Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Milk Sample Collection
2.2. miRNA Analysis
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Expression Levels of miRNAs and Leptin and Adiponectin Concentrations in Breast Milk
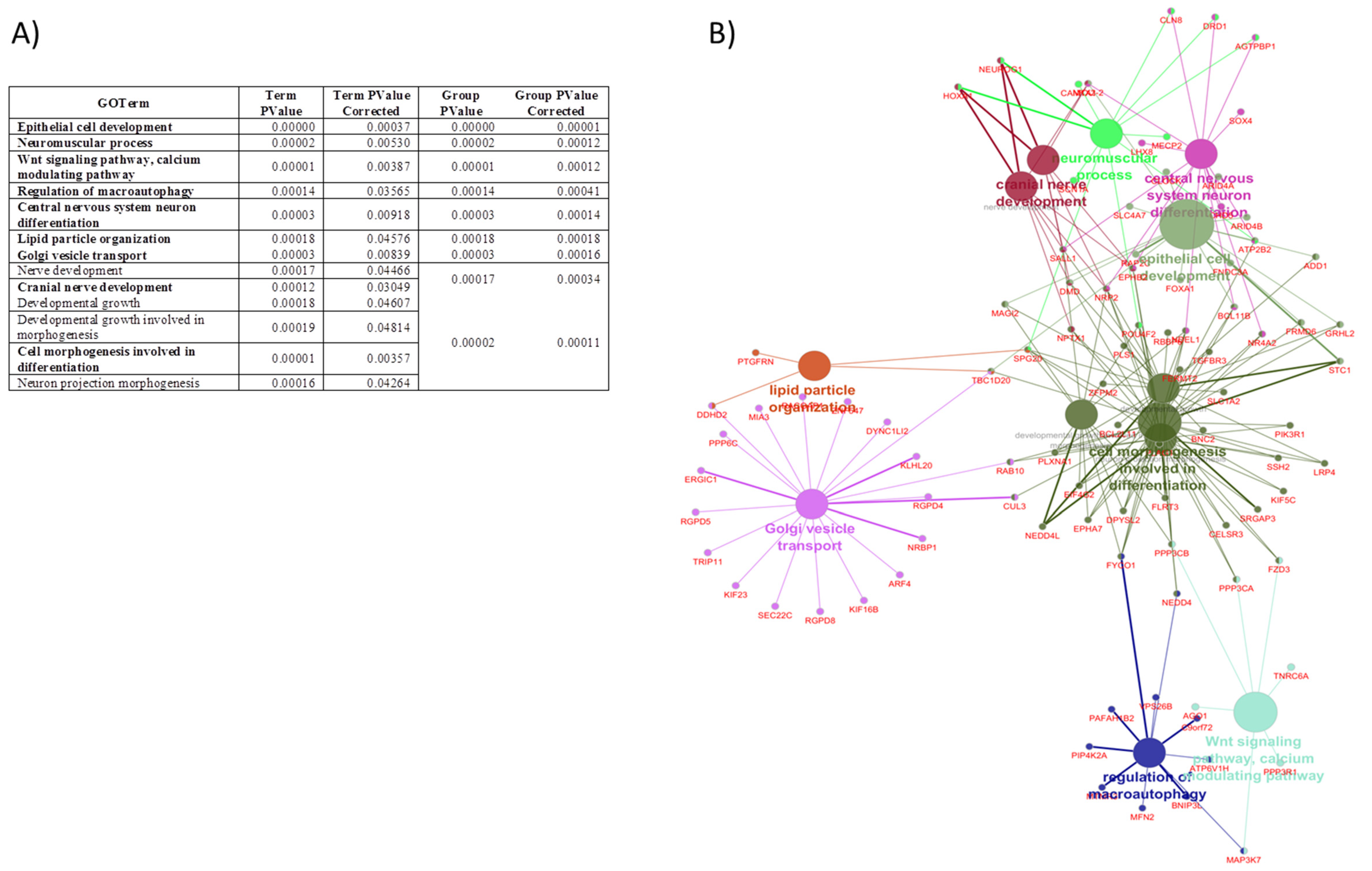
3.3. In Silico Analysis of Potential miRNA Target Genes
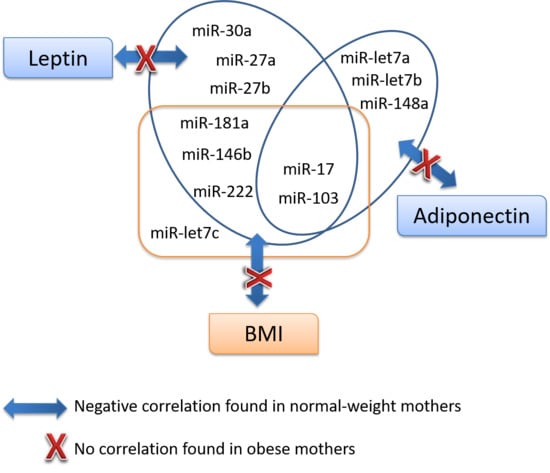
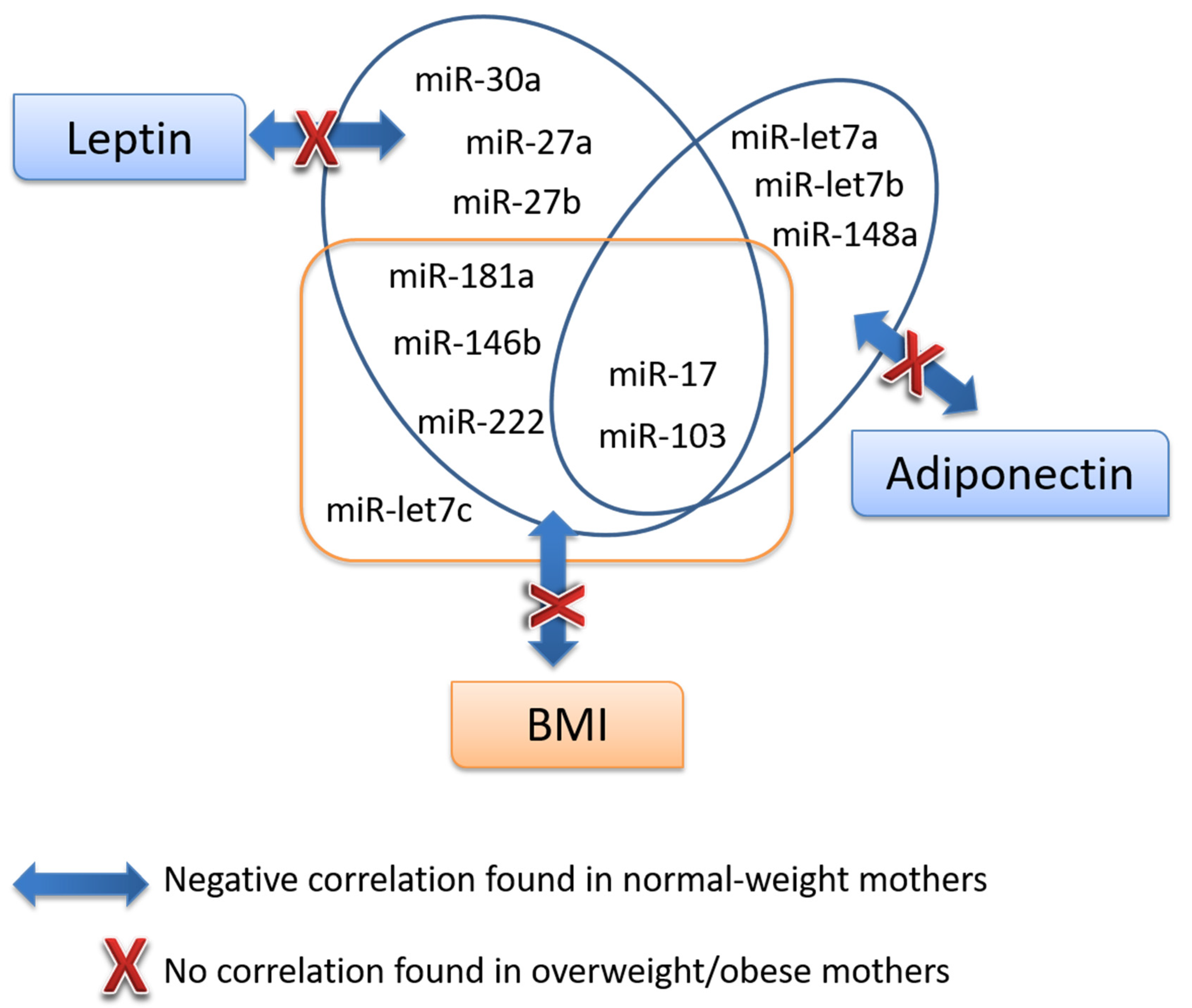
3.4. Association Analysis of miRNAs with Key Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk factors for childhood obesity in the first 1000 days: A systematic review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. MicroRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Na, R.S.; E, G.X.; Sun, W.; Sun, X.W.; Qiu, X.Y.; Chen, L.P.; Huang, Y.F. Expressional analysis of immune-related miRNAs in breast milk. Genet. Mol. Res. 2015, 14, 11371–11376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells and lipids conserve numerous known and novel miRNAs, some of which are differentially expressed during lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef]
- Benmoussa, A.; Lee, C.H.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J. Nutr. 2016, 146, 2206–2215. [Google Scholar] [CrossRef]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [Green Version]
- Pomar, C.A.; Castro, H.; Pico, C.; Serra, F.; Palou, A.; Sanchez, J. Cafeteria diet consumption during lactation in rats, rather than obesity Per Se, alters miR-222, miR-200a, and miR-26a levels in milk. Mol. Nutr. Food Res. 2019, 63, e1800928. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Bonmati, A.; Ortega, F.J.; Mercader, J.M.; Guindo-Martinez, M.; Torrents, D.; Prats-Puig, A.; Martinez-Calcerrada, J.M.; Platero-Gutierrez, E.; De Zegher, F.; et al. Altered circulating miRNA expression profile in pregestational and gestational obesity. J. Clin. Endocrinol. Metab. 2015, 100, E1446–E1456. [Google Scholar] [CrossRef]
- Can, U.; Buyukinan, M.; Yerlikaya, F.H. The investigation of circulating microRNAs associated with lipid metabolism in childhood obesity. Pediatr. Obes. 2016, 11, 228–234. [Google Scholar] [CrossRef]
