Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Nutritional Assessment
2.3. Dietary Indices Calculation
2.4. Blood Biochemical Analyses
2.5. Fecal Collection and Microbial Analysis
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, M.J.R.; Simmonds, M.S.J. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ocké, M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.M.; Bassett, J.K.; Dugué, P.A.; Shivappa, N.; Hébert, J.R.; Milne, R.L.; English, D.R.; Giles, G.G. Dietary inflammatory index or Mediterranean diet score as risk factors for total and cardiovascular mortality. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 461–469. [Google Scholar] [CrossRef]
- Roy, R.; Hebden, L.; Rangan, A.; Allman-Farinelli, M. The development, application and validation of a Healthy eating index for Australian Adults (HEIFA-2013). Nutrition 2016, 32, 432–440. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and validation of an empirical dietary inflammatory index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Giovannucci, E.L. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J. Nutr. 2017, 147, 1567–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.R.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 113, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Onvani, S.; Haghighatdoost, F.; Surkan, P.J.; Larijani, B.; Azadbakht, L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: A meta-analysis of observational studies. J. Hum. Nutr. Diet. 2017, 30, 216–226. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Ciancarelli, M.; Massimo, C.; Amicis, D.; Ciancarelli, I. Mediterranean Diet and Health Promotion: Evidence and current concerns. Med. Res. Arch. 2017, 9, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Sánchez, B.; Margolles, A.; González, S. Mediterranean diet and faecal microbiota: A transversal study. Food Funct. 2016, 7, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot Study of Diet and Microbiota: Interactive Associations of Fibers and Polyphenols with Human Intestinal Bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef]
