Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
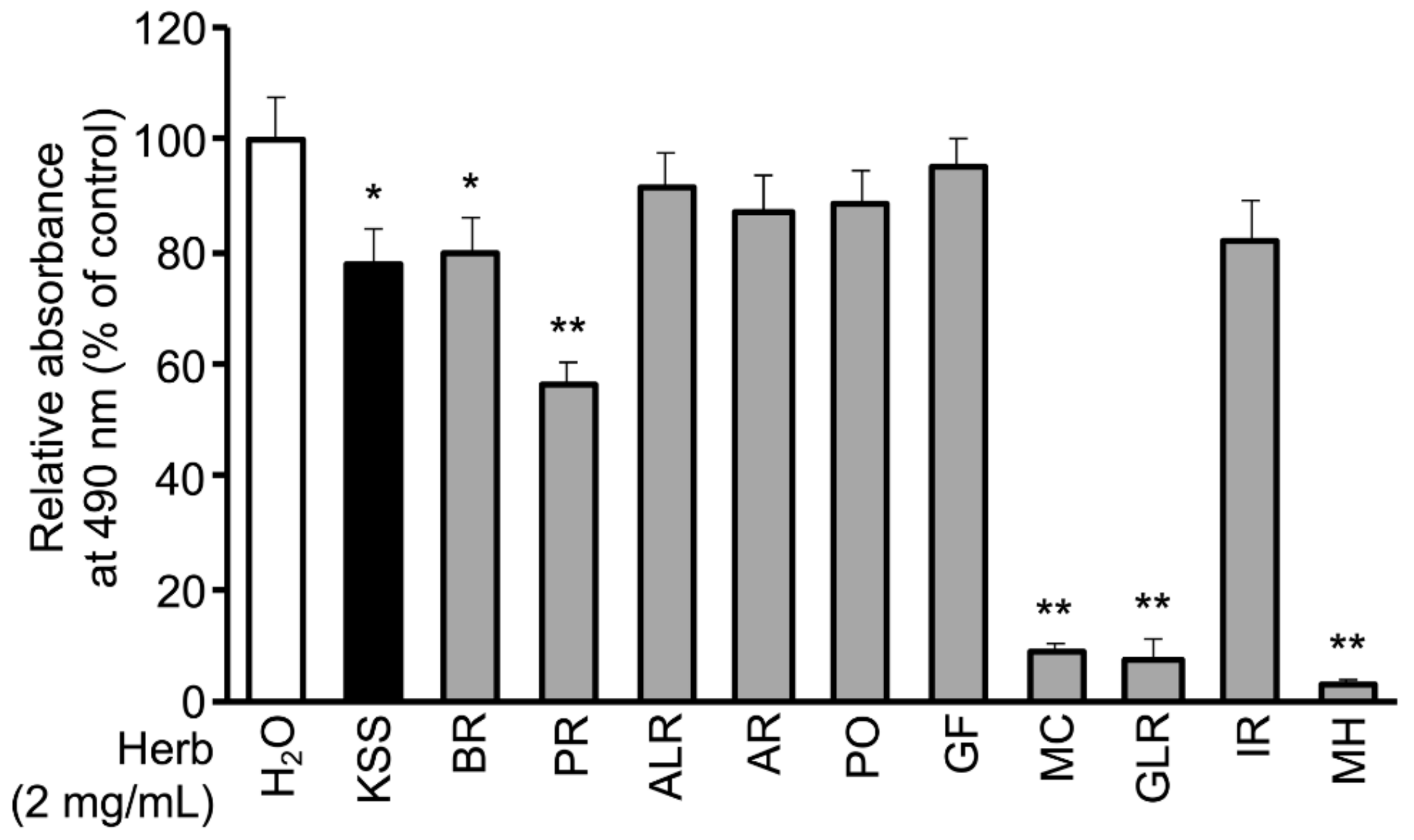
2.2. Preparation of Kampo Medicine and Composition of the Ten Herbs and Eight Major Components of KSS
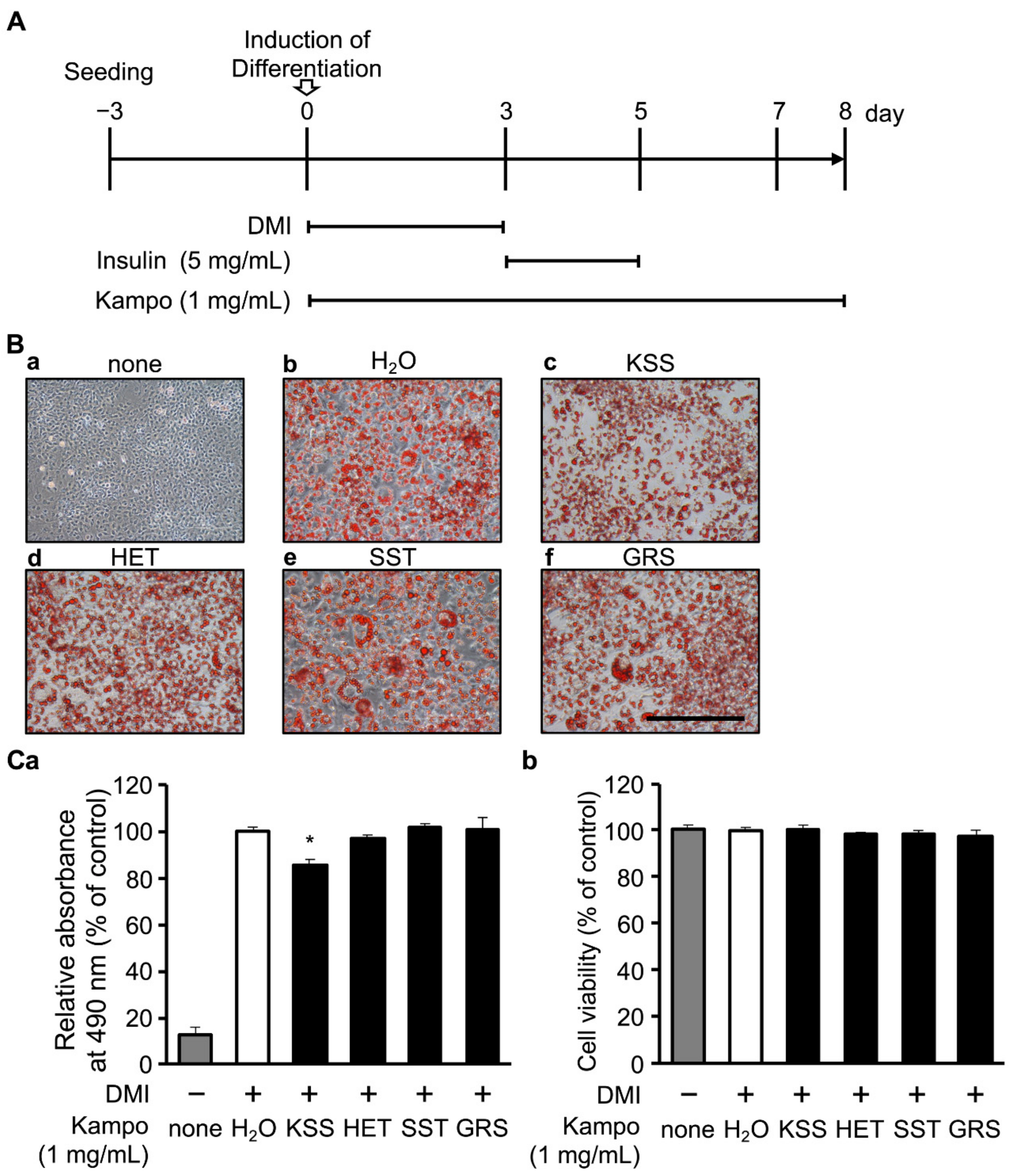
2.3. Evaluation of Intracellular Lipid Accumulation
2.4. Assessment of Cell Viability
2.5. Quantitative PCR (qPCR)
2.6. Luciferase Reporter Assay
2.7. Statistical Analysis
3. Results
3.1. KSS Reduces Intracellular Lipid Accumulation
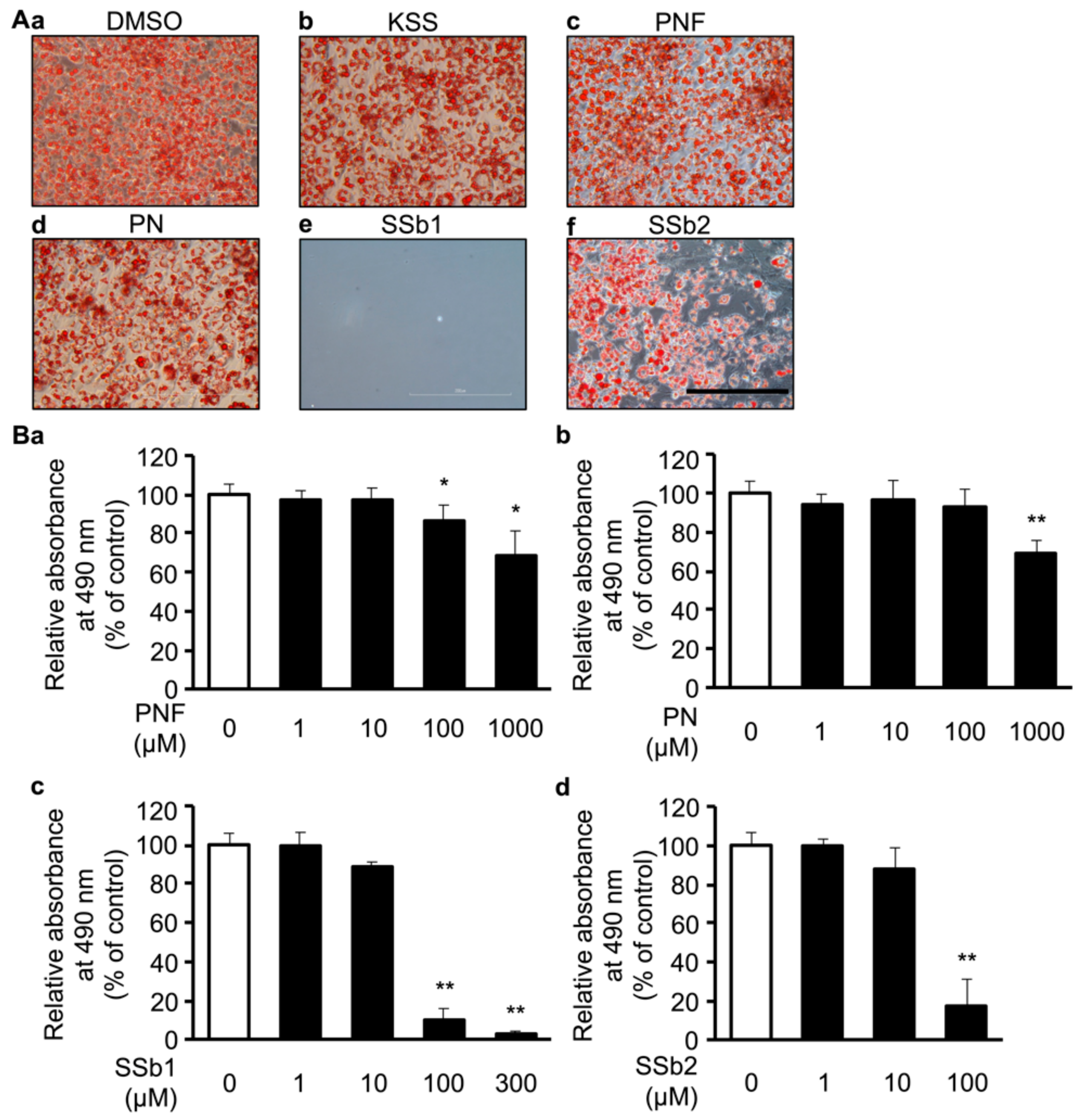
3.2. MC, Paeoniflorin, and Paeonol Inhibit Differentiation of 3T3-L1 Cells into Mature Adipocytes
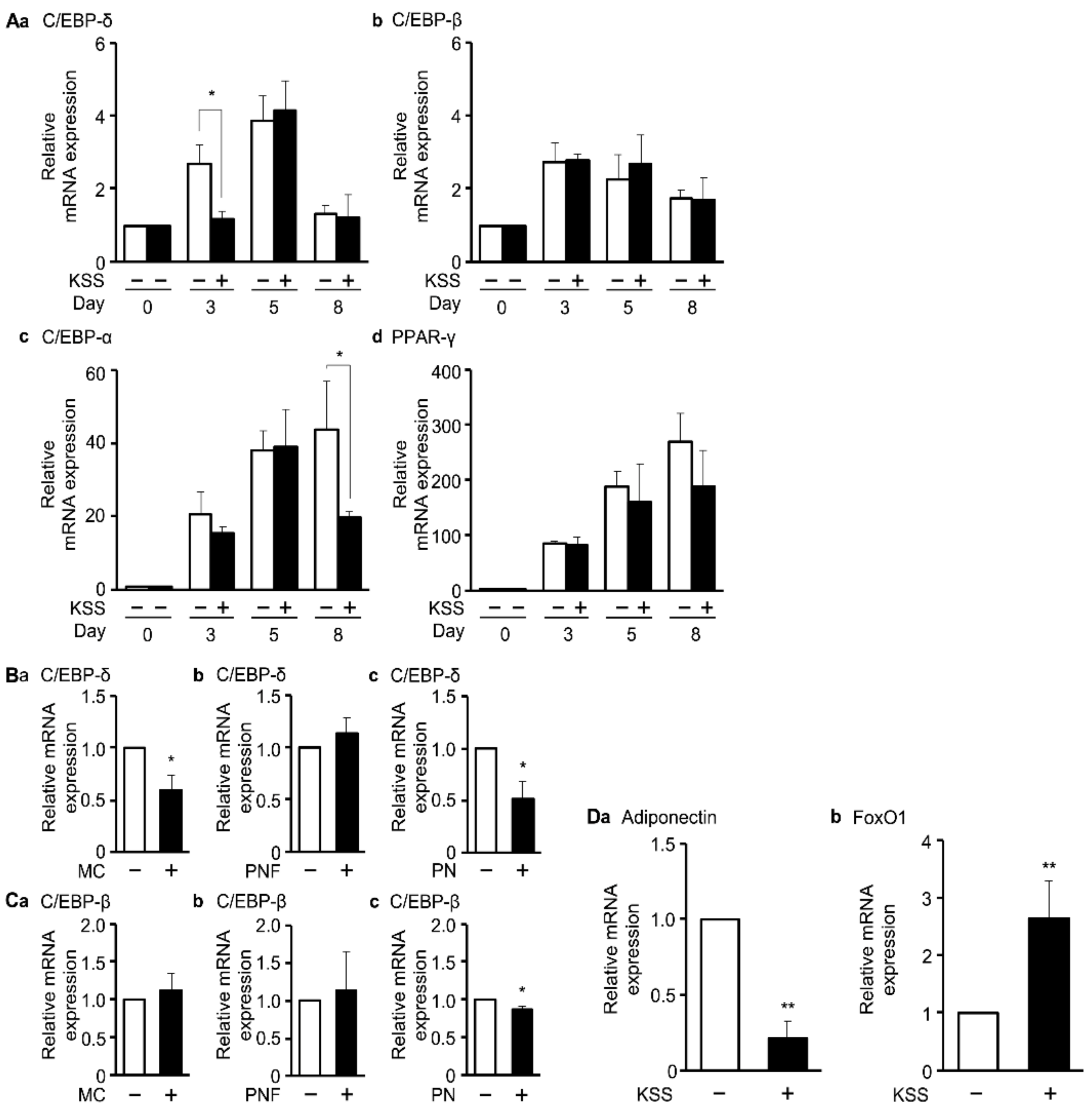
3.3. KSS Inhibits Early Differentiation into Mature Adipocytes by Inhibiting the Action of Dex
3.4. KSS, MC, and Paeonol Alter the Expression of Adipocyte Differentiation Marker Genes
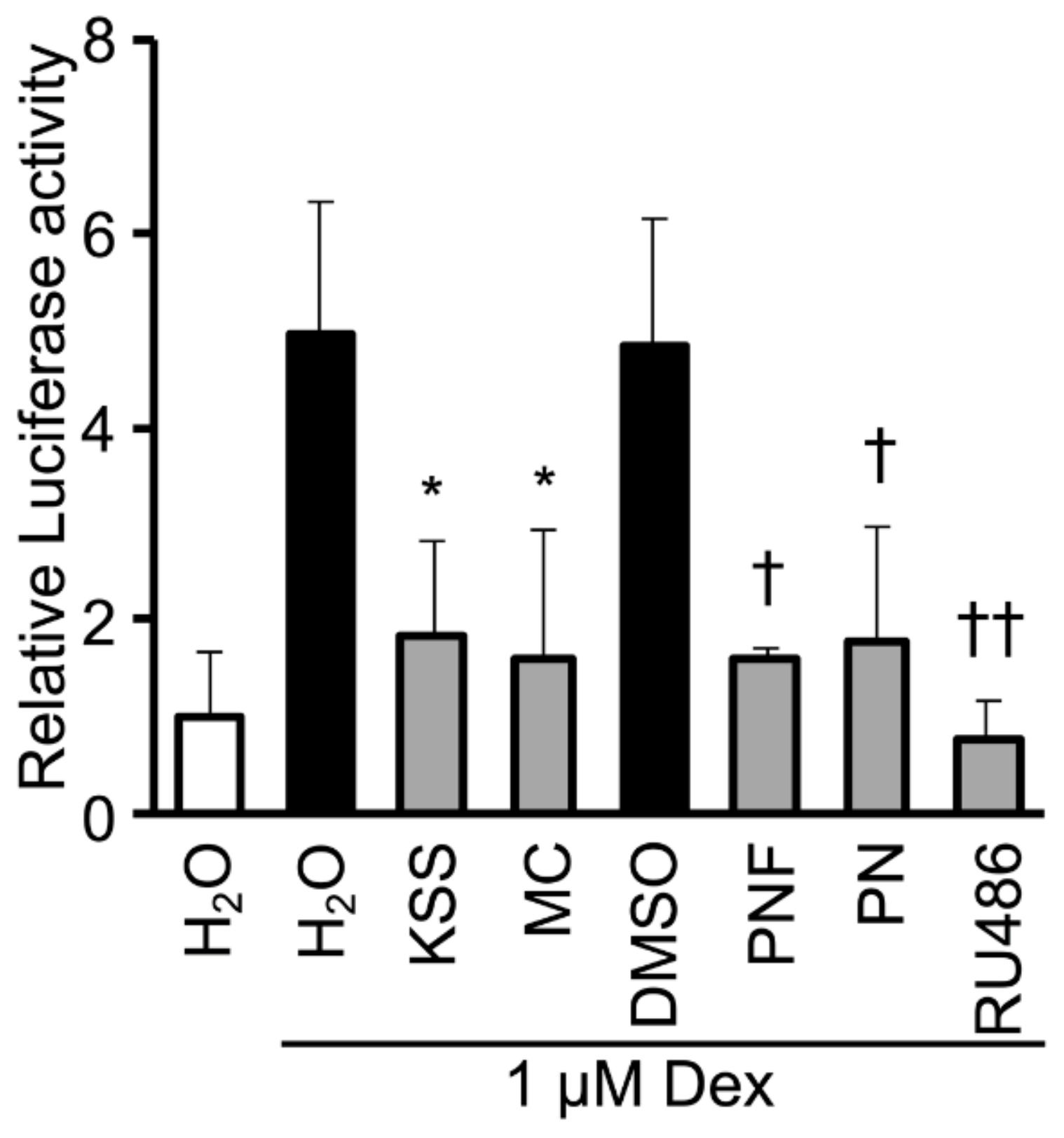
3.5. KSS, MC, Paeoniflorin, and Paeonol Inhibit Promoter Activity of GR
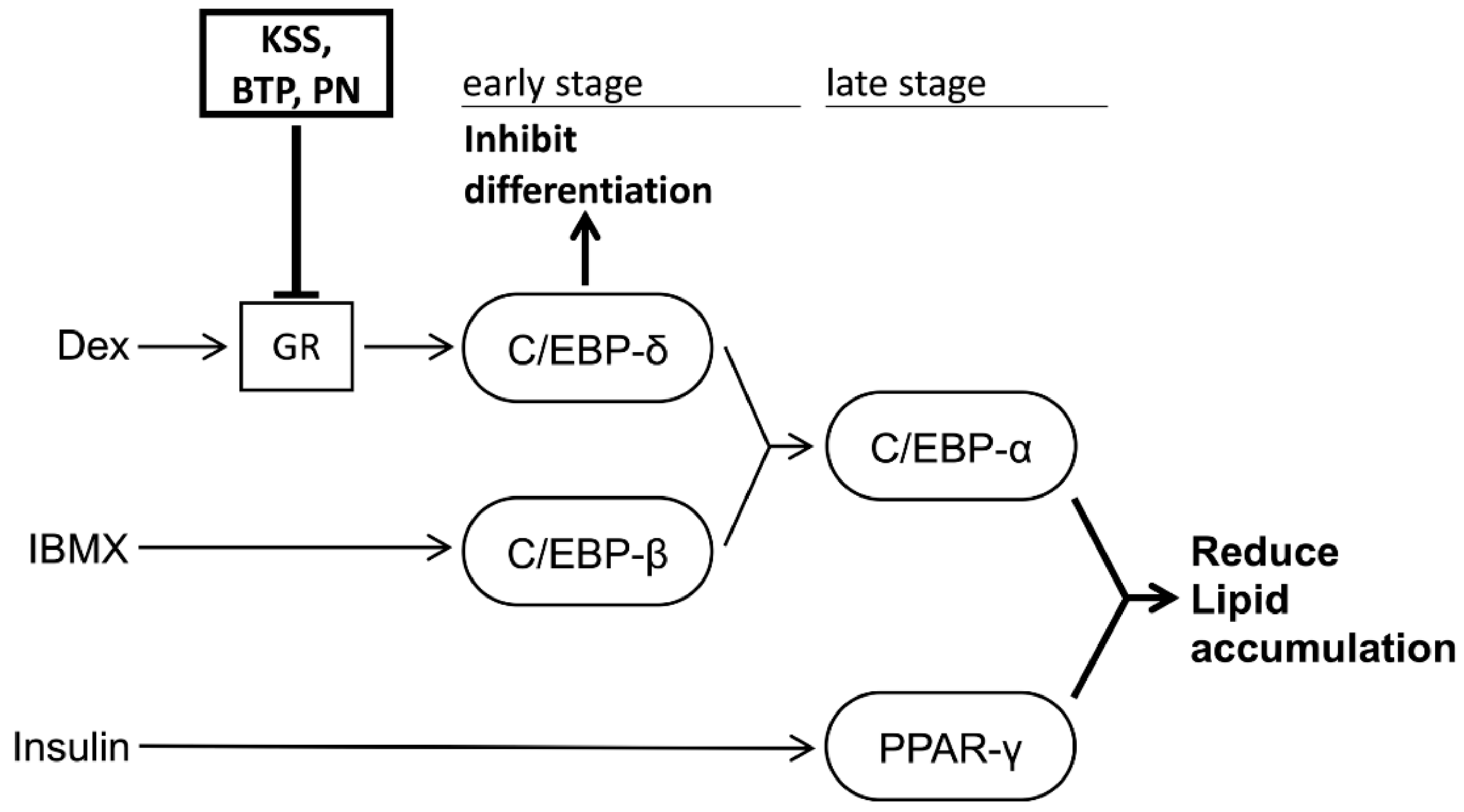
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haslam, D.; Sattar, N.; Lean, M. ABC of obesity: Obesity—Time to wake up. BMJ 2006, 333, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kareholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Despres, J.P.; Koh, K.K. Obesity and cardiovascular disease: Friend or foe? Eur. Heart J. 2016, 37, 3560–3568. [Google Scholar] [CrossRef] [PubMed]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, H.; Matsui, H.; Ueno, M.; Maeno, T.; Iso, T.; Syamsunarno, M.R.; Anjo, S.