, K.-H. The Role of Malic Enzyme on Promoting Total Lipid and Fatty Acid Production in Phaeodactylum tricornutum. Front. Plant Sci. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Mühlroth, A.; Li, K.; Røkke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.; Vadstein, O.; Bones, A. Pathways of Lipid Metabolism in Marine Algae, Co-Expression Network, Bottlenecks and Candidate Genes for Enhanced Production of EPA and DHA in Species of Chromista. Marine Drugs 2013, 11, 4662–4697. [Google Scholar] [CrossRef]
- Rovadoscki, G.A.; Pertile, S.F.N.; Alvarenga, A.B.; Cesar, A.S.M.; Pértille, F.; Petrini, J.; Franzo, V.; Soares, W.V.B.; Morota, G.; Spangler, M.L.; et al. Estimates of genomic heritability and genome-wide association study for fatty acids profile in Santa Inês sheep. BMC Genom. 2018, 19, 375. [Google Scholar] [CrossRef]
- Benítez, R.; Fernández, A.; Isabel, B.; Núñez, Y.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Óvilo, C. Modulatory Effects of Breed, Feeding Status, and Diet on Adipogenic, Lipogenic, and Lipolytic Gene Expression in Growing Iberian and Duroc Pigs. Int. J. Med. Sci. 2017, 19, 22. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Feltham, B.A.; Balogun, K.A.; Cheema, S.K. Perinatal and postweaning diets high in omega-3 fatty acids have age- and sex-specific effects on the fatty acid composition of the cerebellum and brainstem of C57BL/6 mice. Prostaglandins Leukot. Essent. Fatty Acids 2019, 148, 16–24. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Portolés, O.; Sotos-Prieto, M.; Fernández-Carrión, R.; Ramirez-Sabio, J.; Zanón-Moreno, V.; Mattei, J.; Sorlí, J.; Ordovas, J. A Guide to Applying the Sex-Gender Perspective to Nutritional Genomics. Nutrients 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Shibata, S.; Ueda, T.; Sasaki, K.; Shimoida, Y.; Senda-Murata, K.; Sugimoto, K. Characterization of nucleolar localization and exclusion signals in terminal deoxynucleotidyltransferase interacting factor 2/estrogen receptor α-binding protein. Biosci. Biotechnol. Biochem. 