The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women
Abstract
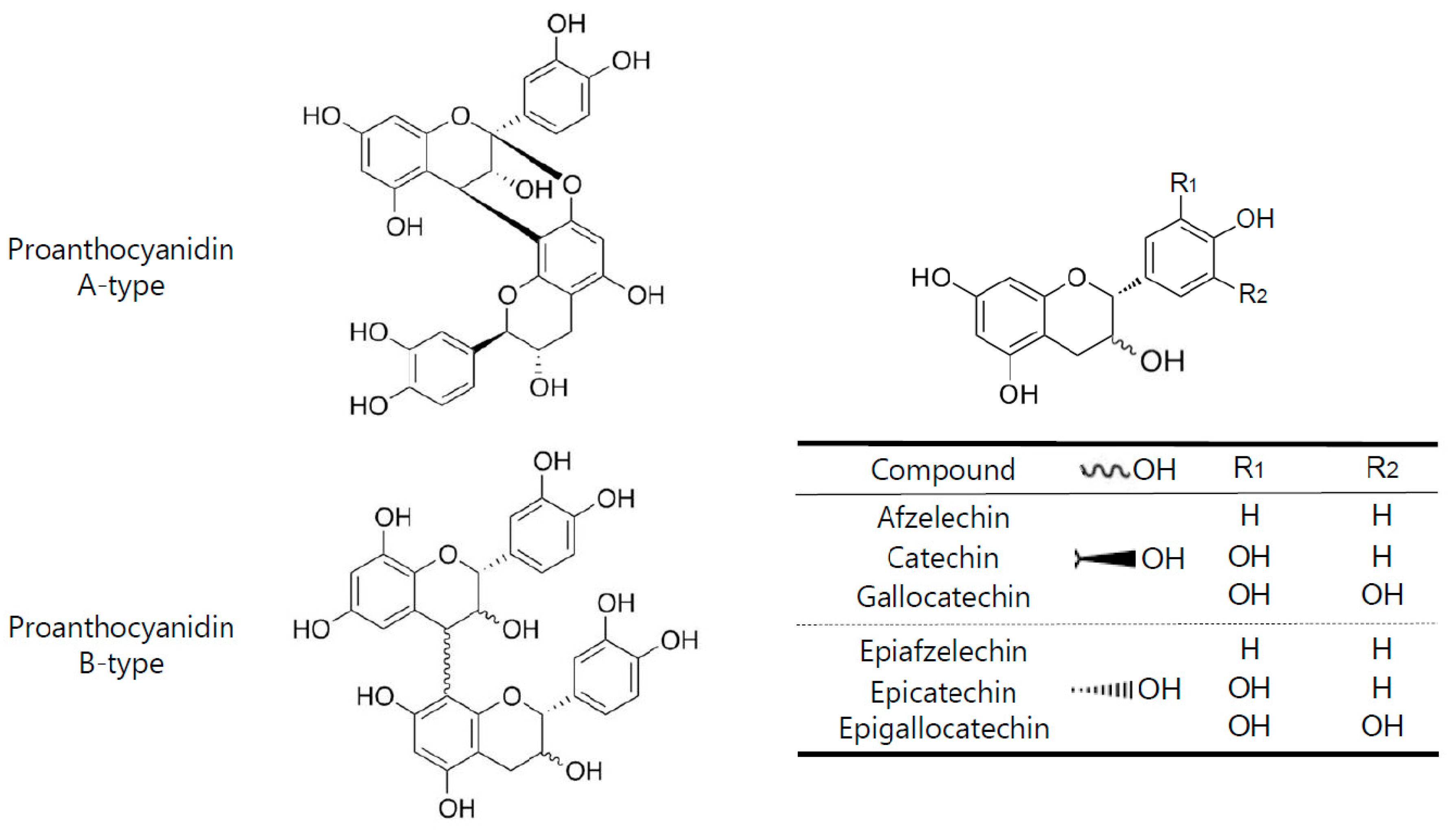
1. Introduction
2. Search Strategy and Study Selection
3. Results
3.1. Menopausal Disorders
3.2. Cancer
3.3. Hypertension
3.4. Cardiovascular Disease
3.5. Obesity
3.6. Osteoporosis
3.7. Urinary Tract Infection
3.8. Renal Function
3.9. Skin Damage
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Thom, T.; Haase, N.; Rosamond, W. Heart disease and stroke statistics-2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid changes during the menopause transition in relation to age and weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef]
- Taddei, S. Blood pressure through aging and menopause. Climacteric 2009, 12 (Suppl. S1), 36–40. [Google Scholar] [CrossRef]
- Brand, J.S.; van der Schouw, Y.T.; Onland-Moret, N.C. Age at menopause, reproductive life span, and type 2 diabetes risk: Results from the EPIC-InterAct study. Diabetes Care 2013, 36, 1012–1019. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- De la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Raufa, A.; Imranb, M.; Abu-Izneidc, T.; Ul-Haqd, I.; Patele, S.; Panf, X.; Nazg, S.; Silvah, A.S.; Saeedi, F.; Suleriaj, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Hellstorm, J.K.; Torronen, A.R.; Mattila, P.H. Proanthocyanidins in Common Food Products of Plant Origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed. Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Kohama, T.; Negami, M. Effect of Low-dose French Maritime Pine Bark Extract on Climacteric Syndrome in 170 Perimenopausal Women A Randomized, Double-blind, Placebo-controlled Trial. J. Reprod. Med. 2013, 58, 39–46. [Google Scholar]
- Yang, H.M.; Liao, M.F.; Zhu, S.Y.; Liao, M.N.; Rohdewald, P. A randomised, double-blind, placebo-controlled trial on the effect of Pycnogenol® on the climacteric syndrome in peri-menopausal women. Acta Obstet. Gynecol. 2007, 86, 978–985. [Google Scholar] [CrossRef]
- Chang, S.C.; Cassidy, A.; Willett, W.C.; Rimm, E.B.; O’Reilly, E.J.; Okereke, O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016, 104, 704–714. [Google Scholar] [CrossRef]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Serge Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: A prospective cohort study. Breast Cancer Res. Treat. 2013, 137, 225–236. [Google Scholar] [CrossRef]
- Rossi, M.; Edefonti, V.; Parpinel, M.; Lagiou, P.; Franchi, M.; Ferraroni, M.; Decarli, A.; Zucchetto, A.; Serraino, D.; Maso, L.D.; et al. Proanthocyanidins and other flavonoids in relation n to endometrial cancer risk: A case–control study in Italy. Br. J. Cancer 2013, 109, 1914–1920. [Google Scholar] [CrossRef][Green Version]
- Cutler, G.J.; Jennifer, A.; Nettleton, J.A.; Ross, J.A.; Harnack, L.J.; Jacobs, D.R., Jr.; Scrafford, C.G.; Barraj, L.M.; Pamela, J.; Mink, P.J.; et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 664–671. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Asuka Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef]