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; et al. Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nat. Commun. 2013, 4, 2563. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, R.; Wakil, S.J. Increased synthesis and accumulation of phospholipids during differentiation of 3T3-L1 cells into adipocytes. J. Biol. Chem. 1983, 258, 3559–3564. [Google Scholar]
- Rubin, C.S.; Hirsch, A.; Fung, C.; Rosen, O.M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem. 1978, 253, 7570–7578. [Google Scholar] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Madsen, M.S.; Siersbaek, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome proliferator-activated receptor gamma and C/EBPalpha synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef]
- Inokawa, A.; Inuzuka, T.; Takahara, T.; Shibata, H.; Maki, M. Tubby-like protein superfamily member PLSCR3 functions as a negative regulator of adipogenesis in mouse 3T3-L1 preadipocytes by suppressing induction of late differentiation stage transcription factors. Biosci. Rep. 2015, 36, e00287. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Chen, Y.; Zheng, X.; Li, Y.; Zhang, Q.; Mo, D.; Yang, G. MicroRNA-139-5p Suppresses 3T3-L1 Preadipocyte Differentiation Through Notch and IRS1/PI3K/Akt Insulin Signaling Pathways. J. Cell. Biochem. 2015, 116, 1195–1204. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H.; Arden, K.C.; Accili, M. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
- Iwasaki, K.; Satoh-Nakagawa, T.; Maruyama, M.; Monma, Y.; Nemoto, M.; Tomita, N.; Tanji, H.; Fujiwara, H.; Seki, T.; Fujii, M.; et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J. Clin. Psychiatry 2005, 66, 248–252. [Google Scholar] [CrossRef]
- Takayama, S.; Arita, R.; Kikuchi, A.; Ohsawa, M.; Kaneko, S.; Ishii, T. Clinical Practice Guidelines and Evidence for the Efficacy of Traditional Japanese Herbal Medicine (Kampo) in Treating Geriatric Patients. Front. Nutr. 2018, 5, 66. [Google Scholar] [CrossRef]
- Yamaguchi, K. Traditional Japanese herbal medicines for treatment of odontopathy. Front. Pharmacol. 2015, 6, 176. [Google Scholar] [CrossRef]
- Huang, K.C.; Yen, H.R.; Chiang, J.H.; Su, Y.C.; Sun, M.F.; Chang, H.H.; Huang, S.T. Chinese Herbal Medicine as an Adjunctive Therapy Ameliorated the Incidence of Chronic Hepatitis in Patients with Breast Cancer: A Nationwide Population-Based Cohort Study. Evid. Based Complement. Altern. Med. 2017, 2017, 1052976. [Google Scholar] [CrossRef]