2019, 83, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kato, K.; Oguri, M.; Horibe, H.; Fujimaki, T.; Yasukochi, Y.; Takeuchi, I.; Sakuma, J. Identification of nine genes as novel susceptibility loci for early-onset ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. Biomed. Rep. 2018, 9, 8–20. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 426) | Men (n = 187) | Women (n = 239) | p |
|---|---|---|---|---|
| Age (years) | 65.1 ± 0.2 | 64.0 ± 0.4 | 66.0 ± 0.3 | <0.001 |
| Weight (Kg) | 84.2 ± 0.7 | 92.5 ± 1.0 | 77.7 ± 0.6 | <0.001 |
| BMI (Kg/m2) | 32.3 ± 0.2 | 32.2 ± 0.3 | 32.3 ± 0.2 | 0.836 |
| Waist circumference (cm) | 105.8 ± 0.5 | 110.9 ± 0.6 | 101.8 ± 0.6 | <0.001 |
| SBP (mm Hg) | 141.9 ± 0.9 | 144.1 ± 1.4 | 140.1 ± 1.2 | 0.029 |
| DBP (mm Hg) | 81.0 ± 0.5 | 82.7 ± 0.7 | 79.6 ± 0.6 | 0.001 |
| Total cholesterol (mg/dL) | 196.4 ± 1.8 | 188.3 ± 2.9 | 202.7 ± 2.3 | <0.001 |
| LDL-C (mg/dL) | 124.9 ± 1.5 | 121.9 ± 2.4 | 127.2 ± 1.9 | 0.078 |
| HDL-C (mg/dL) | 51.6 ± 0.6 | 47.3 ± 0.8 | 55.0 ± 0.7 | <0.001 |
| Triglycerides (mg/dL) | 140.7 ± 2.9 | 137.6 ± 3.9 | 143.1 ± 4.1 | 0.334 |
| Fasting glucose (mg/dL) | 112.4 ± 1.3 | 113.5 ± 2.1 | 111.5 ± 1.6 | 0.441 |
| Total fatty acids (mmol/L) | 11.09 ± 0.10 | 10.63 ± 0.14 | 14.46 ± 0.14 | <0.001 |
| SFA (mmol/L) | 4.02 ± 0.04 | 3.85 ± 0.05 | 4.16 ± 0.05 | <0.001 |
| MUFA (mmol/L) | 3.09 ± 0.03 | 2.97 ± 0.04 | 3.20 ± 0.05 | <0.001 |
| PUFA (mmol/L) | 3.97 ± 0.04 | 3.81 ± 0.05 | 4.10 ± 0.05 | <0.001 |
| Omega-3 (mmol/L) | 0.46 ± 0.01 | 0.43 ± 0.01 | 0.48 ± 0.01 | <0.001 |
| Omega-6 (mmol/L) | 3.52 ± 0.03 | 3.38 ± 0.04 | 3.63 ± 0.04 | <0.001 |
| DHA (mmol/L) | 0.146 ± 0.002 | 0.140 ± 0.003 | 0.150 ± 0.003 | 0.013 |
| LA (mmol/L) | 2.88 ± 0.03 | 2.78 ± 0.04 | 2.97 ± 0.04 | 0.001 |
| SFA (%) 1 | 36.27 ± 0.06 | 36.25 ± 0.09 | 36.29 ± 0.08 | 0.722 |
| MUFA (%) 2 | 27.82 ± 0.09 | 27.85 ± 0.13 | 27.79 ± 0.13 | 0.754 |
| PUFA (%) 3 | 35.90 ± 0.11 | 35.89 ± 0.16 | 35.91 ± 0.15 | 0.950 |
| Omega-3 (%) 4 | 4.09 ± 0.03 | 4.04 ± 0.05 | 4.13 ± 0.05 | 0.182 |
| Omega-6 (%) 5 | 31.80 ± 0.10 | 31.85 ± 0.15 | 31.78 ± 0.14 | 0.723 |
| DHA (%) 6 | 1.30 ± 0.01 | 1.31 ± 0.02 | 1.30 ± 0.02 | 0.710 |