- Prats-Puig, A.; Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Bonet, N.; Ricart, W.; Lopez-Bermejo, A.; Fernandez-Real, J.M. Changes in circulating microRNAs are associated with childhood obesity. J. Clin. Endocrinol. Metab. 2013, 98, E1655–E1660. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, X.; Li, R.; Chen, M.; Song, W.; Li, X. The levels of human milk microRNAs and their association with maternal weight characteristics. Eur. J. Clin. Nutr. 2016, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Munch, E.M.; Harris, R.A.; Mohammad, M.; Benham, A.L.; Pejerrey, S.M.; Showalter, L.; Hu, M.; Shope, C.D.; Maningat, P.D.; Gunaratne, P.H.; et al. Transcriptome profiling of microRNA by Next-Gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS ONE 2013, 8, e50564. [Google Scholar] [CrossRef] [PubMed]
- Palou, A.; Sanchez, J.; Pico, C. Nutrient-gene interactions in early life programming: Leptin in breast milk prevents obesity later on in life. Adv. Exp. Med. Biol. 2009, 646, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Pico, C.; Palou, A. Leptin as a breast milk component for the prevention of obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef]
- Miralles, O.; Sanchez, J.; Palou, A.; Pico, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006, 14, 1371–1377. [Google Scholar] [CrossRef]
- Pico, C.; Oliver, P.; Sanchez, J.; Miralles, O.; Caimari, A.; Priego, T.; Palou, A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int. J. Obes. (Lond) 2007, 31, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Pico, C.; Palou, M.; Priego, T.; Sanchez, J.; Palou, A. Metabolic programming of obesity by energy restriction during the perinatal period: Different outcomes depending on gender and period, type and severity of restriction. Front. Physiol. 2012, 3, 436. [Google Scholar] [CrossRef]
- Sanchez, J.; Priego, T.; Palou, M.; Tobaruela, A.; Palou, A.; Pico, C. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 2008, 149, 733–740. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- Bao, B.; Rodriguez-Melendez, R.; Wijeratne, S.S.; Zempleni, J. Biotin regulates the expression of holocarboxylase synthetase in the miR-539 pathway in HEK-293 cells. J. Nutr. 2010, 140, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Friedman, Y.; Naamati, G.; Linial, M. MiRror: A combinatorial analysis web tool for ensembles of microRNAs and their targets. Bioinformatics 2010, 26, 1920–1921. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Laraichi, S.; Parra, P.; Zamanillo, R.; El Amarti, A.; Palou, A.; Serra, F. Dietary supplementation of calcium may counteract obesity in mice mediated by changes in plasma fatty acids. Lipids 2013, 48, 817–826. [Google Scholar] [CrossRef]