- Herva, A.; Laitinen, J.; Miettunen, J.; Veijola, J.; Karvonen, J.T.; Laksy, K.; Joukamaa, M. Obesity and depression: Results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int. J. Obes. (Lond.) 2006, 30, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Cochran, C.J.; Gallicchio, L.; Miller, S.R.; Flaws, J.A.; Zacur, H. Serum leptin levels, hormone levels, and hot flashes in midlife women. Fertil. Steril. 2010, 94, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Jones, M.; Mishra, G.D. A U-Shaped Relationship between Body Mass Index and Dysmenorrhea: A Longitudinal Study. PLoS ONE 2015, 10, e0134187. [Google Scholar] [CrossRef] [PubMed]
- Thanakun, S.; Pornprasertsuk-Damrongsri, S.; Izumi, Y. Increased oral inflammation, leukocytes, and leptin, and lower adiponectin in overweight or obesity. Oral Dis. 2017, 23, 956–965. [Google Scholar] [CrossRef]
- Go, H.; Ryuk, J.A.; Hwang, J.T.; Ko, B.S. Effects of three different formulae of Gamisoyosan on lipid accumulation induced by oleic acid in HepG2 cells. Integr. Med. Res. 2017, 6, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Tajima, M.; Suzuki, K.; Toda, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Inhibition of preadipocyte differentiation and lipid accumulation by Orengedokuto treatment of 3T3-L1 cultures. Phytother. Res. 2012, 26, 91–100. [Google Scholar] [CrossRef]
- Izumi, M.; Seki, T.; Iwasaki, K.; Sakamoto, K. Chinese herbal medicine Yi-Gan-San decreases the lipid accumulation in mouse 3T3-L1 adipocytes by modulating the activities of transcription factors SREBP-1c and FoxO1. Tohoku J. Exp. Med. 2009, 219, 53–62. [Google Scholar] [CrossRef][Green Version]