| LA (%) 7 | 26.04 ± 0.11 | 26.18 ± 0.18 | 25.90 ± 0.15 | 0.272 |
| Omega-6/Omega-3 ratio | 7.98 ± 0.07 | 8.07 ± 0.10 | 7.91 ± 0.09 | 0.254 |
| Type 2 diabetes: n, % | 160 (37.6) | 70 (37.4) | 90 (37.7) | 0.962 |
| Current smokers: n, % | 46 (10.8) | 28 (15.0) | 18 (7.5) | <0.001 |
| Antihypertensive drugs | 334 (78.4) | 148 (79.1) | 186 (77.8) | 0.742 |
| Hypolipidemic drugs | 274 (64.3) | 121 (64.7) | 153 (64.0) | 0.883 |
| Metformin treatment | 128 (30.0) | 56 (29.9) | 72 (30.1) | 0.968 |
| Insulin treatment | 19 (4.5) | 11 (5.9) | 8 (3.3) | 0.208 |
| Physical Activity (MET.min/wk) | 1724 ± 76 | 1950 ± 132 | 1547 ± 85 | 0.008 |
| Adherence to MedDiet (P17) 8 | 8.1 ± 0.1 | 7.8 ± 0.2 | 8.2 ± 0.2 | 0.145 |
| Unadjusted 1 | Adjusted 2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | SNP | Gene | Position 3 | Allele | MAF | Beta 1 | SE 1 | R2 (1) | p1 | Chr | SNP | Gene | MAF | Beta 2 | p2 |
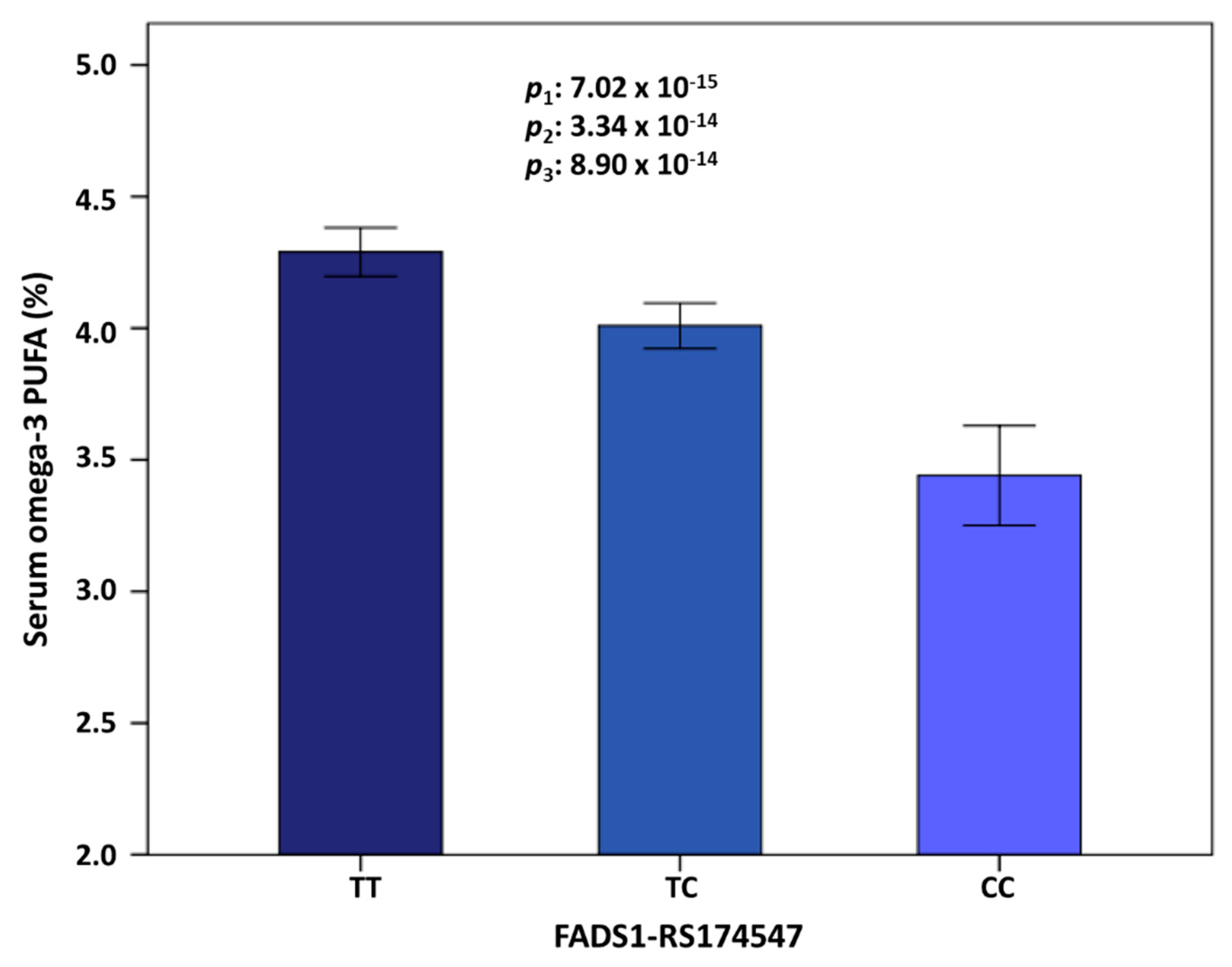
| 11 | rs174547 | FADS1 | 61570783 | C | 0.298 | −0.372 | 0.046 | 0.134 | 7.02 × 10−15 | 11 | rs174547 | FADS1 | 0.298 | −0.366 | 3.34 × 10−14 |
| 11 | rs174550 | FADS1 | 61571478 | C | 0.298 | −0.369 | 0.046 | 0.132 | 1.10 × 10−14 | 11 | rs174550 | FADS1 | 0.298 | −0.364 | 5.35 × 10−14 |
| 11 | rs174546 | FADS1 | 61569830 | T | 0.298 | −0.368 | 0.046 | 0.131 | 1.34 × 10−14 | 11 | rs1535 | FADS2 | 0.322 | −0.356 | 5.85 × 10−14 |
| 11 | rs1535 | FADS2 | 61597972 | G | 0.322 | −0.361 | 0.045 | 0.131 | 1.51 × 10−14 | 11 | rs174546 | FADS1 | 0.298 | −0.362 | 6.72 × 10−14 |
| 11 | rs174583 | FADS2 | 61609750 | T | 0.369 | −0.352 | 0.044 | 0.129 | 2.38 × 10−14 | 11 | rs174583 | FADS2 | 0.369 | −0.346 | 9.75 × 10−14 |
| 11 | rs174576 | FADS2 | 61603510 | A | 0.363 | −0.352 | 0.045 | 0.129 | 2.49 × 10−14 | 11 | rs174576 | FADS2 | 0.363 | −0.346 | 1.17 × 10−13 |
| 11 | rs174577 | FADS2 | 61604814 | A | 0.392 | −0.339 | 0.045 | 0.119 | 2.56 × 10−13 | 11 | rs174577 | FADS2 | 0.392 | −0.332 | 1.12 × 10−12 |
| 11 | rs174535 | MYRF | 61551356 | C | 0.340 | −0.344 | 0.046 | 0.117 | 3.56 × 10−13 | 11 | rs174535 | MYRF | 0.340 | −0.339 | 1.49 × 10−12 |