- Ley, S.H.; Hanley, A.J.; Stone, D.; O'Connor, D.L. Effects of pasteurization on adiponectin and insulin concentrations in donor human milk. Pediatr. Res. 2011, 70, 278–281. [Google Scholar] [CrossRef]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological activities of Extracellular Vesicles and their cargos from bovine and human milk in humans and implications for infants. J. Nutr. 2017, 147, 3–10. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Pico, C.; Serra, F.; Rodriguez, A.M.; Keijer, J.; Palou, A. Biomarkers of nutrition and health: New tools for new approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Bonmati, A.; Ortega, F.J.; Mercader, J.M.; Guindo-Martinez, M.; Torrents, D.; Prats-Puig, A.; Martinez-Calcerrada, J.M.; de Zegher, F.; Ibanez, L.; et al. Dysregulation of placental miRNA in maternal obesity is associated with Pre- and postnatal growth. J. Clin. Endocrinol. Metab. 2017, 102, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.A.; Choi, M.S. MicroRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 2011, 11, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Parra, P.; Serra, F.; Palou, A. Expression of adipose microRNAs is sensitive to dietary conjugated linoleic acid treatment in mice. PLoS ONE 2010, 5, e13005. [Google Scholar] [CrossRef] [PubMed]
- Carney, M.C.; Tarasiuk, A.; DiAngelo, S.L.; Silveyra, P.; Podany, A.; Birch, L.L.; Paul, I.M.; Kelleher, S.; Hicks, S.D. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr. Res. 2017, 82, 226–236. [Google Scholar] [CrossRef]
- Do, D.N.; Li, R.; Dudemaine, P.L.; Ibeagha-Awemu, E.M. MicroRNA roles in signalling during lactation: An insight from differential expression, time course and pathway analyses of deep sequence data. Sci. Rep. 2017, 7, 44605. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in breastmilk and the lactating Breast: potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef]
- Lonnerdal, B. Human milk microRNAs/exosomes: Composition and biological effects. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 83–92. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hepworth, A.R.; Lefevre, C.; Hartmann, P.E.; Geddes, D.T.; Hassiotou, F. Human milk microRNA and total RNA differ depending on milk fractionation. J. Cell. Biochem. 2015, 116, 2397–2407. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Armstrong, J.; Reilly, J.J. Breastfeeding and lowering the risk of childhood obesity. Lancet 2002, 359, 2003–2004. [Google Scholar] [CrossRef]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Schmid, D.; Zang, K.; Much, D.; Knoeferl, B.; Kratzsch, J.; Amann-Gassner, U.; Bader, B.L.; Hauner, H. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr. Obes. 2015, 10, 67–73. [Google Scholar] [CrossRef]
