Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Preparation
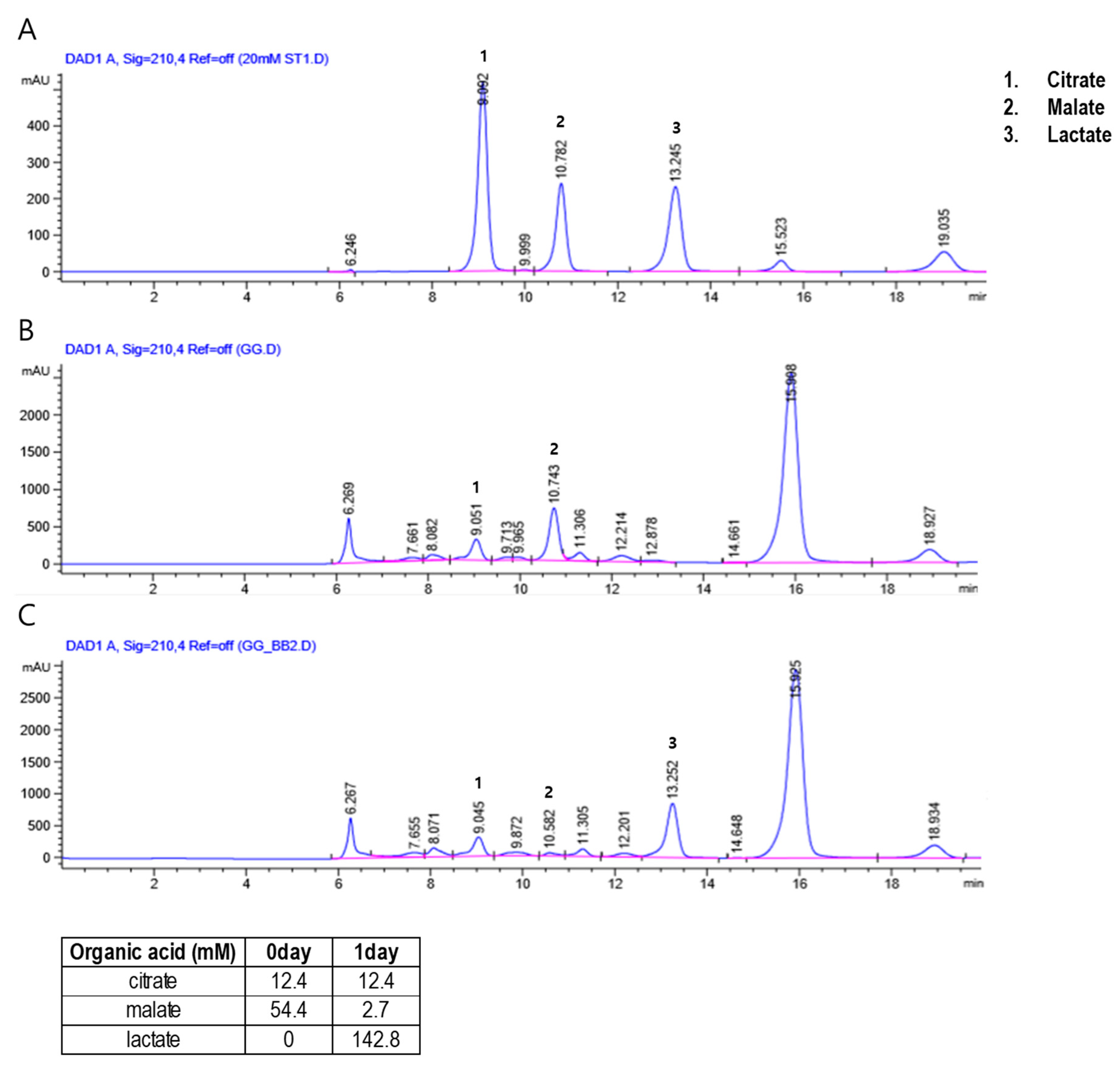
2.2. High-Performance Liquid Chromatography (HPLC)-Based Analysis of PR and fPR
2.3. Animals and Treatment
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Immunoblotting
2.6. H&E Staining
2.7. Oral Glucose Tolerance Test (OGTT)
2.8. Sequencing of 16S rRNA Gene Amplicon of Fecal Microbiota
2.9. Sequence Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Analysis of fPR Preparation
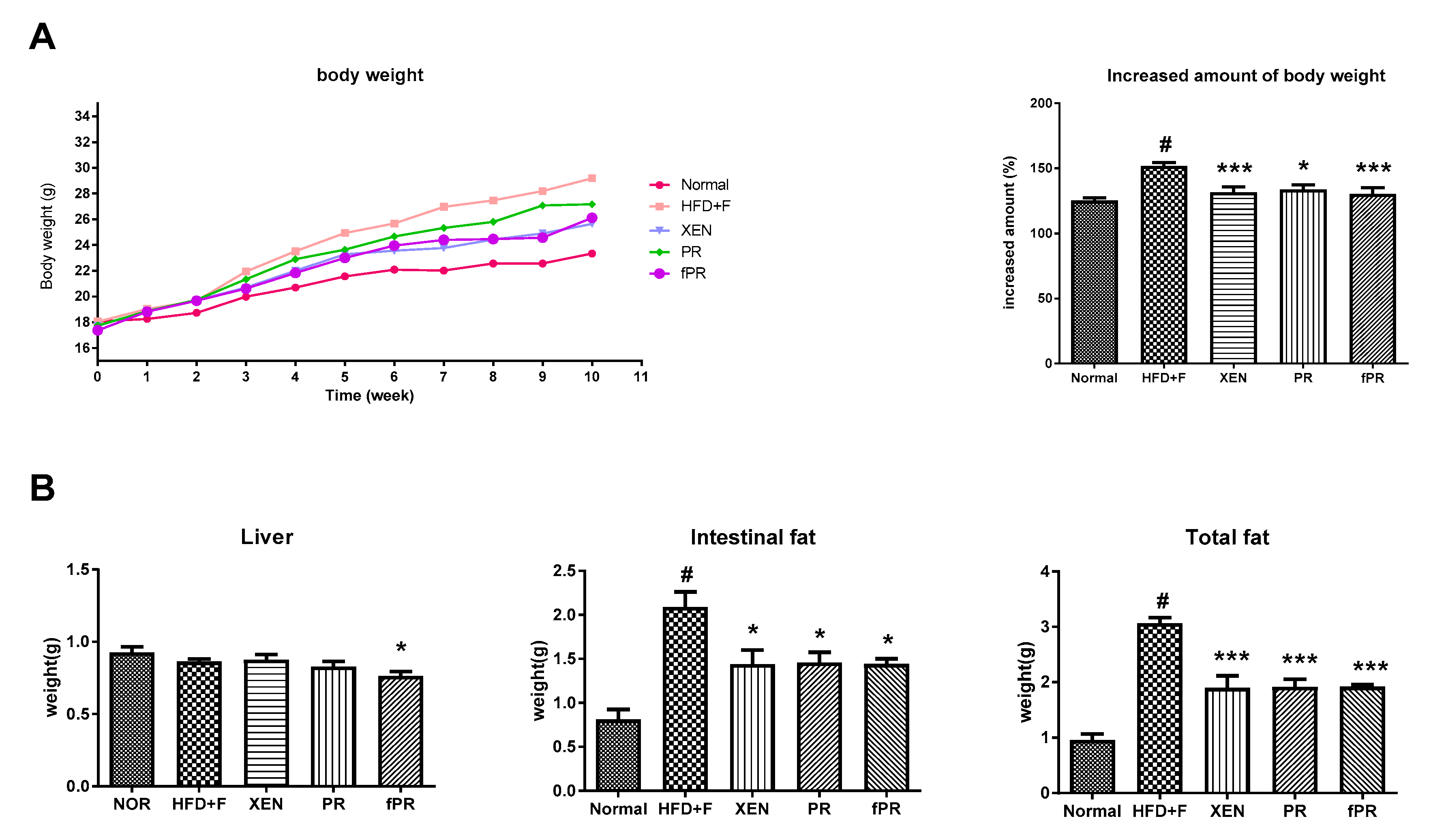
3.2. Effects of PR and fPR on the Body Weight, Liver Weight, and Intestinal and Total Fat Weights in the HFD+F Group
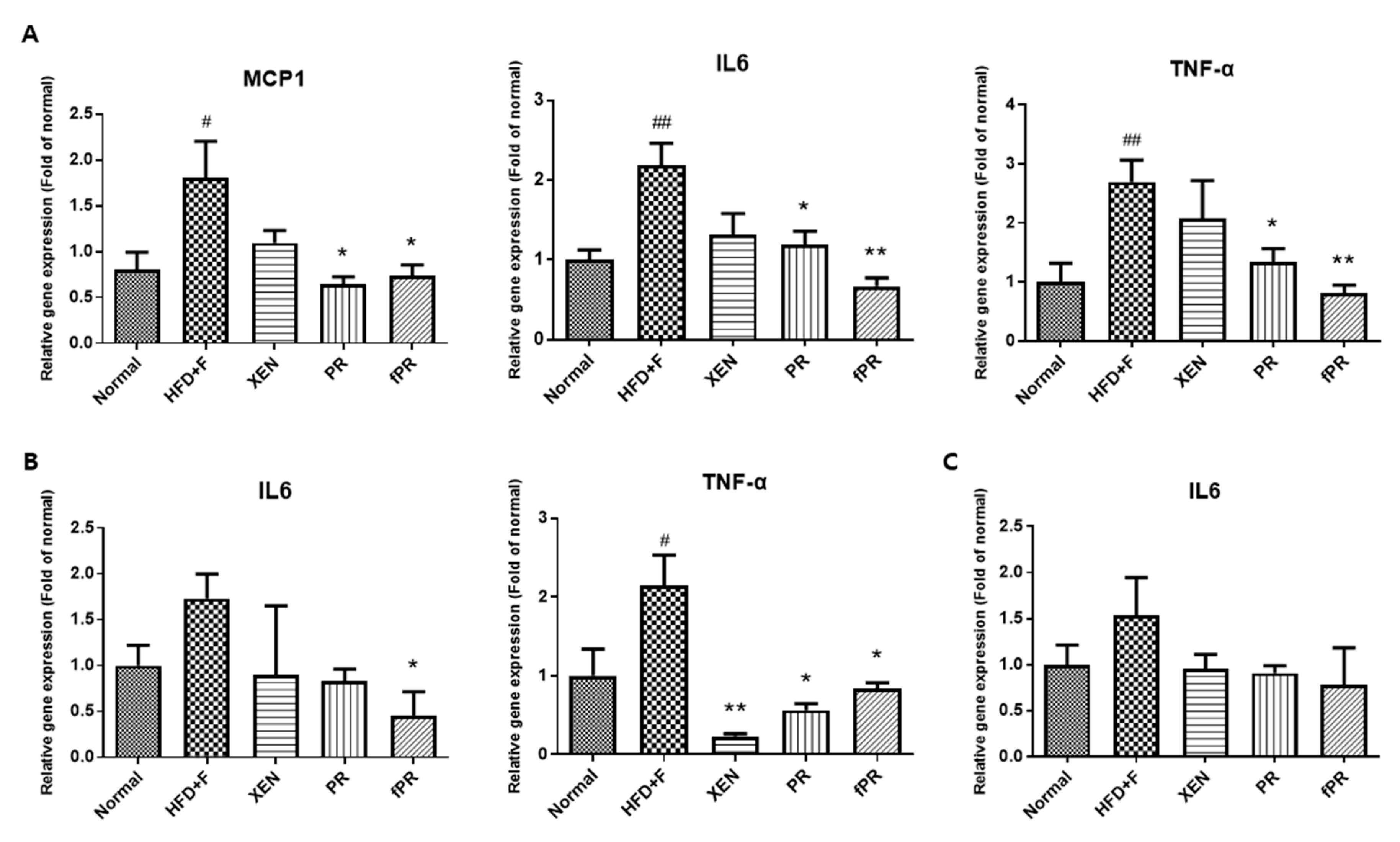
3.3. Anti-Inflammatory Effects of PR and fPR on the Liver, Intestine, and Intestinal Fat Tissue in HFD+F-Fed Animals
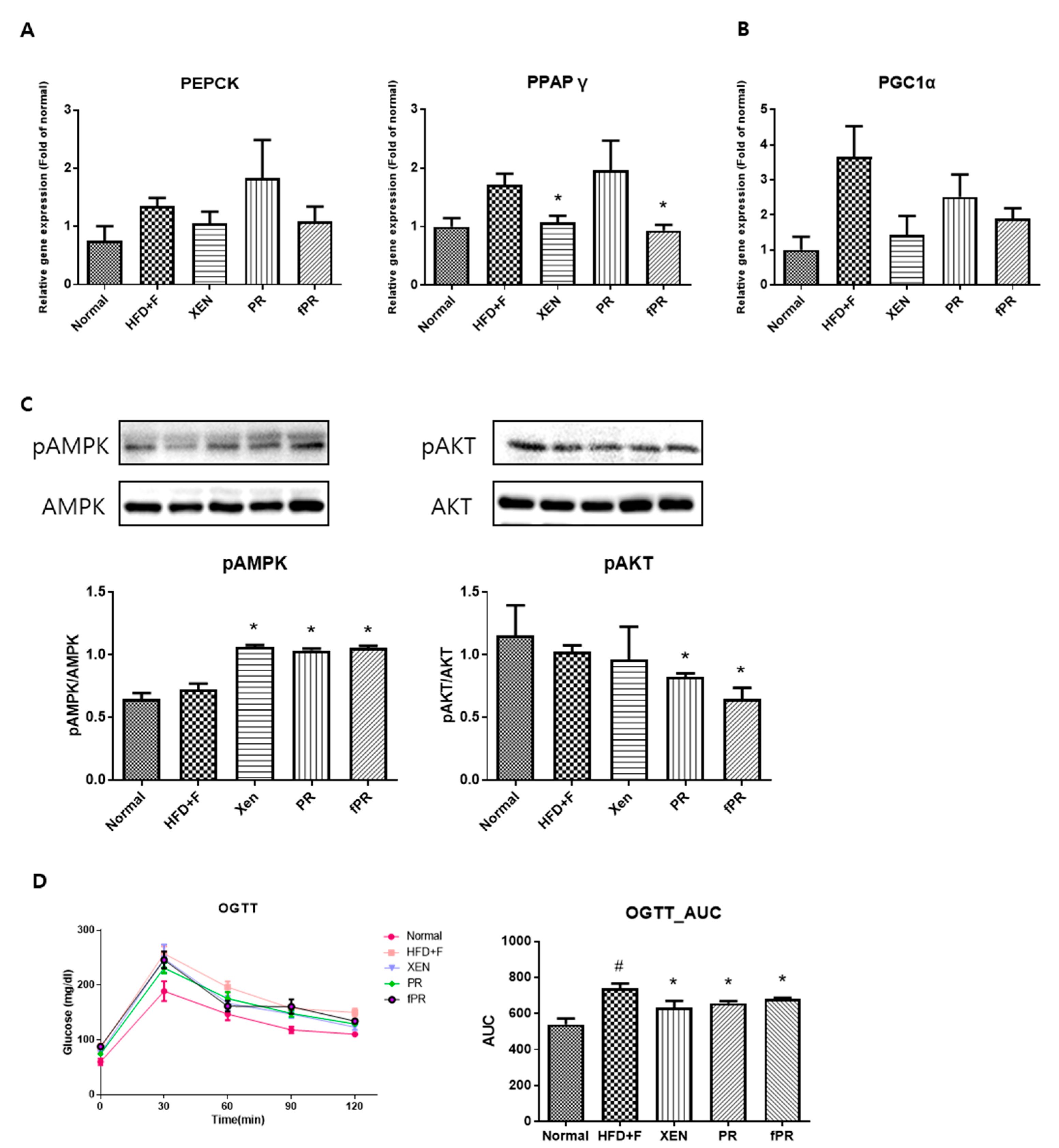
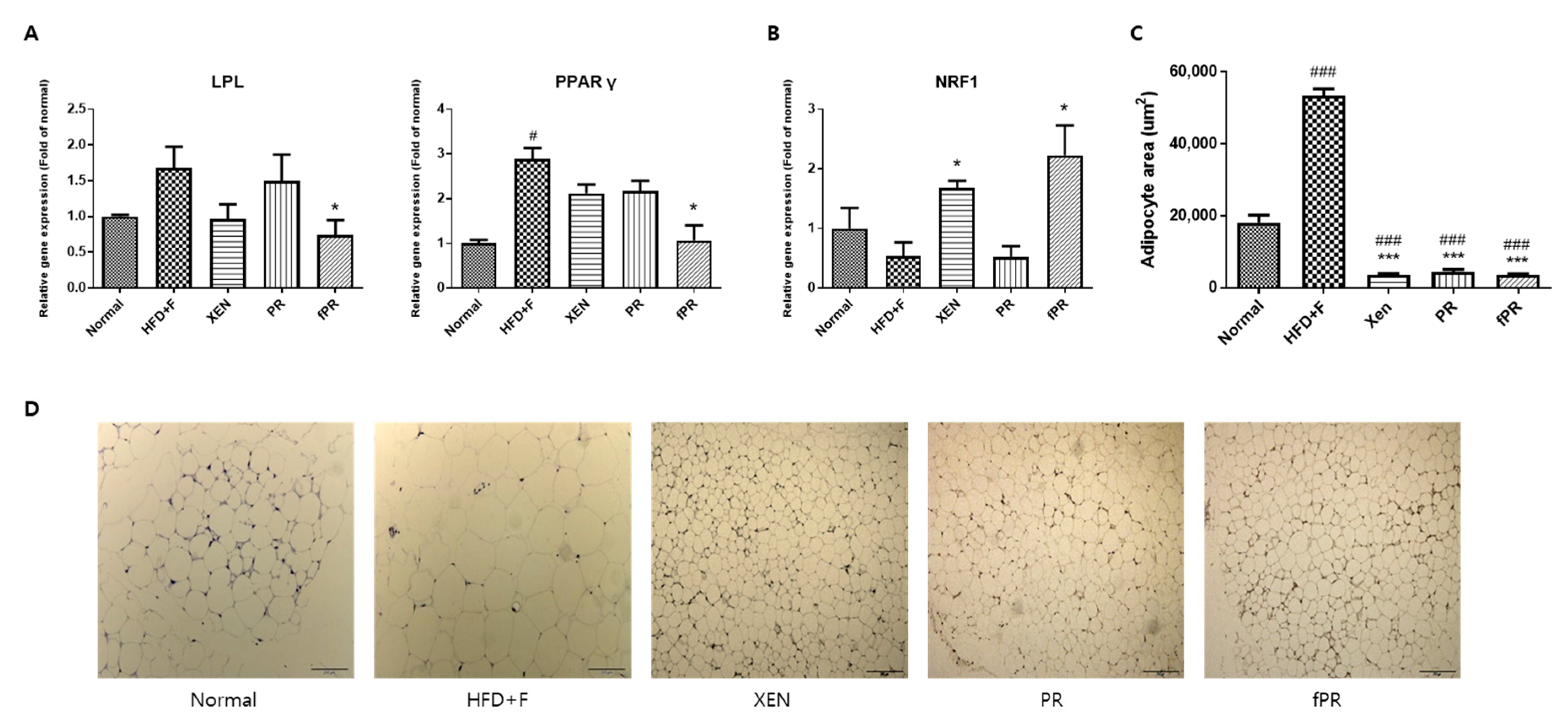
3.4. Effects of PR and fPR on Adipocyte Size and Pathways Related to Glucose and Lipid Metabolism in the HFD+F Group
3.5. Effects of PR and fPR on the Gut Microbial Communities of HFD-Fed Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PR | Puerariae Radix |
| fPR | Fermented Puerariae Radix |
| HFD | High-fat diet |
| XEN | Orlistat |
| MCP1 | Monocyte chemoattractant protein 1 |
| IL-6 | Interleukin 6 |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| TNFα | tumor necrosis factor-α |