- Cuervo, A.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Lopez, P.; Suarez, A.; Gueimonde, M.; González, S. Red Wine Consumption Is Associated with Fecal Microbiota and Malondialdehyde in a Human Population. J. Am. Coll. Nutr. 2015, 34, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Takahashi, H.; Suzuki, K.; Hirano, A.; Kamei, M.; Goto, T.; Takahashi, N.; Kawada, T. Theobromine enhances absorption of cacao polyphenol in rats. Biosci. Biotechnol. Biochem. 2014, 78, 2059–2063. [Google Scholar] [CrossRef]
- Centro de Ensenanza Superior de Nutricion Humana y Dietetica (CESNID). Tablas de Composicion de Alimentos Por Medidas Caseras de Consumo Habitual en España; McGrawHill, Ed.; Publicaciones y Ediciones de la Universidad de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Marlett, J.A.; Cheung, T.-F. Database and Quick Methods of Assessing Typical Dietary Fiber Intakes using data for 228 Commonly Consumed Foods. J. Am. Diet. Assoc. 1997, 97, 1139–1151. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Marks, G.C. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br. J. Nutr. 2003, 90, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudo, A.; Ibáñez, R.; Amiano, P.; Ardanaz, E.; Barricarte, A.; Berenguer, A.; Chirlaque, M.D.; Dorronsoro, M.; Jakszyn, P.; Larrañaga, N.; et al. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur. J. Clin. Nutr. 2008, 62, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research of Cancer (IARC). Cruciferous Vegetables, Isothicynates and Indoles. In Handbooks of Cancer Prevention; IARC: Lyon, France, 2004. [Google Scholar]
- Fernández-Navarro, T.; Díaz, I.; Gutiérrez-Díaz, I.; Rodríguez-Carrio, J.; Suárez, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; Salazar, N.; González, S. Exploring the interactions between serum free fatty acids and fecal microbiota in obesity through a machine learning algorithm. Food Res. Int. 2019, 121, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Arcas, M.; Romaguera, D.; Rivas, A.; Feriche, B.; Pons, A.; Tur, J.A.; Olea-Serrano, F. Diet quality of young people in southern Spain evaluated by a Mediterranean