- Lajous, M.; Rossignol, E.; Fagherazzi, G.; Perquier, F.; Scalbert, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Flavonoid intake and incident hypertension in women. Am. J. Clin. Nutr. 2016, 103, 1091–1098. [Google Scholar] [CrossRef]
- Zhao, S.; Bomser, J.; Joseph, E.L.; DiSilvestro, R.A. Intakes of apples or apple polyphenols decease plasma values for oxidized low-density lipoprotein/beta2-glycoprotein I complex. J. Funct. Food 2013, 5, 493–497. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Higher dietary flavonoid intakes are associated with lower objectively measured body composition in women: Evidence from discordant monozygotic twins. Am. J. Clin. Nutr. 2017, 105, 626–634. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Vitelli-Storelli, F.; Becerra-Tomas, N.; Vázquez-Ruiz, Z.; Díaz-López, A.; Corella, D.; Castañer, O.; Romaguera, D.; Jesús Vioque, J.; et al. Associations between Dietary Polyphenols and Type 2 Diabetes in a Cross-Sectional Analysis of the PREDIMED-Plus Trial: Role of Body Mass Index and Sex. Antioxidants 2019, 8, 537. [Google Scholar] [CrossRef]
- Kim, S.-A.; Kim, J.; Jun, S.; Wie, G.-A.; Shin, S.; Joung, H. Association between dietary flavonoid intake and obesity among adults in Korea. Appl. Physiol. Nutr. Metab. 2020, 45, 203–212. [Google Scholar] [CrossRef]
- Panahande, S.B.; Maghbooli, Z.; Hossein-nezhad4, A.; Qorbani, M.; Moeini-Nodeh, S.; Haghi-Aminjan, H.; Hosseini, S. Effects of French maritime pine bark extract (Oligopin®) supplementation on bone remodeling markers in postmenopausal osteopenic women: A randomized clinical trial. Phytother. Res. 2019, 33, 1233–1240. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; He, L.-P.; Liu, Y.-H.; Liu, J.; Su, Y.-X.; Chen, Y.-M. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporos. Int. 2014, 25, 2417–2425. [Google Scholar] [CrossRef]
- Takahashi, S.; Hamasuna, R.; Yasuda, M.; Arakawa, S.; Tanaka, K.; Ishikawa, K.; Hiroshi Kiyota, H.; Hayami, H.; Yamamoto, S.; Kubo, T.; et al. A randomized clinical trial to evaluate the preventive effect of cranberry juice (UR65) for patients with recurrent urinary tract infection. J. Infect. Chemother. 2013, 19, 112–117. [Google Scholar] [CrossRef]
- Maki, K.C.; Kaspar, K.L.; Khoo, C.; Derrig, L.H.; Schild, A.L.; Gupta, K. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am. J. Clin. Nutr. 2016, 103, 1434–1442. [Google Scholar] [CrossRef]
- Vostalova, J.; Vidlar, A.; Simanek, V.; Galandakova, A.; Kosina, P.; Vacek, J.; Jana Vrbkova, J.; Zimmermann, B.F.; Ulrichova, J.; Student, V. Are High Proanthocyanidins Key to Cranberry Efficacy in the Prevention of Recurrent Urinary Tract Infection? Phytother. Res. 2015, 29, 1559–1567. [Google Scholar] [CrossRef]
- Ivey, K.L.; Lewis, J.R.; Lim, W.H.; Lim, E.M.; Hodgson, J.M.; Prince, R.L. Associations of Proanthocyanidin Intake with Renal Function and Clinical Outcomes in Elderly Women. PLoS ONE 2013, 8, e71166. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral Intake of Proanthocyanidin-Rich Extract from Grape Seeds Improves Chloasma. Phytother. Res. 2004, 18, 895–899. [Google Scholar] [CrossRef]