| PPAR γ | Peroxisome proliferator-activated receptor gamma |
| AKT | Protein kinase B |
| OGTT | Oral glucose tolerance test |
| B.breve | Bifidobacterium breve |
| GAPDH | Glyceraldehyde-3-phosphatase dehydrogenase |
| AUC | Area under the curve |
| PCoA | principal coordinated analysis |
| LEfSe | linear discriminant analysis effect size |
References
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Holguin, F. Metabolic Dysregulation, Systemic Inflammation, and Pediatric Obesity-related Asthma. Ann. Am. Thorac. Soc. 2017, 14, S363–S367. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Yan, G.-L.; Wu, F.-F.; Wang, X.-J. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018, 8, 42380–42389. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Baek, J.-H.; Kim, N.-J.; Song, J.-K.; Chun, K.-H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566. [Google Scholar] [CrossRef]
- Prasain, J.K.; Peng, N.; Rajbhandari, R.; Wyss, J.M. The Chinese Pueraria root extract (Pueraria lobata) ameliorates impaired glucose and lipid metabolism in obese mice. Phytomedicine 2012, 20, 17–23. [Google Scholar] [CrossRef]
- Zheng, G.; Lin, L.; Zhong, S.; Zhang, Q.; Li, D. Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS ONE 2015, 10, e0122925. [Google Scholar] [CrossRef]