adaptation of the Diet Quality Index-International (DQI-I). Br. J. Nutr. 2007, 98, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Agudo, A.; Travier, N.; María Huerta, J.; Cirera, L.; Tormo, M.J.; Navarro, C.; Dolores Chirlaque, M.; Moreno-Iribas, C.; Ardanaz, E.; et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br. J. Nutr. 2011, 330, 991–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.M.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. Br. Med. J. 2005, 330, 991–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudière, J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. CUPRAC total antioxidant capacity assay of lipophilic antioxidants in combination with hydrophilic antioxidants using the macrocyclic oligosaccharide methyl β-cyclodextrin as the solubility enhancer. React. Funct. Polym. 2007, 67, 1548–1560. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Salazar, N.; Arboleya, S.; Ruas-Madiedo, P.; Mancabelli, L.; Suarez, A.; Martinez-Faedo, C.; Ventura, M.; Tochio, T.; Hirano, K.; et al. In vitro evaluation of different prebiotics on the modulation of gut microbiota composition and function in morbid obese and normal-weight subjects. Int. J. Mol. Sci. 2020, 21, 906. [Google Scholar] [CrossRef] [Green Version]
- Valdés, L.; Salazar, N.; González, S.; Arboleya, S.; Ríos-Covián, D.; Genovés, S.; Ramón, D.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M. Selection of potential probiotic bifidobacteria and prebiotics for elderly by using in vitro faecal batch cultures. Eur. Food Res. Technol. 2017, 243, 157–165. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; Hernández-Barranco, A.M.; Ruas-Madiedo, P.; De Los Reyes-Gavilán, C.G. Exopolysaccharides produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Derrien, M.; Isolauri, E.; De Vos, W.M.; Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, P.W. Changes in the intestinal microbiota from adulthood through to old age. Clin. Microbiol. Infect. 2012, 18, 44–46. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: A cross-sectional study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowyer, R.C.E.; Jackson, M.A.; Pallister, T.; Skinner, J.; Spector, T.D.; Welch, A.A.; Steves, C.J. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 16, 35–56. [Google Scholar] [CrossRef] [Green Version]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. MBio 2017, 8, e00770-17. [Google Scholar] [CrossRef] [Green Version]
- Lyra, A.; Forssten, S.; Rolny, P.; Wettergren, Y.; Lahtinen, S.J.; Salli, K.; Cedgård, L.; Odin, E.; Gustavsson, B.; Ouwehand, A.C. Comparison of bacterial quantities in left and right colon biopsies and faeces. World J. Gastroenterol. 2012, 18, 4404–4411. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Shanahan, F.; Guarner, F.; De Vos, W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Theissig, F.; Rückert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kühn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, M.; Yang, X.; Hong, N.; Yu, C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohn’s Colitis 2013, 7, e558–e568. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, S4586–S4591. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Legarrea, P.; Fuller, N.R.; Zulet, M.A.; Martinez, J.A.; Caterson, I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac. J. Clin. Nutr. 2014, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Thissen, J.P.; Delzenne, N.M. Gut microbial metabolites of polyunsaturated fatty acids correlate with specific fecal bacteria and serum markers of metabolic syndrome in obese women. Lipids 2014, 49, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, T.; Sarwar, A.; Fahim, M.; ud Din, J.; Al-Dalali, S.; Ud Din, Z.; Khan, A.A.; Jian, Z.; Yang, Z. Dose-dependent production of linoleic acid analogues in food derived Lactobacillus plantarum K25 and in silico characterization of relevant reactions. Acta Biochim. Pol. 2020, 67, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Carrio, J.; Salazar, N.; Margolles, A.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A. Free fatty acids profiles are related to gut microbiota signatures and short-chain fatty acids. Front. Immunol. 2017, 8, 823. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Brann, L.; Shivappa, N.; Hébert, J.R. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition 2019, 63–64, 155–161. [Google Scholar] [CrossRef]