- Palou, A.; Pico, C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite 2009, 52, 249–252. [Google Scholar] [CrossRef]
- Pico, C.; Sanchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Palou, A. Role of leptin present in maternal milk in the control of energy balance during the post-natal period. Genes Nutr. 2007, 2, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Andreas, N.J.; Hyde, M.J.; Gale, C.; Parkinson, J.R.; Jeffries, S.; Holmes, E.; Modi, N. Effect of maternal body mass index on hormones in breast milk: A systematic review. PLoS ONE 2014, 9, e115043. [Google Scholar] [CrossRef]
- Schuster, S.; Hechler, C.; Gebauer, C.; Kiess, W.; Kratzsch, J. Leptin in maternal serum and breast milk: Association with infants' body weight gain in a longitudinal study over 6 months of lactation. Pediatr. Res. 2011, 70, 633–637. [Google Scholar] [CrossRef]
- Konieczna, J.; Sanchez, J.; Palou, M.; Pico, C.; Palou, A. Blood cell transcriptomic-based early biomarkers of adverse programming effects of gestational calorie restriction and their reversibility by leptin supplementation. Sci. Rep. 2015, 5, 9088. [Google Scholar] [CrossRef] [Green Version]
- Palou, M.; Pico, C.; McKay, J.A.; Sanchez, J.; Priego, T.; Mathers, J.C.; Palou, A. Protective effects of leptin during the suckling period against later obesity may be associated with changes in promoter methylation of the hypothalamic pro-opiomelanocortin gene. Br. J. Nutr. 2011, 106, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Pico, C.; Jilkova, Z.M.; Kus, V.; Palou, A.; Kopecky, J. Perinatal programming of body weight control by leptin: Putative roles of AMP kinase and muscle thermogenesis. Am. J. Clin. Nutr. 2011, 94, 1830S–1837S. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.J.; Power, C.; Manor, O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: Longitudinal study. BMJ 2001, 323, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
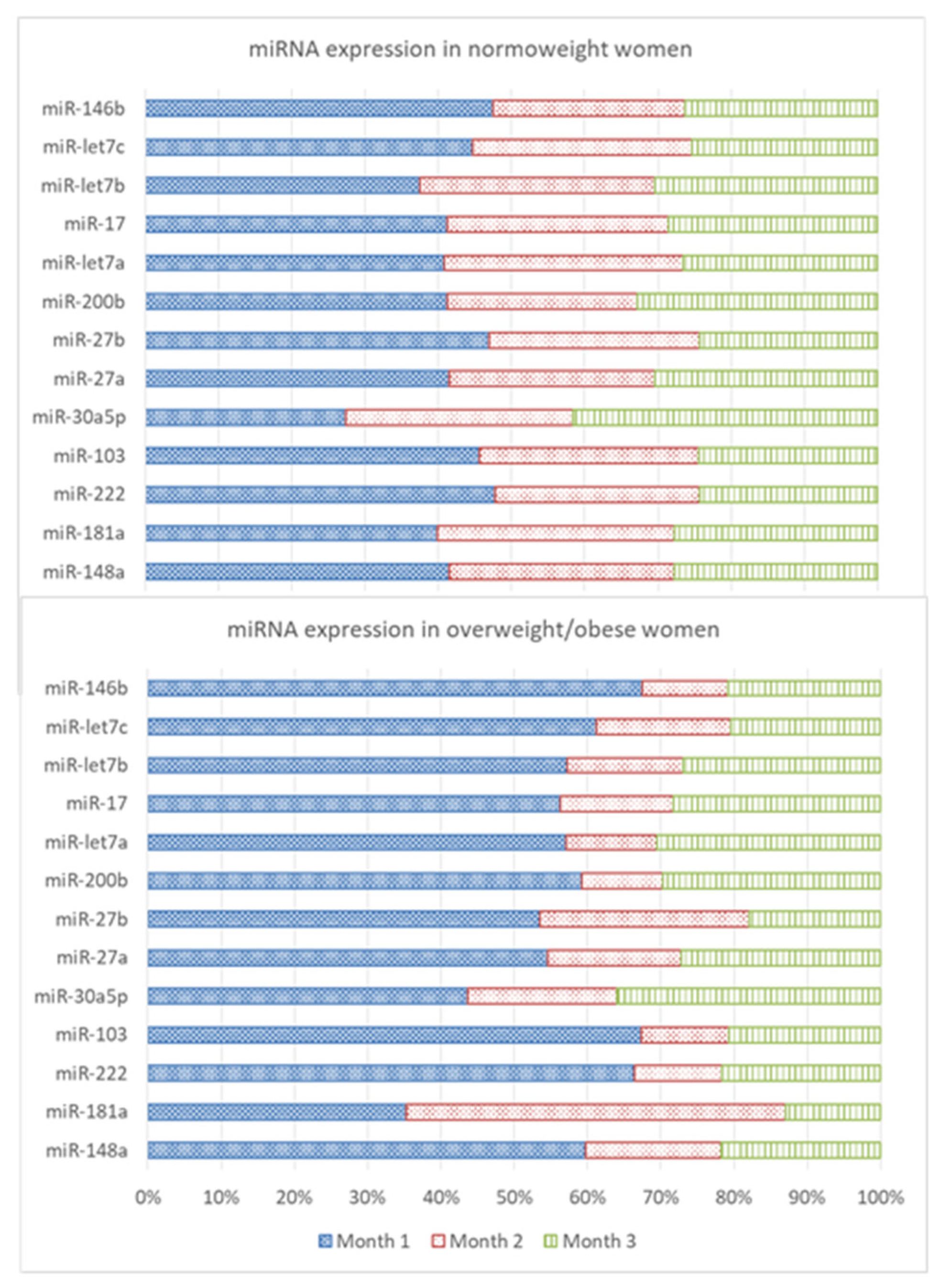
| Month 1 | Month 2 | Month 3 | Statistical Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | Overall | Normal/Obese Weight | ||