| 11 | rs102275 | TMEM258 | 61557803 | C | 0.493 | −0.338 | 0.045 | 0.116 | 5.79 × 10−13 | 11 | rs102275 | TMEM258 | 0.493 | −0.332 | 2.43 × 10−12 |
| 11 | rs174538 | FEN1 | 61560081 | A | 0.282 | −0.346 | 0.048 | 0.107 | 4.25 × 10−12 | 11 | rs174538 | FEN1 | 0.282 | −0.339 | 1.96 × 10−11 |
| 11 | rs108499 | MYRF | 61547237 | T | 0.288 | −0.332 | 0.048 | 0.103 | 1.31 × 10−11 | 11 | rs108499 | MYRF | 0.288 | −0.327 | 4.21 × 10−11 |
| 11 | rs174532 | MYRF | 61548874 | A | 0.100 | 0.267 | 0.046 | 0.074 | 1.12 × 10−08 | 11 | rs174532 | MYRF | 0.100 | 0.267 | 1.20 × 10−08 |
| 11 | rs2727270 | FADS2 | 61603237 | T | 0.163 | −0.402 | 0.077 | 0.060 | 2.88 × 10−07 | 11 | rs2727270 | FADS2 | 0.163 | −0.391 | 8.01 × 10−07 |
| 6 | rs2092556 | LINC00271 | 135899344 | C | 0.188 | 0.409 | 0.085 | 0.052 | 2.23 × 10−06 | 6 | rs2092556 | LINC00271 | 0.188 | 0.413 | 1.85 × 10−06 |
| 14 | rs7149414 | intergenic | 31237170 | A | 0.133 | 0.381 | 0.080 | 0.052 | 2.31 × 10−06 | 4 | rs2736982 | DSPP | 0.326 | 0.220 | 2.09 × 10−06 |
| 5 | rs7736605 | WDR70 | 37642044 | A | 0.059 | 0.775 | 0.163 | 0.051 | 2.72 × 10−06 | 5 | rs7736605 | WDR70 | 0.059 | 0.782 | 2.26 × 10−06 |
| 6 | rs10499212 | intergenic | 140027475 | T | 0.100 | 0.642 | 0.136 | 0.051 | 3.11 × 10−06 | 14 | rs7149414 | intergenic | 0.133 | 0.382 | 2.31 × 10−06 |
| 6 | rs4896151 | LINC00271 | 135829796 | T | 0.141 | 0.398 | 0.085 | 0.049 | 3.93 × 10−06 | 1 | rs11164689 | intergenic | 0.065 | 0.500 | 3.09 × 10−06 |
| 1 | rs11164689 | intergenic | 103603452 | T | 0.065 | 0.491 | 0.106 | 0.049 | 4.32 × 10−06 | 6 | rs4896151 | LINC00271 | 0.141 | 0.402 | 3.42 × 10−06 |
| 6 | rs1547079 | AHI1 | 135749202 | C | 0.177 | 0.389 | 0.085 | 0.047 | 5.91 × 10−06 | 6 | rs1547079 | AHI1 | 0.177 | 0.634 | 4.69 × 10−06 |
| 6 | rs9494274 | LINC00271 | 0.234 | 0.394 | 7.95 × 10−06 | ||||||||||
| Unadjusted 1 | Adjusted 2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | SNP | Gene | Position 3 | Allele | MAF | Beta 1 | SE 1 | R2 (1) | p1 | Chr | SNP | Gene | MAF | Beta 2 | p2 |
| 11 | rs174547 | FADS1 | 61570783 | C | 0.298 | −0.122 | 0.017 | 0.106 | 6.12 × 10−12 | 11 | rs174547 | FADS1 | 0.298 | −0.121 | 1.28 × 10−11 |