- Handog, E.B.; Galang, D.A.V.F.; Leon-Godinez, M.A.; Chan, G.P. A randomized, double-blind, placebo-controlled trial of oral procyanidin with vitamins A, C, E for melasma among Filipino women. Int. J. Dermat. 2009, 48, 896–901. [Google Scholar] [CrossRef]
- Furumura, M.; Sato, N.; Kusaba, N.; Takagaki, K.; Nakayama, J. Oral administration of French maritime pine bark extract (Flavangenol®) improves clinical symptoms in photoaged facial skin. Clin. Interv. Aging 2012, 7, 275–286. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Goetz, M.E.; Judd, S.E.; Safford, M.M.; Hartman, T.J.; McClellan, W.M. Vaccarino, Dietary flavonoid intake and incident coronary heart disease: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am. J. Clin. Nutr. 2016, 104, 1236–1244. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Raman, G.; Shams-White, M.; Avendano, E.E.; Chen, F.; Novotny, J.A.; Cassidy, A. Dietary intake of flavan-3-ols and cardiovascular health: A field synopsis using evidence Mapping of randomized trials and prospective cohort studies. Syst. Rev. 2018, 7, 100. [Google Scholar] [CrossRef]
- Raman, G.; Avendano, E.E.; Chen, S.; Wang, J.; Matson, J.; Gayer, B.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiometabolic health: Systematic review and meta-analysis of randomized trials and prospective cohort studies. Am. J. Clin. Nutr. 2019, 110, 1067–1078. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H.; Rossouw, J.E.; Prentice, R.L.; Manson, J.E. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: A randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016, 293, 609–615. [Google Scholar] [CrossRef]
- Moreira, E.L.; Rial, D.; Duarte, F.S. Central nervous system activity of the proanthocyanidin-rich fraction obtained from Croton celtidifolius in rats. J. Pharm. Pharmacol. 2010, 62, 1061–1068. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Chen, R.; Li, G.; Barish, P.A.; You, W.; Chen, L.; Lin, M.; Ku, B.; Pan, J.; et al. Antidepressant-like effect of low molecular proanthocyanidin in mice: Involvement of monoaminergic system. Pharmacol. Biochem. Behav. 2010, 94, 447–453. [Google Scholar] [CrossRef]
- Jiangb, X.; Liua, J.; Lin, Q.; Mao, K.; Tian, F.; Jing, C.; Wang, C.; Ding, L.; Pang, C. Proanthocyanidin prevents lipopolysaccharide-induced depressive-like behavior in mice via neuroinflammatory pathway. Brain Res. Bull. 2017, 135, 40–46. [Google Scholar] [CrossRef]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ge, B.; Yang, X.L.; Zhai, J.; Yang, L.N.; Wang, X.X.; Liu, X.; Shi, J.C.; Wu, Y.J. Proanthocyanidins from grape seeds modulates the nuclear factor kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int. Immunopharmacol. 2011, 11, 1620–1627. [Google Scholar] [CrossRef]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Han, W.Q.; Chen, J.; Zhu, D.L.; Chen-Yan Gao, P.J. Anti-stiffness effect of apocynin in deoxycorticosterone acetate-salt hypertensive rats via inhibition of oxidative stress. Hypertens. Res. 2013, 36, 306–312. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- Yochum, L.; Kushi, L.H.; Meyer, K.; Folsom, A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999, 149, 943–949. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C.B. Proposed mechanisms of the effects of proanthocyanidins on glucose homeostasis. Nutr. Rev. 2017, 75, 8–642. [Google Scholar] [CrossRef]