- Song, M.-Y. The effects of cinnamomum cassia blume, aconitum carmichaeli debx, and pueraria lobata benth on glucose and energy metabolism in C2C12 myotubes. J. Korean Med. Obes. Res. 2015, 15, 131–136. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, N.; Zhang, Y.; Xu, X. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr. J. Microbiol. Res. 2013, 7, 1644–1650. [Google Scholar]
- Bose, S.; Jeon, S.; Eom, T.; Song, M.Y.; Kim, H. Evaluation of the in vitro and in vivo protective effects of unfermented and fermented Rhizoma coptidis formulations against lipopolysaccharide insult. Food Chem. 2012, 135, 452–459. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gomez-Valades, A.G.; Matamoros, S.; Ramirez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Bose, S.; Song, M.Y.; Nam, J.K.; Lee, M.J.; Kim, H. In vitro and in vivo protective effects of fermented preparations of dietary herbs against lipopolysaccharide insult. Food Chem. 2012, 134, 758–765. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Kim, G.-C.; Hong, S.-U.; Kim, J.-H.; Kim, J.-E.; Kim, H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Ray, M.; Hor, P.; Ojha, D.; Soren, J.; Singh, S.; Mondal, K. Bifidobacteria and its rice fermented products on diet induced obese mice: Analysis of physical status, serum profile and gene expressions. Benef. Microbes 2018, 9, 441–452. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol Hepatol 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, C.; Carlotta Tagliacarne, S.; Castellazzi, A. Gut Microbiota and Obesity. J. Clin. Gastroenterol. 