| miR-148a | Whole | 52 | 0.004 | 237.21 | 21.98 | 45.40 | 53 | 0.061 | 69.52 | 11.28 | 13.73 | 55 | 0.029 | 88.37 | 11.13 | 15.46 | ||
| Normal-weight | 34 | 0.004 | 124.38 | 15.77 | 24.35 | 36 | 0.063 | 69.52 | 11.69 | 14.62 | 36 | 0.262 | 62.59 | 10.54 | 12.13 | |||
| Overweight/obese | 18 | 0.018 | 237.21 | 33.71 | 69.36 | 17 | 0.061 | 42.12 | 10.41 | 12.02 | 19 | 0.029 | 88.37 | 12.24 | 20.72 | |||
| miR-181a | Whole | 53 | 0.024 | 34.72 | 2.93 | 5.36 | 54 | 0.060 | 53.90 | 3.33 | 10.11 | 55 | 0.041 | 14.48 | 1.49 | 2.22 | ||
| Normal-weight | 34 | 0.024 | 10.14 | 1.99 | 2.14 | 36 | 0.060 | 7.97 | 1.61 | 1.94 | 36 | 0.067 | 7.64 | 1.38 | 1.41 | |||
| Overweight/obese | 19 | 0.115 | 34.72 | 4.63 | 8.36 | 18 | 0.068 | 53.90 | 6.78 | 17.09 | 19 | 0.041 | 14.48 | 1.69 | 3.29 | |||
| miR-222 | Whole | 51 | 0.017 | 31.63 | 2.99 a | 5.89 | 53 | 0.032 | 6.46 | 1.04 a,b | 1.14 | 54 | 0.024 | 9.75 | 1.20 b | 1.63 | T (p = 0.020) | |
| Normal-weight | 34 | 0.017 | 12.48 | 1.88 | 2.43 | 35 | 0.065 | 6.46 | 1.10 | 1.20 | 36 | 0.056 | 2.46 | 0.96 | 0.73 | T (p = 0.006) | ||
| Overweight/obese | 17 | 0.108 | 31.63 | 5.22 a | 9.40 | 18 | 0.032 | 4.09 | 0.94 ab | 1.05 | 18 | 0.024 | 9.75 | 1.70 b | 2.60 | T (p = 0.029) | ||
| miR-103 | Whole | 52 | 0.019 | 21.09 | 2.21 a | 3.81 | 54 | 0.036 | 7.05 | 0.90 b | 1.25 | 54 | 0.013 | 7.70 | 0.91 b | 1.28 | T (p = 0.019) | |
| Normal-weight | 34 | 0.019 | 11.80 | 1.60 | 2.27 | 36 | 0.036 | 7.05 | 1.05 | 1.47 | 36 | 0.053 | 4.52 | 0.85 | 0.91 | TxW (p = 0.022) | ||
| Overweight/obese | 18 | 0.105 | 21.09 | 3.36 a | 5.61 | 18 | 0.070 | 2.02 | 0.59 b | 0.52 | 18 | 0.013 | 7.70 | 1.03 b | 1.83 | T (p = 0.017) | ||
| miR-30a | Whole | 51 | 0.474 | 410.65 | 47.84 | 71.53 | 53 | 0.355 | 220.17 | 37.26 | 41.28 | 55 | 1.66 | 510.41 | 54.88 | 78.87 | ||
| Normal-weight | 33 | 0.474 | 112.54 | 34.48 a | 34.18 | 35 | 1.64 | 220.17 | 39.04 b | 44.04 | 36 | 2.85 | 252.94 | 52.31 b | 51.76 | TxW (p = 0.023) | T (p = 0.008) | |
| Overweight/obese | 18 | 1.70 | 410.65 | 72.33 | 108.94 | 18 | 0.355 | 128.04 | 33.81 | 36.27 | 19 | 1.66 | 510.41 | 59.75 | 115.82 | |||
| miR-27a | Whole | 52 | 0.004 | 4.68 | 0.40 | 0.81 | 54 | 0.008 | 1.84 | 0.18 | 0.27 | 55 | 0.008 | 4.09 | 0.23 | 0.56 | ||
| Normal-weight | 34 | 0.004 | 1.56 | 0.24 | 0.29 | 36 | 0.009 | 0.67 | 0.16 | 0.15 | 36 | 0.016 | 0.91 | 0.17 | 0.21 | T (p = 0.049) | ||
| Overweight/obese | 18 | 0.056 | 4.68 | 0.70 | 1.29 | 18 | 0.008 | 1.84 | 0.23 | 0.42 | 19 | 0.008 | 4.09 | 0.35 | 0.92 | |||