- Melton, L.J., III; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. How many women have osteoporosis? J. Bone Miner. Res. 2005, 20, 886–892. [Google Scholar] [CrossRef]
- Liu, J.; Ho, S.C.; Su, Y.X.; Chen, W.Q.; Zhang, C.X.; Chen, Y.M. Effect of long-term intervention of soy isoflavones on bone mineral density in women: A meta-analysis of randomized controlled trials. Bone 2009, 44, 948–953. [Google Scholar] [CrossRef]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-type cranberry proanthocyanidins inhibit the RANKL-dependent differentiation and function of human osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef]
- Ishikawa, M.; Maki, K.; Tofani, I.; Kimura, K.; Kimura, M. Grape seed proanthocyanidins extract promotes bone formation in rat’s mandibular condyle. Eur. J. Oral Sci. 2005, 113, 47–52. [Google Scholar] [CrossRef]
- Foxman, B.; Barlow, R.; D’Arcy, H.; Gillespie, B.; Sobel, J.D. Urinary tract infection: Self-reported incidence and associated costs. Ann. Epidemiol. 2000, 10, 509–515. [Google Scholar] [CrossRef]
- Litwin, M.S.; Saigal, C.S.; Yano, E.M.; Avila, C.; Gescgwind, S.A.; Hanley, J.M.; Joyce, G.F.; Madison, R.; Pace, J.; Polich, S.M. Urologic Diseases in America Project: Analytical methods and principal findings. J. Urol. 2005, 173, 933–937. [Google Scholar] [CrossRef]
- Wang, C.H.; Fang, C.C.; Chen, N.C.; Liu, S.S.; Yu, P.H.; Wu, T.Y.; Chen, W.T.; Lee, C.C.; Chen, S.C. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: A systemic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar] [CrossRef]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD001321. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W. Prevalence of chronic kidney disease in the United States. J. Am. Med. Assoc. 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Annuk, M.; Zilmer, M.; Lind, L.; Linde, T.; Fellstro¨m, B. Oxidative stress and endothelial function in chronic renal failure. J. Am. Soc. Nephrol. 2001, 12, 2747–2752. [Google Scholar] [PubMed]
- Kielstein, J.T.; Frölich, J.C.; Haller, H.; Fliser, D. ADMA (asymmetric dimethylarginine): An atherosclerotic disease mediating agent in patients with renal disease? Nephrol. Dial. Transplant. 2001, 16, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimburger, O.; Paultre, F.; Diczfalusy, U.; Wang, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Bagi, Z.; Hamar, P.; Antus, B.; Rosivall, L.; Koller, A. Chronic renal failure leads to reduced flow-dependent dilation in isolated rat skeletal muscle arterioles due to lack of NO mediation. Kidney Blood Press. Res. 2003, 26, 19–26. [Google Scholar] [CrossRef]
- Liao, J.; Bettmann, M.; Sandor, T.; Tucker, J.; Coleman, S. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ. Res. 1991, 68, 1027–1034. [Google Scholar] [CrossRef]
- Anderson, S.; Eldadah, B.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J. Acute kidney injury in older adults. J. Am. Soc. Nephrol. 2011, 22, 28–38. [Google Scholar] [CrossRef]