2016, 50 (Suppl. 2), 3–15. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. On behalf of the Obesity Programs of nutrition ER, Assessment g: Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ma, F.; Wang, G.; Wang, Y.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria attenuate the development of metabolic disorders, with inter- and intra-species differences. Food Funct. 2018, 9, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Montandon, S.A.; Jornayvaz, F.R. Effects of Antidiabetic Drugs on Gut Microbiota Composition. Genes (Basel) 2017, 8, 250. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Shin, N.R.; Chin, Y.W.; Choi, Y.H.; Kim, H. Pharmaceutical Impact of Houttuynia Cordata and Metformin Combination on High-Fat-Diet-Induced Metabolic Disorders: Link to Intestinal Microbiota and Metabolic Endotoxemia. Front. Endocrinol. (Lausanne) 2018, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Bose, S.; Kim, H.-G.; Han, K.-S.; Kim, H. Fermented RhizomaAtractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci. Rep. 2015, 5, 8391. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Kim, Y.-M.; Chin, Y.-W.; Kim, B.-S.; Wang, J.-H.; Lee, J.-H.; Kim, H. Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food Funct. 2015, 6, 2684–2692. [Google Scholar] [CrossRef]
- Hussain, A.; Yadav, M.K.; Bose, S.; Wang, J.-H.; Lim, D.; Song, Y.-K.; Ko, S.-G.; Kim, H. Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS ONE 2016, 11, e0165483. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-E.; Pyun, C.; Jeong, S.-M.; Han, K.-H.; Lee, C.-H. Effects of fermented pueraria radix by lactobacillus acidophilus on lipid and bone metabolism in ovariectomized rats. Asian J. Anim. Vet. Adv. 2014, 9, 556–567. [Google Scholar]
- Krotkiewski, M.; Bjorntorp, P.; Sjostrom, L.; Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Investig. 1983, 72, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Kanter, R.; Caballero, B. Global gender disparities in obesity: A review. Adv. Nutr. 2012, 3, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bogarin, R.; Chanoine, J.-P. Efficacy, safety and tolerability of orlistat, a lipase inhibitor, in the treatment of adolescent weight excess. Therapy 2009, 6, 23–30. [Google Scholar] [CrossRef]
- Sun, L.; Xing, D.; Sun, H.; Li, M.; Jin, W.; Du, L. Effect of pueraria flavonoid on diabetes in mice complicated by hyperlipidemia. Tsinghua Sci. Technol. 2002, 7, 369–373. [Google Scholar]
- Oh, S.A.; Ok, H.M.; Kim, H.J.; Lee, W.J.; Kwon, O. Effects of a Pueraria lobata-root based combination supplement containing Rehmannia glutinosa and aerobic exercise on improvement of metabolic dysfunctions in ovariectomized rats. J. Nutr. Health 2015, 48, 133–139. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P.; Consortium NIHMMPC. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Jensen, T.L.; Kiersgaard, M.K.; Sorensen, D.B.; Mikkelsen, L.F. Fasting of mice: A review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef]
- Ikeda, I.; Metoki, K.; Yamahira, T.; Kato, M.; Inoue, N.; Nagao, K.; Yanagita, T.; Shirakawa, H.; Komai, M. Impact of fasting time on hepatic lipid metabolism in nutritional animal studies. Biosci. Biotechnol. Biochem. 2014, 78, 1584–1591. [Google Scholar] [CrossRef]
- Shin, N.R.; Bose, S.; Wang, J.-H.; Ansari, A.; Lim, S.-K.; Chin, Y.-W.; Choi, H.-S.; Kim, H. Flos lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front. Microbiol. 2017, 8, 2271. [Google Scholar] [CrossRef]
- Ansari, A.; Bose, S.; Patra, J.K.; Na, R.S.; Lim, D.-W.; Kim, K.-W.; Wang, J.-H.; Kim, Y.-M.; Chin, Y.-W.; Kim, H. A controlled fermented-Samjunghwan herbal formula ameliorates nonalcoholic-hepatosteatosis in HepG2 cells and OLETF rats. J. Front. Pharmacol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Ayala, J.E.; Bracy, D.P.; McGuinness, O.P.; Wasserman, D.H. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 2006, 55, 390–397. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Galaxy. Available online: http://huttenhower.sph.harvard.edu/galaxy (accessed on 23 December 2019).