- Kwak, D.H.; Lee, J.H.; Kim, D.G.; Kim, T.; Lee, K.J.; Ma, J.Y. Inhibitory Effects of Hwangryunhaedok-Tang in 3T3-L1 Adipogenesis by Regulation of Raf/MEK1/ERK1/2 Pathway and PDK1/Akt Phosphorylation. Evid. Based Complement. Altern. Med. 2013, 2013, 413906. [Google Scholar] [CrossRef]
- Xu, F.; Xiao, H.; Liu, R.; Yang, Y.; Zhang, M.; Chen, L.; Chen, Z.; Liu, P.; Huang, H. Paeonol Ameliorates Glucose and Lipid Metabolism in Experimental Diabetes by Activating Akt. Front. Pharmacol. 2019, 10, 261. [Google Scholar] [CrossRef]
- Kim, H.; Hiraishi, A.; Tsuchiya, K.; Sakamoto, K. (-) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology 2010, 62, 245–255. [Google Scholar] [CrossRef][Green Version]
- Kopinke, D.; Roberson, E.C.; Reiter, J.F. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell 2017, 170, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vale, M.I.; Peres, S.B.; Vernochet, C.; Farmer, S.R.; Lima, F.B. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta transcriptional activity. J. Pineal Res. 2009, 47, 221–227. [Google Scholar] [CrossRef]