| 11 | rs174550 | FADS1 | 61571478 | C | 0.298 | −0.121 | 0.017 | 0.105 | 7.35 × 10−12 | 11 | rs174550 | FADS1 | 0.298 | −0.121 | 1.50 × 10−11 |
| 11 | rs174546 | FADS1 | 61569830 | T | 0.298 | −0.121 | 0.017 | 0.104 | 8.24 × 10−12 | 11 | rs174546 | FADS1 | 0.298 | −0.120 | 1.66 × 10−11 |
| 11 | rs1535 | FADS2 | 61597972 | G | 0.322 | −0.118 | 0.017 | 0.103 | 1.29 × 10−11 | 11 | rs1535 | FADS2 | 0.322 | −0.117 | 2.87 × 10−11 |
| 11 | rs174583 | FADS2 | 61609750 | T | 0.369 | −0.115 | 0.017 | 0.102 | 1.54 × 10−11 | 11 | rs174583 | FADS2 | 0.369 | −0.114 | 3.34 × 10−11 |
| 11 | rs174576 | FADS2 | 61603510 | A | 0.363 | −0.115 | 0.017 | 0.101 | 2.11 × 10−11 | 11 | rs174576 | FADS2 | 0.363 | −0.114 | 4.42 × 10−11 |
| 11 | rs174577 | FADS2 | 61604814 | A | 0.392 | −0.109 | 0.017 | 0.092 | 1.66 × 10−10 | 11 | rs174538 | FEN1 | 0.282 | −0.117 | 3.40 × 10−10 |
| 11 | rs174538 | FEN1 | 61560081 | A | 0.282 | −0.117 | 0.018 | 0.092 | 1.77 × 10−10 | 11 | rs174577 | FADS2 | 0.392 | −0.109 | 3.47 × 10−10 |
| 11 | rs174535 | MYRF | 61551356 | C | 0.340 | −0.111 | 0.017 | 0.091 | 1.91 × 10−10 | 11 | rs174535 | MYRF | 0.340 | −0.111 | 3.89 × 10−10 |
| 11 | rs102275 | TMEM258 | 61557803 | C | 0.493 | −0.108 | 0.017 | 0.088 | 4.14 × 10−10 | 11 | rs102275 | TMEM258 | 0.493 | −0.108 | 8.79 × 10−10 |
| 11 | rs108499 | MYRF | 61547237 | T | 0.288 | −0.112 | 0.018 | 0.087 | 5.76 × 10−10 | 11 | rs108499 | MYRF | 0.288 | −0.111 | 1.22 × 10−09 |
| 11 | rs174532 | MYRF | 61548874 | A | 0.100 | 0.089 | 0.017 | 0.061 | 2.19 × 10−07 | 11 | rs174532 | MYRF | 0.100 | 0.088 | 3.43 × 10−07 |
| 6 | rs2092556 | LINC00271 | 135899344 | C | 0.188 | 0.154 | 0.031 | 0.055 | 1.13 × 10−06 | 6 | rs2092556 | LINC00271 | 0.188 | 0.154 | 1.24 × 10−06 |
| 6 | rs10499212 | intergenic | 140027475 | T | 0.100 | 0.239 | 0.050 | 0.052 | 2.27 × 10−06 | 4 | rs2736982 | DSPP | 0.326 | 0.081 | 2.25 × 10−06 |
| 1 | rs3001080 | intergenic | 163434750 | G | 0.426 | −0.082 | 0.017 | 0.050 | 2.90 × 10−06 | 6 | rs10499212 | intergenic | 0.100 | 0.238 | 2.64 × 10−06 |