- Suzuki, K.; Shivappa, N.; Kawado, M.; Yamada, H.; Hashimoto, S.; Wakai, K.; Iso, H.; Okada, E.; Fujii, R.; Hébert, J.R.; et al. Association between dietary inflammatory index and serum C-reactive protein concentrations in the Japan collaborative cohort study. Nagoya J. Med. Sci. 2020, 82, 237–249. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as Regulator of Gut Microbiota and its Role in Health and Disease. Arch. Med. Res. 2019, 50, 259–268. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
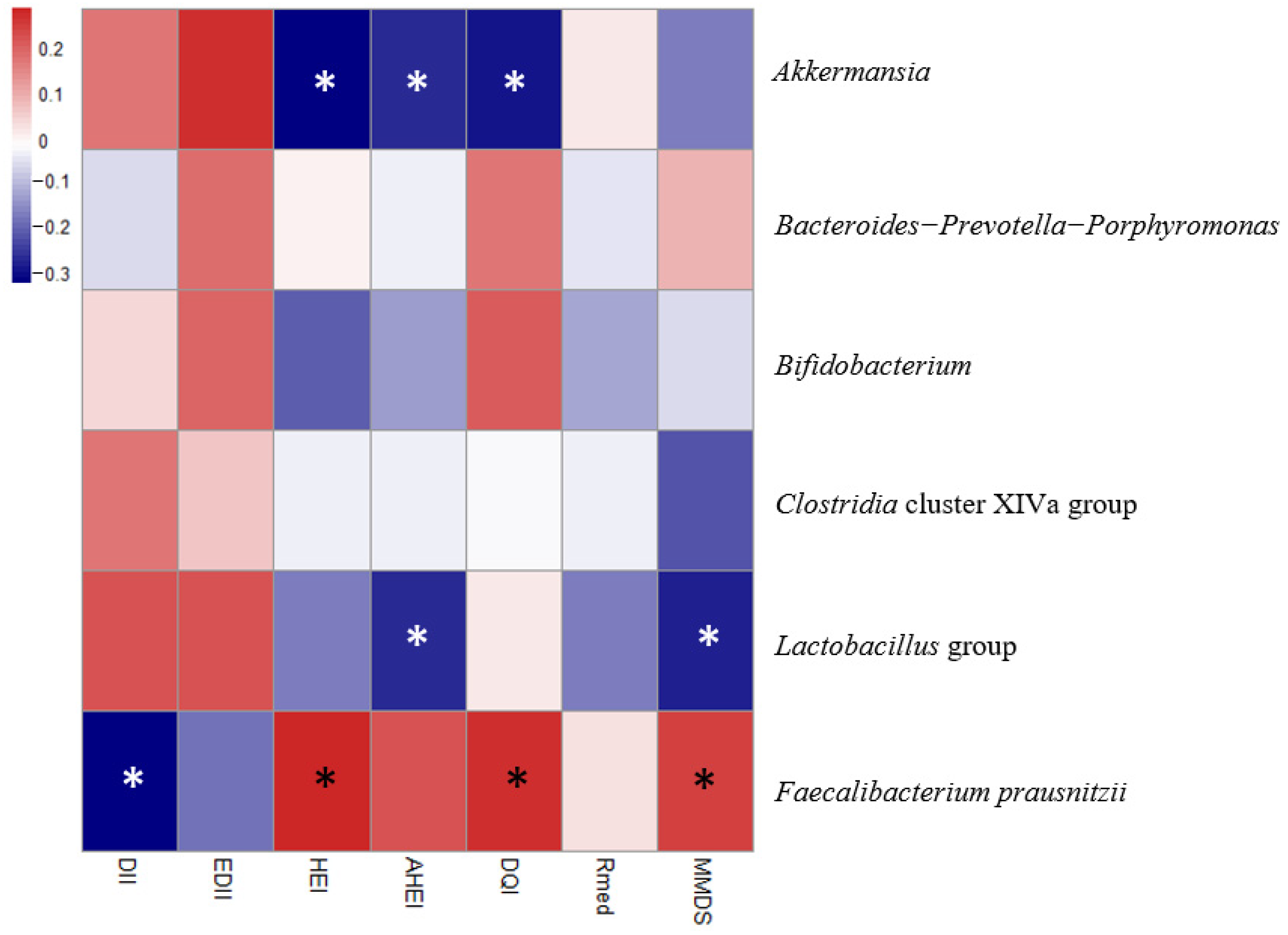
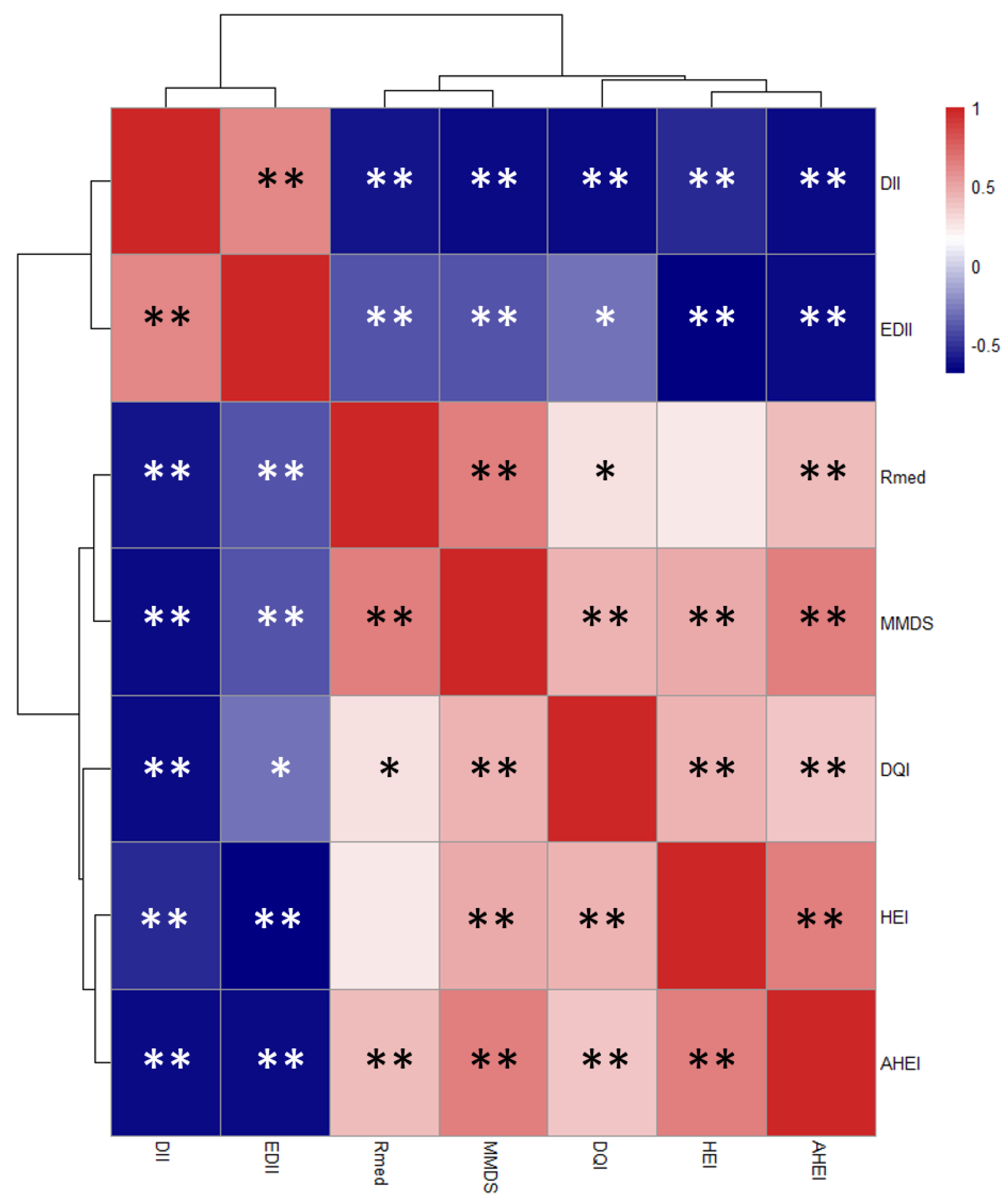
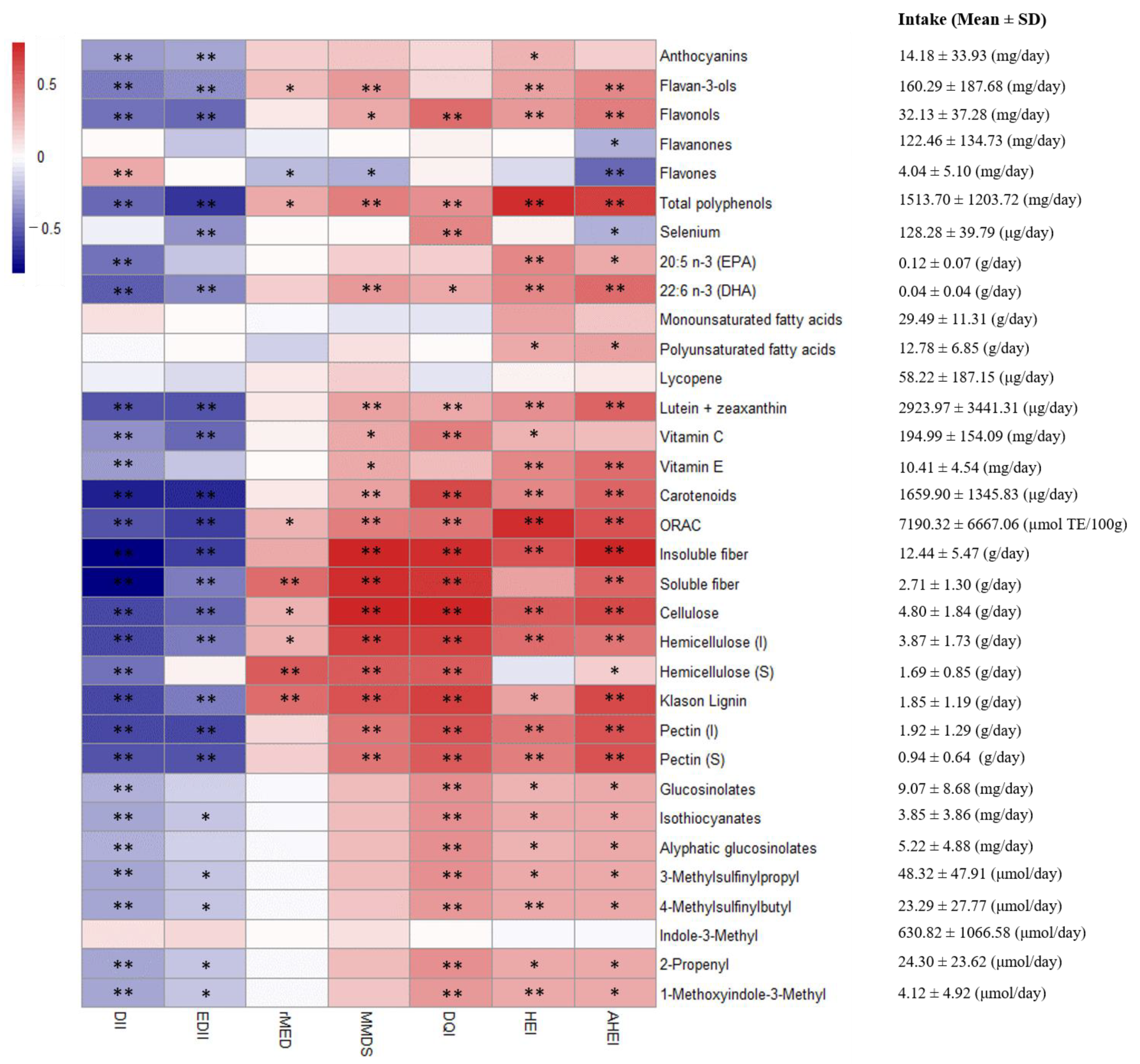
| Variable | n | DII | EDII | HEI | AHEI | DQI-I | rMED | MMDS |
|---|---|---|---|---|---|---|---|---|
| Mean (IQR) | −0.35 (1.07–−2.25) | 0.67 (1.17–0.19) | 58.16 (65.68–49.95) | 60.99 (67.97–55.08) | 49.03 (55–44) | 7.16 (9–6) | 3.37 (4–2) | |
| Gender | ||||||||
| Male | 20 | −1.04 ± 2.27 a | 0.64 ± 0.73 a | 55.92 ± 11.47 a | 63.48 ± 8.51 a | 47.15 ± 9.53 a | 7.80 ± 2.48 a | 3,65 ± 1,31 a |
| Female | 53 | −0.10 ± 2.54 a | 0.68 ± 0.78 a | 59.00 ± 10.00 a | 60.10 ± 10.52 a | 49.74 ± 7.04 a | 6.92 ± 2.28 a | 3.26 ± 1.46 a |
| Age (years) | ||||||||
| 50–65 | 33 | −1.92 ± 2.05 a | 0.24 ± 0.62 a | 62.63 ± 9.01 a | 64.07 ± 8.94 a | 51.97 ± 0.83 a | 8.39 ± 2.14 a | 3.67 ± 1.29 a |
| >65 | 40 | 0.98 ± 2.02 b | 1.02 ± 0.69 b | 54.46 ± 10.16 b | 58.39 ± 10.37 b | 46.60 ± 5.96 b | 6.15 ± 2.03 b | 3.13 ± 1.49 a |
| Energy intake (kcal/day) | ||||||||
| ≤1538.9 | 25 | 0.01 ± 2.3 a,b | 0.41 ± 0.67 a | 58.95 ± 11.43 a | 63.49 ± 8.21 a | 46.36 ± 7.76 a | 7.08 ± 2.33 a | 3.52 ± 1.66 a |
| 1539–1994.8 | 24 | 0.40 ± 2.19 a | 0.77 ± 0.62 a | 57.22 ± 9.42 a | 60.17 ± 9.80 a | 47.46 ± 7.17 a | 6.79 ± 1.86 a | 3 ± 1.02 a |
| >1994.9 | 24 | −1.47 ± 2.65 b | 0.84 ± 0.92 a | 58.27 ± 10.67 a | 59.32 ± 11.84 a | 53.38 ± 6.88 b | 7.63 ± 2.76 a | 3.58 ± 1.47 a |
| BMI (kg/m2) | ||||||||