| miR-27b | Whole | 52 | 0.003 | 1.44 | 0.19 | 0.27 | 54 | 0.002 | 1.50 | 0.11 | 0.21 | 52 | 0.002 | 0.65 | 0.08 | 0.11 | ||
| Normal-weight | 34 | 0.003 | 0.89 | 0.15 | 0.18 | 36 | 0.002 | 0.57 | 0.09 | 0.11 | 35 | 0.003 | 0.40 | 0.08 | 0.08 | T (p = 0.022) | ||
| Overweight/obese | 18 | 0.007 | 1.44 | 0.26 | 0.39 | 18 | 0.004 | 1.50 | 0.14 | 0.34 | 17 | 0.002 | 0.65 | 0.09 | 0.16 | |||
| miR-200b | Whole | 52 | 0.041 | 78.38 | 9.06 a | 14.69 | 49 | 0.002 | 24.40 | 3.68 b | 4.48 | 53 | 0.066 | 40.98 | 5.87 ab | 7.71 | T (p = 0.026) | |
| Normal-weight | 34 | 0.088 | 36.53 | 6.87 | 8.35 | 32 | 0.007 | 24.40 | 4.33 | 5.17 | 34 | 0.138 | 23.98 | 5.46 | 5.12 | |||
| Overweight/obese | 18 | 0.041 | 78.38 | 13.20 | 22.01 | 17 | 0.002 | 8.00 | 2.46 | 2.46 | 19 | 0.066 | 40.98 | 6.60 | 11.07 | |||
| miR-let7a | Whole | 51 | 0.063 | 56.35 | 5.44 | 10.03 | 48 | 0.058 | 18.65 | 2.64 | 3.74 | 51 | 0.050 | 26.57 | 3.25 | 4.56 | ||
| Normal-weight | 34 | 0.063 | 29.79 | 3.85 | 5.78 | 31 | 0.058 | 18.65 | 3.06 | 4.33 | 33 | 0.050 | 8.91 | 2.50 | 2.01 | TxW (p = 0.045) | ||
| Overweight/obese | 17 | 0.200 | 56.35 | 8.62 a | 15.14 | 17 | 0.063 | 8.38 | 1.87 b | 2.24 | 18 | 0.079 | 26.57 | 4.62 ab | 7.11 | |||
| miR-17 | Whole | 52 | 0.047 | 34.02 | 4.88 a | 6.39 | 52 | 0.083 | 17.68 | 2.55 b | 3.31 | 55 | 0.069 | 17.34 | 2.95 b | 3.42 | T (p = 0.029) | |
| Normal-weight | 34 | 0.047 | 21.32 | 4.01 | 4.50 | 34 | 0.083 | 17.68 | 2.94 | 3.79 | 36 | 0.126 | 13.37 | 2.78 | 2.74 | TxW (p = 0.034) | ||
| Overweight/obese | 18 | 0.144 | 34.02 | 6.52 a | 8.89 | 18 | 0.180 | 6.48 | 1.80 b | 2.01 | 19 | 0.069 | 17.34 | 3.27 ab | 4.50 | T (p = 0.048) | ||
| miR-let7b | Whole | 52 | 0.157 | 244.25 | 26.50 | 41.50 | 54 | 0.316 | 85.16 | 14.79 | 17.44 | 55 | 0.584 | 89.15 | 16.86 | 19.25 | ||
| Normal-weight | 34 | 0.157 | 93.52 | 19.64 | 19.91 | 36 | 0.316 | 85.16 | 16.77 | 19.66 | 36 | 0.584 | 89.15 | 15.94 | 16.30 | |||
| Overweight/obese | 18 | 0.659 | 244.25 | 39.47 | 64.23 | 18 | 0.773 | 39.07 | 10.83 | 11.30 | 19 | 0.714 | 83.21 | 18.60 | 24.30 | |||
| miR-let7c | Whole | 51 | 0.035 | 23.88 | 2.88 a | 4.54 | 54 | 0.024 | 9.21 | 1.37 ab | 1.76 | 55 | 0.053 | 7.04 | 1.27 b | 1.48 | T (p = 0.014) | |
| Normal-weight | 33 | 0.035 | 9.31 | 2.10 | 1.97 | 36 | 0.024 | 6.70 | 1.41 | 1.62 | 36 | 0.053 | 5.85 | 1.19 | 1.12 | T (p = 0.016) | ||
| Overweight/obese | 18 | 0.079 | 23.88 | 4.32 a | 7.07 | 18 | 0.067 | 9.21 | 1.30 a,b | 2.07 | 19 | 0.057 | 7.04 | 1.44 b | 2.03 | |||