| 6 | rs4896151 | LINC00271 | 135829796 | T | 0.141 | 0.148 | 0.031 | 0.050 | 2.90 × 10−06 | 6 | rs4896151 | LINC00271 | 0.141 | 0.149 | 3.04 × 10−06 |
| 4 | rs2736982 | DSPP | 88534235 | A | 0.326 | 0.079 | 0.017 | 0.050 | 3.13 × 10−06 | 1 | rs3001080 | intergenic | 0.426 | −0.081 | 3.57 × 10−06 |
| 1 | rs16856236 | DISC1 | 232160058 | G | 0.062 | 0.188 | 0.040 | 0.050 | 3.38 × 10−06 | 6 | rs1547079 | AHI1 | 0.177 | 0.146 | 3.91 × 10−06 |
| 6 | rs1547079 | AHI1 | 135749202 | C | 0.177 | 0.146 | 0.031 | 0.049 | 3.85 × 10−06 | 1 | rs16856236 | DISC1 | 0.062 | 0.186 | 4.01 × 10−06 |
| 5 | rs7736605 | WDR70 | 37642044 | A | 0.059 | 0.280 | 0.060 | 0.049 | 3.86 × 10−06 | 5 | rs7736605 | WDR70 | 0.059 | 0.281 | 4.05 × 10−06 |
| 6 | rs9494274 | LINC00271 | 135880480 | G | 0.234 | 0.135 | 0.029 | 0.048 | 5.55 × 10−06 | 5 | rs9312754 | CTNND2 | 0.261 | 0.123 | 5.24 × 10−06 |
| 5 | rs9312754 | CTNND2 | 11088028 | C | 0.261 | 0.122 | 0.027 | 0.047 | 6.18 × 10−06 | 6 | rs9494274 | LINC00271 | 0.234 | 0.135 | 6.35 × 10−06 |
| 18 | rs165155 | intergenic | 75341905 | C | 0.163 | −0.110 | 0.024 | 0.047 | 6.53 × 10−06 | 18 | rs165155 | intergenic | 0.163 | −0.109 | 8.42 × 10−06 |
| 10 | rs16911516 | intergenic | 59751257 | C | 0.086 | −0.337 | 0.074 | 0.047 | 7.04 × 10−06 | 10 | rs16911516 | intergenic | 0.086 | −0.336 | 8.92 × 10−06 |
| 1 | rs11164689 | intergenic | 0.065 | 0.175 | 9.18 × 10−06 | ||||||||||
| Adjusted 1 | |||||||
|---|---|---|---|---|---|---|---|
| Chr | SNP | Gene | Position2 | Alleles | MAF | Beta | p |
| 11 | rs174611 | FADS2 | 61627881 | C | 0.116 | 0.679 | 4.86 × 10−04 |
| 11 | rs174618 | FADS2 | 61629322 | C | 0.349 | 0.550 | 1.21 × 10−03 |
| 11 | rs174547 | FADS1 | 61570783 | C | 0.298 | 0.527 | 2.78 × 10−03 |
| 11 | rs174583 | FADS2 | 61609750 | T | 0.369 | 0.507 | 2.88 × 10−03 |
| 11 | rs174546 | FADS1 | 61569830 | T | 0.298 | 0.521 | 3.19 × 10−03 |
| 11 | rs174550 | FADS1 | 61571478 | C | 0.298 | 0.512 | 3.82 × 10−03 |
| 11 | rs174576 | FADS2 | 61603510 | A | 0.363 | 0.493 | 3.83 × 10−03 |
| 11 | rs174577 | FADS2 | 61604814 | A | 0.392 | 0.491 | 3.89 × 10−03 |
| 11 | rs174450 | FADS3 | 61641542 | T | 0.435 | 0.481 | 5.15 × 10−03 |