- Silva, F.G. The aging kidney: A review—Part I. Int. Urol. Nephrol. 2005, 37, 185–205. [Google Scholar] [CrossRef]
- Bökenkamp, A.; Herget-Rosenthal, S.; Bökenkamp, R. Cystatin C, kidney function and cardiovascular disease. Pediatr. Nephrol. 2006, 21, 1223–1230. [Google Scholar] [CrossRef]
- Yanarates, O.; Guven, A.; Sizlan, A.; Uysal, B.; Akgul, O. Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren. Fail. 2008, 30, 931–938. [Google Scholar] [CrossRef]
- Lee, Y.A.; Kim, Y.J.; Cho, E.J.; Yokozawa, T. Ameliorative effects of proanthocyanidin on oxidative stress and inflammation in streptozotocin induced diabetic rats. J. Agric. Food Chem. 2007, 55, 9395–9400. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.H.; Yoon, K.H.; Lee, E.-S. Melasma: Histopathological characteristics in 56 Korean patients. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Blazsó, G.; Gabor, M.; Rohdewald, P. Antiinflammatory activities and superoxide radical scavenging activities of a procyanidin containing extract from the bark of Pinus pinaster Sol. and its fractions. Pharm. Pharmacol. Lett. 1994, 3, 217–220. [Google Scholar]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of Proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F. Procyanidin dimer B2 (epicatechin-(4beta-8)-epicatechin) in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Remesy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]

| Studies (Ref. No.) | Study Design | Period | Age | Contents | Main Results |
|---|---|---|---|---|---|
| Menopausal Disorders (Including Mental Symptoms) | |||||
| Terauchi [17] | RCT | 8 weeks | 40–60 | grape seed extract proanthocyanidins 100 mg/day or 200 mg/day | improvement of HADS-anxiety, HADS-depression and AIS score |
| Kohama [18] | RCT | 12 weeks | 42–58 | pine bark extract proanthocyanidins 30 mg/day | improvement of vasomotor and insomnia/sleep problem symptoms and Kupperman’s index |
| Yang [19] | RCT | 6 months | 45–55 | pine bark extract proanthocyanidins 200 mg/day | improvement of climacteric symptoms |
| Chang [20] | Prospective cohort | 10 years | 65- | diet-derived proanthocyanidins | lowering of depression risk |
| Cancer | |||||
| Touvier [21] | Prospective cohort | 14 years | 55- | diet-derived proanthocyanidins | lowering of breast cancer risks |
| Rossi [22] | case-control | 15 years | 60–61 (median) | diet-derived proanthocyanidins | lowering of endometrial cancer risk |
| Culter [23] | Prospective cohort | 19 years | 55–69 | diet-derived proanthocyanidins | decreasing of lung cancer incidence |
| Hypertension | |||||
| Terauchi [17] | RCT | 8 weeks | 40–60 | grape seed extract proanthocyanidins 100 mg/day or 200 mg/day | reducing of SBP and DBP |
| Odai [24] | RCT | 12 weeks | 40–64 | grape seed extract proanthocyanidins 200 mg/day or 400 mg/day | reducing of SBP and DBP |
| Lajous [25] | Prospective cohort | 16 years | 45–58 | diet-derived proanthocyanidins | lowering of hypertension rate |
| Cardiovascular disease | |||||
| Odai [24] | RCT | 12 weeks | 40–64 | grape seed extract proanthocyanidins 200 mg/day or 400 mg/day | Improvement of stiffness parameter, distensibility, Eincand PWV |