- Bottacini, F.; Milani, C.; Turroni, F.; Sanchez, B.; Foroni, E.; Duranti, S.; Serafini, F.; Viappiani, A.; Strati, F.; Ferrarini, A.; et al. Bifidobacterium asteroides PRL2011 genome analysis reveals clues for colonization of the insect gut. PLoS ONE 2012, 7, e44229. [Google Scholar] [CrossRef] [PubMed]
- Gerz, H.O. On 7 years of clinical experience with the logotherapeutic technic of paradoxic intention. A contribution to the treatment of phobic and obsessive-compulsive patients. Z. Psychother Med. Psychol. 1966, 16, 25–32. [Google Scholar] [PubMed]
- Leite, T.C.; Da Silva, D.; Coelho, R.G.; Zancan, P.; Sola-Penna, M. Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem. J. 2007, 408, 123–130. [Google Scholar]
- Caesar, K.; Hashemi, P.; Douhou, A.; Bonvento, G.; Boutelle, M.G.; Walls, A.B.; Lauritzen, M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J. Physiol. 2008, 586, 1337–1349. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Zhuang, L.; Chen, X.; Min, H.; Song, S.; Liang, Q.; Li, A.D.; Gao, Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS ONE 2019, 14, e0218490. [Google Scholar] [CrossRef]
- Jung, H.; Kang, A.; Kang, S.; Park, Y.-K.; Song, M. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J. Inflammatory concepts of obesity. Int. J. Inflam. 2011, 2011, 529061. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Gorgun, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-T.; Cheng, P.-C.; Pan, T.-M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adubra, L.; Le Port, A.; Kameli, Y.; Fortin, S.; Mahamadou, T.; Ruel, M.T.; Martin-Prevel, Y.; Savy, M. Conditional cash transfer and/or lipid-based nutrient supplement targeting the first 1000 d of life increased attendance at preventive care services but did not improve linear growth in young children in rural Mali: Results of a cluster-randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1476–1490. [Google Scholar] [PubMed]
- Joh, E.-H.; Trinh, H.-T.; Han, M.-J.; Kim, D.-H. Anti-Inflammatory effect of fermented Artemisia princeps Pamp in mice. Biomol. Ther. 2010, 18, 308–315. [Google Scholar] [CrossRef]
- Stumvoll, M.; Mitrakou, A.; Pimenta, W.; Jenssen, T.; Yki-Järvinen, H.; Van Haeften, T.; Renn, W.; Gerich, J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000, 23, 295–301. [Google Scholar] [CrossRef]
- Matsuoka, H.; Shima, A.; Kuramoto, D.; Kikumoto, D.; Matsui, T.; Michihara, A. Phosphoenolpyruvate carboxykinase, a key enzyme that controls blood glucose, is a target of retinoic acid receptor-related orphan receptor α. PLoS ONE 2015, 10, e0137955. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Wu, H.; Deng, X.; Shi, Y.; Su, Y.; Wei, J.; Duan, H. PGC-1α, glucose metabolism and type 2 diabetes mellitus. J. Endocrinol. 2016, 229, R99–R115. [Google Scholar] [CrossRef]
- D’Errico, I.; Salvatore, L.; Murzilli, S.; Sasso, G.L.; Latorre, D.; Martelli, N.; Egorova, A.V.; Polishuck, R.; Madeyski-Bengtson, K.; Lelliott, C. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) is a metabolic regulator of intestinal epithelial cell fate. Proc. Natl. Acad. Sci. USA 2011, 108, 6603–6608. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, T.Y.; Kwok, C.F.; Hsu, Y.P.; Shih, K.C.; Lin, Y.J.; Ho, L.T. Cannabinoid receptor type 1 mediates high-fat diet-induced insulin resistance by increasing forkhead box O1 activity in a mouse model of obesity. Int. J Mol. Med. 2016, 37, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T.J.B.; et al. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.-H.; Satoh, H.; Herzig, S.; Lee, C.-H.; Hedrick, S.; Kulkarni, R.; Evans, R.M.; Olefsky, J.; Montminy, M. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat. Med. 2004, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Xu, G.; Deeney, J.T.; Yang, S.-N.; Rhee, J.; Puigserver, P.; Levens, A.R.; Yang, R.; Zhang, C.-Y.; Lowell, B.B. Suppression of β cell energy metabolism and insulin release by PGC-1α. Dev. Cell 2003, 5, 73–83. [Google Scholar] [CrossRef]
- Park, S.-S.; Yang, G.; Kim, E. Lactobacillus acidophilus NS1 Reduces Phosphoenolpyruvate Carboxylase Expression by Regulating HNF4α Transcriptional Activity. Korean J. Food Sci. Anim. Resour. 2017, 37, 529. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-W.; Bose, S.; Wang, J.-H.; Choi, H.S.; Kim, Y.-M.; Chin, Y.-W.; Jeon, S.-H.; Kim, J.-E.; Kim, H. Modified SJH alleviates FFAs-induced hepatic steatosis through leptin signaling pathways. Sci. Rep. 2017, 7, 45425. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Bhatnagar, M.S. Non-Alcoholic Fatty Liver Disease. J. Indian Pract. 2018, 71, 17–23. [Google Scholar]