| 11 | rs1535 | FADS2 | 61597972 | G | 0.322 | 0.482 | 5.49 × 10−03 |
| 11 | rs17156442 | FADS2 | 61614023 | T | 0.098 | 0.976 | 8.04 × 10−03 |
| 11 | rs174455 | FADS3 | 61656117 | A | 0.407 | 0.401 | 1.93 × 10−02 |
| 11 | rs1000778 | FADS3 | 61655305 | A | 0.477 | 0.329 | 7.15 × 10−02 |
| 11 | rs174570 | FADS2 | 61597212 | T | 0.228 | 0.219 | 3.84 × 10−01 |
| 11 | rs2727270 | FADS2 | 61603237 | T | 0.163 | 0.236 | 4.08 × 10−01 |
| 11 | rs482548 | FADS2 | 61633182 | T | 0.030 | 0.216 | 4.19 × 10−01 |
| 11 | rs17764288 | FADS2 | 61633736 | A | 0.003 | 0.520 | 5.00 × 10−01 |
| 11 | rs12807005 | FADS2 | 61591059 | A | 0.006 | 0.357 | 6.30 × 10−01 |
| 11 | rs2851682 | FADS2 | 61616012 | G | 0.149 | 0.133 | 6.75 × 10−01 |
| 11 | rs498793 | FADS2 | 61624705 | T | 0.313 | −0.048 | 7.77 × 10−01 |
| 11 | rs7942717 | FADS3 | 61647288 | G | 0.097 | 0.040 | 8.83 × 10−01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Barragán, R.; González, J.I.; Giménez-Alba, I.M.; Zanón-Moreno, V.; Estruch, R.; Ramírez-Sabio, J.B.; Pascual, E.C.; et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients 2020, 12, 310. https://doi.org/10.3390/nu12020310
Coltell O, Sorlí JV, Asensio EM, Barragán R, González JI, Giménez-Alba IM, Zanón-Moreno V, Estruch R, Ramírez-Sabio JB, Pascual EC, et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients. 2020; 12(2):310. https://doi.org/10.3390/nu12020310
Chicago/Turabian StyleColtell, Oscar, Jose V. Sorlí, Eva M. Asensio, Rocío Barragán, José I. González, Ignacio M. Giménez-Alba, Vicente Zanón-Moreno, Ramon Estruch, Judith B. Ramírez-Sabio, Eva C. Pascual, and et al. 2020. "Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome" Nutrients 12, no. 2: 310. https://doi.org/10.3390/nu12020310
APA StyleColtell, O., Sorlí, J. V., Asensio, E. M., Barragán, R., González, J. I., Giménez-Alba, I. M., Zanón-Moreno, V., Estruch, R., Ramírez-Sabio, J. B., Pascual, E. C., Ortega-Azorín, C., Ordovas, J. M., & Corella, D. (2020). Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients, 12(2), 310. https://doi.org/10.3390/nu12020310