| <25 | 19 | −0.99 ± 2.77 a | 0.37 ± 0.72 a | 10.02 ± 19 a | 62.74 ± 9.90 a | 48.95 ± 9.20 a | 7.79 ± 2.32 a | 3.68 ± 1.60 a |
| 25.0–29.9 | 37 | −0.39 ± 2.37 a | 0.62 ± 0.77 a.b | 10.10 ± 37 a | 61.83 ± 10.16 a | 48.43 ± 7.65 a | 7.11 ± 2.50 a | 3.22 ± 1.38 a |
| ≥30 | 17 | 0.44 ± 2.33 a | 1.10 ± 0.62 b | 10.35 ± 17 a | 57.28 ± 9.77 a | 50.41 ± 6.75 a | 6.59 ± 1.97 a | 3.35 ± 1.32 a |
| Depositions (times/week) | ||||||||
| ≤3 | 8 | 0.51 ± 2.12 a | 0.85 ± 0.58 a | 53.09 ± 12.71 a | 60.29 ± 6.12 a | 47.50 ± 7.96 a | 7.00 ± 2.51 a | 3.00 ± 1.60 a |
| >3–7 | 57 | −0.11 ± 2.48 a | 0.71 ± 0.76 a | 58.40 ± 10.02 a | 60.74 ± 10.44 a | 49.19 ± 7.89 a | 6.93 ± 2.34 a | 3.35 ± 1.38 a |
| >7 | 8 | −2.82 ± 1.28 a | 0.16 ± 0.85 b | 61.51 ± 10.59 a | 63.44 ± 10.92 a | 49.38 ± 8.07 a | 9.00 ± 1.51 a | 3.88 ± 1.55 a |
| Smoking status | ||||||||
| Never | 9 | −2.27 ± 1.74 a | 0.11 ± 0.51 a | 67.14 ± 8.34 a | 64.66 ± 7.90 a | 49.22 ± 10.94 a | 8.78 ± 2.54 a | 4.0 ± 1.32 a |
| Current | 17 | −0.40 ± 2.55 a | 0.63 ± 0.67 a | 60.79 ± 9.12 a | 61.14 ± 8.21 a | 50.35 ± 6.72 a | 7.53 ± 2.24 a | 3.65 ± 1.37 a |
| Former | 16 | −1.11 ± 2.04 a | 0.38 ± 0.59 a | 60.17 ± 10.28 a | 65.93 ± 7.43 a | 49.81 ± 8.23 a | 8.25 ± 1.77 a | 3.75 ± 1.18 a |
| Educational level | ||||||||
| None or primary school | 2 | −2.46 ± 0.42 a | 0.45 ± 0.49 a | 66.19 ± 5.27 a | 63.13 ± 7.38 a | 57.00 ± 7.07 a | 7.00 ± 1.41 a | 3.50 ± 0.71 a |
| Technical school | 5 | −1.73 ± 1.80 a | −0.03 ± 0.34 a | 63.81 ± 6.21 a | 63.80 ± 1.99 a | 51.60 ± 6.35 a | 9.60 ± 1.67 a | 3.20 ± 1.10 a |
| Secondary school | 7 | −1.40 ± 2.86 a | 0.37 ± 0.65 a | 59.20 ± 8.96 a | 60.29 ± 8.83 a | 54.00 ± 8.06 a | 7.71 ± 2.75 a | 3.29 ± 1.25 a |
| University degree | 13 | −2.00 ± 1.61 a | 0.19 ± 0.46 a | 63.55 ± 9.67 a | 66.63 ± 7.77 a | 48.85 ± 9.14 a | 8.54 ± 1.90 a | 4.00 ± 1.22 a |
| Mood feeling | ||||||||
| Really satisfied | 42 | −0.35 ± 2.61 a | 0.70 ± 0.81 a | 57.99 ± 10.07 a | 60.63 ± 9.90 a | 48.88 ± 8.16 a | 7.24 ± 2.54 a | 3.48 ± 1.45 a |
| Satisfied | 21 | −0.68 ± 2.28 a | 0.57 ± 0.65 a | 61.28 ± 10.14 a | 63.27 ± 10.61 a | 49.48 ± 7.61 a | 7.76 ± 1.84 a | 3.38 ± 1.40 a |