- Chiang, D.J.; Pritchard, M.T.; Nagy, L.E. Obesity, diabetes mellitus, and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G697–G702. [Google Scholar] [CrossRef]
- Liu, K.; Xu, W.; Wong, V.W.S. Serum biomarkers for nonalcoholic fatty liver disease: Are we there yet? Hepatology 2017, 65, 8–11. [Google Scholar] [CrossRef][Green Version]
- Arao, T.; Udayama, M.; Kinjo, J.; Nohara, T. Preventive Effects of Saponins from the Pueraria lobata Root on in vitro Immunological Liver Injury of Rat Primary Hepatocyte Cultures1. Planta Med. 1998, 64, 413–416. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Y.; Yuan, J.; Wu, D. Effects of Puerariae radix extract on the increasing intestinal permeability in rat with alcohol-induced liver injury. J. Ethnopharmacol. 2009, 126, 207–214. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, G.; Liu, J. Reversal of chemical-induced liver fibrosis in Wistar rats by puerarin. J. Nutr. Biochem. 2006, 17, 485–491. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Jia, Q.; Chen, L.; Zhong, J.; Pan, Y.; Shen, P.; Shen, Y.; Wang, S.; Wei, Z. NiaoDuQing granules relieve chronic kidney disease symptoms by decreasing renal fibrosis and anemia. Oncotarget 2017, 8, 55920. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, G.S.; Cheon, S.Y.; Cha, Y.Y.; An, H.J. The anti-obesity effects of Tongbi-san in a high-fat diet-induced obese mouse model. BMC Complement. Altern. Med. 2019, 19, 1. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Choi, K.-M.; Jeon, Y.S.; Kim, W.; Lee, A.; Kim, Y.-G.; Lee, J.H.; Kang, Y.E.; Jung, J.-C.; Lee, J.; Min, B. Xanthigen attenuates high-fat diet-induced obesity through down-regulation of PPARγ and activation of the AMPK pathway. Food Sci. Biotechnol. 2014, 23, 931–935. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Amoroso, R. Inhibition of PPARγ by Natural Compounds as a Promising Strategy in Obesity and Diabetes. Open Med. Chem. J. 2019, 13, 1. [Google Scholar] [CrossRef]
- He, P.P.; Jiang, T.; OuYang, X.P.; Liang, Y.Q.; Zou, J.Q.; Wang, Y.; Shen, Q.Q.; Liao, L.; Zheng, X.L. Lipoprotein lipase: Biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin. Chim. Acta 2018, 480, 126–137. [Google Scholar] [CrossRef]
- Ferreira, L.D.-B.; Pulawa, L.K.; Jensen, D.R.; Eckel, R.H. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes 2001, 50, 1064–1068. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Gréchez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pévet, P.; Challet, E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef]
- Zané, F.; Ademola, A.; Emmanuel, M. Control of carbohydrate and lipid metabolism by NRF-1 and sirtuins: Implications on type 2 diabetes and obesity. Chem. Biol. Lett. 2014, 1, 66–76. [Google Scholar]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Ansari, A.; Bose, S.; Yadav, M.; Wang, J.-H.; Song, Y.-K.; Ko, S.-G.; Kim, H. CST, an herbal formula, exerts anti-obesity effects through brain-gut-adipose tissue axis modulation in high-fat diet fed mice. Molecules 2016, 21, 1522. [Google Scholar] [CrossRef]
- Whang, A.; Nagpal, R.; Yadav, H. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine 2019, 39, 591–602. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome. Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio 2017, 8, e00543-17. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Qin, L.; Yang, W.; Yu, G.; Wei, Q. Relationship between Helicobacter pylori infection and obesity in Chinese adults: A systematic review with meta-analysis. PLoS ONE 2019, 14, 9. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Bose, S.; Shin, N.R.; Song, E.-J.; Nam, Y.-D.; Kim, H. Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients 2020, 12, 276. https://doi.org/10.3390/nu12020276
Choi Y, Bose S, Shin NR, Song E-J, Nam Y-D, Kim H. Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients. 2020; 12(2):276. https://doi.org/10.3390/nu12020276
Chicago/Turabian StyleChoi, Yura, Shambhunath Bose, Na Rae Shin, Eun-Ji Song, Young-Do Nam, and Hojun Kim. 2020. "Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities" Nutrients 12, no. 2: 276. https://doi.org/10.3390/nu12020276
APA StyleChoi, Y., Bose, S., Shin, N. R., Song, E.-J., Nam, Y.-D., & Kim, H. (2020). Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients, 12(2), 276. https://doi.org/10.3390/nu12020276