| Zhao [26] | RCT | 4 weeks | 42–53 | apple proanthocyanidins | decreasing of oxLDL-b2GPI |
| Mink [27] | Prospective cohort | 13 years | 55–69 | diet-derived proanthocyanidins | inverse association with coronary heart disease mortality |
| McCullough [28] | Prospective cohort | 7 years | 68.9 ± 6.2 | diet-derived proanthocyanidins | reduction in cardiovascular disease risk |
| Jennings [29] | Prospective cohort | 12 years | 53 (median) | diet-derived proanthocyanidins | lowering of fat mass ratio (FMR) |
| Obesity | |||||
| Tresserra-Rimbau [30] | Prospective cohort | 4 years | 60–75 | diet-derived proanthocyanidins | inverse association with overweight and obese |
| Kim [31] | Prospective cohort | 15 years | 45.0 ± 0.2 | diet-derived proanthocyanidins | inverse association with abdominal obesity |
| Osteoporosis | |||||
| Panahande [32] | RCT | 12 weeks | 50–65 | pine bark extract proanthocyanidins 250 mg/day | increase of P1NP and BAP levels, decrease of CTx1 levels |
| Zhang [33] | Prospective cohort | 12 weeks | 56–63 | diet-derived proanthocyanidins | Increase of bone mineral density at the whole body, femoral neck and lumbar spine |
| Urinary Tract Infection (UTI) | |||||
| Takahashi [34] | RCT | 6 months | 50- | cranberry proanthocyanidins 40 mg/day (cranberry juice) | prevention of the recurrence of UTI |
| Maki [35] | RCT | 24 weeks | 40–41 (average) | cranberry proanthocyanidins 41 mg/day (cranberry juice) | lowered the number of clinical UTI episodes |
| Vostalova [36] | RCT | 6 months | 18–75 | cranberry powder 500 mg/day (proanthocyanidins 0.56%) | reduction of the risk of symptomatic UTI |
| Renal Function | |||||
| Ivey [37] | Prospective cohort | 5 years | 80 ± 3 | diet-derived proanthocyanidins | improvement of renal function and reduction of risk of chronic kidney disease and renal disease associated events |
| Skin Damage | |||||
| Yamakoshi [38] | single-armed | 12 months | 34–58 | grape seed extract proanthocyanidins 160 mg/d | improvement of chloasma and decreasing melanin index |
| Evangeline [39] | RCT | 8 weeks | 18–60 | cranberry proanthocyanidins 24 mg/day | decreasing the degree of pigmentation in the malar regions and improvement of the melasma area and severity index |
| Furumura [40] | RCT | 12 weeks | 31–59 | pine bark extract proanthocyanidins 100 mg/day | improvement of scores for solar lentigines, mottled pigmentation, roughness, wrinkles, and swelling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, T.; Terauchi, M. The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women. Nutrients 2020, 12, 3833. https://doi.org/10.3390/nu12123833
Izumi T, Terauchi M. The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women. Nutrients. 2020; 12(12):3833. https://doi.org/10.3390/nu12123833
Chicago/Turabian StyleIzumi, Toru, and Masakazu Terauchi. 2020. "The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women" Nutrients 12, no. 12: 3833. https://doi.org/10.3390/nu12123833
APA StyleIzumi, T., & Terauchi, M. (2020). The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women. Nutrients, 12(12), 3833. https://doi.org/10.3390/nu12123833