| miR-146b | Whole | 50 | 0.148 | 354.36 | 35.70 a | 65.49 | 48 | 0.427 | 62.18 | 12.02 b | 12.85 | 53 | 0.034 | 78.81 | 14.77 b | 17.66 | T (p = 0.008) | |
| Normal-weight | 32 | 0.148 | 103.03 | 24.10 a | 29.06 | 31 | 0.448 | 62.18 | 13.26 b | 13.15 | 34 | 0.034 | 44.90 | 13.32 b | 12.60 | T (p = 0.001) | T (p = 0.029) | |
| Overweight/obese | 18 | 0.890 | 354.36 | 56.31 a | 100.61 | 17 | 0.427 | 40.83 | 9.75 b | 12.33 | 19 | 0.090 | 78.81 | 17.37 b | 24.48 | T (p = 0.027) | ||
| Month 1 | Month 2 | Month 3 | Statistical Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | Overall | Normal/Obese Weight | ||
| Leptin (ng/mL) | Whole | 53 | 0.020 | 1.92 | 0.376 a | 0.353 | 55 | 0.003 | 1.18 | 0.341 b | 0.314 | 54 | 0.003 | 1.45 | 0.346 b | 0.333 | Normal/Obese weight | T (p = 0.010) |
| Normal-weight | 35 | 0.020 | 0.60 | 0.235 a | 0.153 | 36 | 0.003 | 0.98 | 0.242 b | 0.242 | 35 | 0.020 | 1.08 | 0.209 b | 0.195 | T (p = 0.030); W (p = 0.000) | ||
| Overweight/obese | 18 | 0.156 | 1.92 | 0.652 | 0.462 | 19 | 0.020 | 1.18 | 0.529 | 0.355 | 19 | 0.003 | 1.45 | 0.597 | 0.388 | |||
| Adiponectin (ng/mL) | Whole | 53 | 8.90 | 48.50 | 23.39 | 7.57 | 55 | 7.30 | 44.10 | 20.83 | 6.61 | 53 | 9.30 | 65.70 | 20.32 | 8.02 | T (p = 0.000) | |
| Normal-weight | 35 | 10.70 | 48.50 | 23.16 a | 7.97 | 36 | 7.30 | 44.10 | 19.19 b | 7.03 | 35 | 9.30 | 26.80 | 18.81 b | 4.62 | TxW (p = 0.035) | ||
| Overweight/obese | 18 | 8.90 | 34.00 | 23.03 | 6.94 | 19 | 10.00 | 31.50 | 22.37 | 5.58 | 18 | 13.30 | 65.70 | 23.38 | 11.80 | |||
| Adiponectin | miR-30a | miR-27a | miR-27b | miR-148a | miR-let7a | miR-let7b | miR-103 | miR-17 | miR-181a | miR-222 | miR-let7c | miR-146b | miR-200b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | Whole | r | 0.214 | −0.195 | −0.092 | −0.165 | −0.064 | −0.146 | −0.151 | −0.325 * | −0.198 | −0.172 | −0.162 | −0.110 | −0.263 | −0.184 |
| p | 0.117 | 0.161 | 0.508 | 0.233 | 0.647 | 0.322 | 0.275 | 0.016 | 0.160 | 0.214 | 0.246 | 0.428 | 0.071 | 0.206 | ||
| Normal-weight | r | 0.089 | −0.454 ** | −0.380 * | −0.410 * | −0.150 | −0.328 | −0.276 | −0.574 ** | −0.419 * | −0.470 ** | −0.471 ** | −0.261 | −0.473 ** | −0.297 | |
| p | 0.605 | 0.006 | 0.022 | 0.013 | 0.381 | 0.072 | 0.103 | 0.000 | 0.014 | 0.004 | 0.004 | 0.125 | 0.007 | 0.098 | ||
| Overweight/Obese | r | 0.136 | 0.159 | 0.352 | 0.180 | 0.112 | 0.170 | 0.248 | 0.114 | 0.301 | 0.267 | 0.386 | 0.214 | 0.139 | 0.055 | |