- Renault, V.M.; Thekkat, P.U.; Hoang, K.L.; White, J.L.; Brady, C.A.; Kenzelmann Broz, D.; Venturelli, O.S.; Johnson, T.M.; Oskoui, P.R.; Xuan, Z.; et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene 2011, 30, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nishiyama, T.; Takizawa, I.; Miyashiro, Y.; Hara, N.; Tomita, Y. A carbon 21 steroidal metabolite from progestin, 20beta-hydroxy-5alpha-dihydroprogesterone, stimulates the androgen receptor in prostate cancer cells. Prostate 2018, 78, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Yamada, M.; Uemura, H.; Ueno, S.; Numata, S.; Ohmori, T.; Tsuchiya, N.; Noguchi, M.; Yuzurihara, M.; Kase, Y.; et al. Changes in circulating cytokine levels in midlife women with psychological symptoms with selective serotonin reuptake inhibitor and Japanese traditional medicine. Maturitas 2009, 62, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.C.; Makino, I.; Aono, S.; Yasui, H.; Isai, H.; Nishihara, M.; Hatakeyama, N.; Kawai, T.; Ikemoto, T.; Inoue, S.; et al. Effects of Kamishoyosan, a Traditional Japanese Kampo Medicine, on Pain Conditions in Patients with Intractable Persistent Dentoalveolar Pain Disorder. Evid. Based Complement. Altern. Med. 2015, 2015, 750345. [Google Scholar] [CrossRef]
- Nagai, T.; Arai, Y.; Emori, M.; Nunome, S.Y.; Yabe, T.; Takeda, T.; Yamada, H. Anti-allergic activity of a Kampo (Japanese herbal) medicine “Sho-seiryu-to (Xiao-Qing-Long-Tang)” on airway inflammation in a mouse model. Int. Immunopharmacol. 2004, 4, 1353–1365. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Cornelius, P.; Lin, F.T.; Chen, S.S.; Lane, M.D. Glucocorticoids reciprocally regulate expression of the CCAAT/enhancer-binding protein alpha and delta genes in 3T3-L1 adipocytes and white adipose tissue. J. Biol. Chem. 1994, 269, 19041–19047. [Google Scholar]
- Chivers, J.E.; Cambridge, L.M.; Catley, M.C.; Mak, J.C.; Donnelly, L.E.; Barnes, P.J.; Newton, R. Differential effects of RU486 reveal distinct mechanisms for glucocorticoid repression of prostaglandin E release. Eur. J. Biochem. 2004, 271, 4042–4052. [Google Scholar] [CrossRef]
- Park, Y.K.; Ge, K. Glucocorticoid Receptor Accelerates, but Is Dispensable for, Adipogenesis. Mol. Cell. Biol. 2017, 37, e00260-16. [Google Scholar] [CrossRef]
- Kitagawa, H.; Munekage, M.; Ichikawa, K.; Fukudome, I.; Munekage, E.; Takezaki, Y.; Matsumoto, T.; Igarashi, Y.; Hanyu, H.; Hanazaki, K. Pharmacokinetics of Active Components of Yokukansan, a Traditional Japanese Herbal Medicine after a Single Oral Administration to Healthy Japanese Volunteers: A Cross-Over, Randomized Study. PLoS ONE 2015, 10, e0131165. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, H.; Chen, X.; Hu, Z. Determination of paeonol in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies following oral administration of Moutan cortex decoction. Biomed. Chromatogr. 2003, 17, 504–508. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sense | Antisense | Ref. |