| Unsatisfied | 8 | 0.96 ± 2.17 a | 0.78 ± 0.58 a | 51.65 ± 11.64 a | 57.86 ± 7.67 a | 46.75 ± 6.90 a | 5.25 ± 1.98 a | 2.63 ± 1.30 a |
| Self-health perception | ||||||||
| Excellent-good | 42 | −0.01 ± 2.20 a | 0.75 ± 0.72 a | 57.17 ± 9.85 a | 59.24 ± 7.88 a | 47.12 ± 7.15 a | 6.95 ± 2.39 a,b | 3.26 ± 1.31 a,b |
| Normal | 18 | −1.53 ± 2.54 a | 0.51 ± 0.68 a | 61.38 ± 101.39 a | 66.44 ± 10.49 b | 52.28 ± 7.89 a | 8.33 ± 2.22 a | 4.00 ± 1.57 a |
| Bad | 12 | 0.56 ± 2.76 a | 0.75 ± 0.95 a | 56.43 ± 11.15 a | 58.14 ± 13.62 a,b | 49.92 ± 8.40 a | 6.08 ± 1.88 b | 2.67 ± 1.23 b |
| Variable | Age Groups | |
|---|---|---|
| G1 (≤65) (n = 33) | G2 (>65) (n = 40) | |
| Akkermansia (Log10 n° cells/gram feces) | 6.43 ± 1.88 a | 6.99 ± 1.77 a |
| Bacteroides-Prevotella-Porphyromonas (Log10 n° cells/gram feces) | 9.32 ± 0.82 a | 8.79 ± 0.69 b |
| Bifidobacterium (Log10 n° cells/gram feces) | 7.93 ± 1.53 a | 7.55 ± 1.10 a |
| Clostridia cluster XIVa (Log10 n° cells/gram feces) | 7.57 ± 1.49 a | 6.45 ± 1.54 b |
| Lactobacillus group (Log10 n° cells/gram feces) | 5.91 ± 1.26 a | 6.97 ± 1.83 b |
| Faecalibacterium prausnitzii (Log10 n° cells/gram feces) | 7.07 ± 0.76 a | 6.42 ± 1.31 b |
| Acetic acid (mM) | 29.81 ± 9.25 a | 23.18 ± 14.45 b |
| Propionic acid (mM) | 12.94 ± 5.43 a | 9.50 ± 7.46 b |
| Butyric acid (mM) | 11.76 ± 9.39 a | 8.44 ± 7.94 a |
| Glucose (mg/dL) | 97.76 ± 12.99 a | 106.78 ± 33.85 a |
| Triglycerides (mg/dL) | 118.82 ± 54.23 a | 121.75 ± 48.41 a |
| Cholesterol (mg/dL) | 233.18 ± 39.06 a | 203.39 ± 37.48 b |
| LDL-HDL ratio | 2.76 ± 0.78 a | 2.72 ± 0.81 a |
| Leptin (ng/mL) | 9.62 ± 5.72 a | 12.01 ± 7.87 a |
| Serum MDA (μM) | 2.01 ± 0.53 a | 2.60 ± 0.49 b |
| Antioxidant capacity (mM) | 0.34 ± 0.09 a | 0.35 ± 0.09 a |
| CRP (mg/L) | 1.28 ± 1.22 a | 1.19 ± 1.03 a |
| TGF-β (ng/mL) | 4.44 ± 2.71 a | 6.25 ± 5.70 a |
| IL-10 (pg/mL) | 0.14 ± 0.78 a | 0.80 ± 3.70 a |
| IL-17 (pg/mL) | 1.42 ± 3.10 a | 2.28 ± 11.57 a |
| IL-8 (pg/mL) | 7.09 ± 6.01 a | 20.80 ± 9.91 b |
| IL-12 (pg/mL) | 0.21 ± 1.21 a | 3.54 ± 8.92 b |
| TNF-α (pg/mL) | 0.23 ± 1.23 a | 4.82 ± 7.94 b |