| p | 0.580 | 0.529 | 0.152 | 0.476 | 0.670 | 0.513 | 0.321 | 0.654 | 0.224 | 0.283 | 0.113 | 0.394 | 0.596 | 0.833 | ||
| Adiponectin | Whole | r | 1,000 | −0.345 * | −0.123 | −0.198 | −0.353 ** | −0.374 ** | −0.403 ** | −0.365 ** | −0.425 ** | −0.275 * | −0.310 * | −0.329* | −0.293 * | −0.248 |
| p | 0.011 | 0.374 | 0.152 | 0.010 | 0.009 | 0.003 | 0.007 | 0.002 | 0.044 | 0.024 | 0.015 | 0.043 | 0.086 | |||
| Normal-weight | r | 1,000 | −0.325 | −0.071 | −0.134 | −0.388 * | −0.467 ** | −0.384 * | −0.393* | −0.418 * | −0.204 | −0.299 | −0.319 | −0.240 | −0.207 | |
| p | 0.057 | 0.682 | 0.435 | 0.019 | 0.008 | 0.021 | 0.018 | 0.014 | 0.232 | 0.081 | 0.058 | 0.193 | 0.255 | |||
| Overweight/Obese | r | 1,000 | −0.209 | −0.184 | −0.291 | −0.222 | −0.006 | −0.151 | −0.180 | −0.250 | −0.164 | −0.163 | −0.313 | −0.085 | −0.098 | |
| p | 0.406 | 0.465 | 0.241 | 0.392 | 0.981 | 0.550 | 0.476 | 0.317 | 0.515 | 0.518 | 0.206 | 0.747 | 0.708 | |||
| BMI24 | Whole | r | 0.125 | −0.246 | −0.167 | −0.218 | −0.252 | −0.326 * | −0.278 | −0.425 ** | −0.298 * | −0.238 | −0.298 * | −0.331 * | −0.287 | −0.347 * |
| p | 0.380 | 0.085 | 0.245 | 0.129 | 0.081 | 0.031 | 0.051 | 0.002 | 0.040 | 0.096 | 0.037 | 0.019 | 0.059 | 0.020 | ||
| Normal-weight | r | 0.199 | −0.302 | −0.196 | −0.276 | −0.207 | −0.296 | −0.313 | −0.521 ** | −0.427 * | −0.347 * | −0.385 * | −0.360 * | −0.422 * | −0.327 | |
| p | 0.260 | 0.082 | 0.267 | 0.114 | 0.239 | 0.120 | 0.071 | 0.002 | 0.015 | 0.044 | 0.027 | 0.036 | 0.023 | 0.078 | ||
| Overweight/Obese | r | −0.124 | −0.224 | −0.179 | −0.071 | −0.377 | −0.379 | −0.285 | −0.265 | −0.171 | −0.106 | −0.138 | −0.276 | −0.218 | −0.318 | |
| p | 0.636 | 0.405 | 0.506 | 0.795 | 0.166 | 0.164 | 0.284 | 0.322 | 0.528 | 0.696 | 0.610 | 0.300 | 0.435 | 0.248 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. https://doi.org/10.3390/nu11112589
Zamanillo R, Sánchez J, Serra F, Palou A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients. 2019; 11(11):2589. https://doi.org/10.3390/nu11112589
Chicago/Turabian StyleZamanillo, Rocío, Juana Sánchez, Francisca Serra, and Andreu Palou. 2019. "Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI" Nutrients 11, no. 11: 2589. https://doi.org/10.3390/nu11112589
APA StyleZamanillo, R., Sánchez, J., Serra, F., & Palou, A. (2019). Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients, 11(11), 2589. https://doi.org/10.3390/nu11112589