|---|---|---|---|
| C/EBP-α | 5′-CAAGAACAGCAACGAGTACC-3′ | 5′-GTCACTGGTCAACTCCAGCAC-3′ | [31] |
| C/EBP-β | 5′-ACGACTTCCTCTCCGACCTC-3′ | 5′-CGAGGCTCACGTAACCGTAG-3′ | |
| C/EBP-δ | 5′-CTGCCATGTACGACGACGAGAG-3′ | 5′-GCTTTGTGGTTGCTGTTGAAGA-3′ | |
| PPAR-γ | 5′-CTGATGCACTGCCTATGAGC-3′ | 5′-TCACGGAGAGGTCCACAGAG-3′ | |
| Adiponectin | 5′-GCACTGGCAAGTTCTACTGCAA-3′ | 5′-GTAGGTGAAGAGAACGGCCTTGT-3′ | [32] |
| FoxO1 | 5′-ACGAGTGGATGGTGAAGAGC-3′ | 5′-TGCTGTGAAGGGACAGATTG-3′ | [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, M.; Yoshida, T.; Nakamura, T.; Wakamori, M. Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells. Nutrients 2020, 12, 309. https://doi.org/10.3390/nu12020309
Izumi M, Yoshida T, Nakamura T, Wakamori M. Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells. Nutrients. 2020; 12(2):309. https://doi.org/10.3390/nu12020309
Chicago/Turabian StyleIzumi, Masayuki, Takashi Yoshida, Takashi Nakamura, and Minoru Wakamori. 2020. "Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells" Nutrients 12, no. 2: 309. https://doi.org/10.3390/nu12020309
APA StyleIzumi, M., Yoshida, T., Nakamura, T., & Wakamori, M. (2020). Paeonol, an Ingredient of Kamishoyosan, Reduces Intracellular Lipid Accumulation by Inhibiting Glucocorticoid Receptor Activity in 3T3-L1 Cells. Nutrients, 12(2), 309. https://doi.org/10.3390/nu12020309