| Phagocytosis granulocytes (%) | 72.23 ± 22.13 a | 86.37 ± 18.53 a |
| Phagocytosis granulocytes and monocytes (%) | 71.39 ± 21.32 a | 82.35 ± 17.49 a |
| NK cell activity (%) | 53.09 ± 11.25 a | 52.70 ± 18.19 a |
| Dependent Variable | Independent Variable | R2 | β | p | |
|---|---|---|---|---|---|
| Model 1. Fecal microbiota groups Akkermansia, Bacteroides-Prevotella-Porphyromonas, Bifidobacterium, Clostridia cluster XIVa, Faecalibacterium prausnitzii, Lactobacillus group | Akkermansia | HEI | 0.080 | −0.307 | 0.026 |
| AHEI | 0.059 | −0.256 | 0.050 | ||
| DQI-I | 0.072 | −0.285 | 0.038 | ||
| Faecalibacterium prausnitzii | DII | 0.124 | −0.312 | 0.030 | |
| HEI | 0.128 | 0.284 | 0.035 | ||
| DQI-I | 0.122 | 0.265 | 0.047 | ||
| MMDS | 0.123 | 0.240 | 0.044 | ||
| Lactobacillus group | AHEI | 0.264 | −0.256 | 0.027 | |
| MMDS | 0.283 | −0.275 | 0.012 | ||
| Model 2. Fecal short chain fatty acids Acetic acid, Propionic acid, Butyric acid | Acetic acid | DII | 0.252 | −0.425 | 0.003 |
| EDII | 0.244 | −0.369 | 0.013 | ||
| HEI | 0.239 | 0.320 | 0.016 | ||
| AHEI | 0.335 | 0.478 | 0.000 | ||
| MMDS | 0.356 | 0.451 | 0.000 | ||
| Propionic acid | DII | 0.198 | −0.316 | 0.031 | |
| HEI | 0.246 | 0.348 | 0.009 | ||
| AHEI | 0.303 | 0.441 | 0.000 | ||
| MMDS | 0.292 | 0.378 | 0.001 | ||
| Butyric acid | HEI | 0.189 | 0.289 | 0.034 | |
| AHEI | 0.213 | 0.338 | 0.007 | ||
| MMDS | 0.211 | 0.298 | 0.012 | ||
| Model 3. Blood biomarkers Glucose, Triglycerides, LDL-HDL ratio, Leptin, Serum malondialdehyde (MDA), Antioxidant capacity, C-Reactive protein (CRP), Transforming growth factor-beta (TGF-β), IL-10, IL-17, IL-8, IL-12, TNF-α, % Phagocytosis granulocytes, % Phagocytosis granulocytes and monocytes, NK cell activity | MDA | DII | 0.297 | 0.373 | 0.003 |
| EDII | 0.318 | 0.408 | 0.002 | ||
| IL-8 | rMED | 0.443 | −0.251 | 0.018 | |
| MMDS | 0.443 | −0.221 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Saavedra, S.; Salazar, N.; Suárez, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects. Nutrients 2020, 12, 3828. https://doi.org/10.3390/nu12123828
Ruiz-Saavedra S, Salazar N, Suárez A, de los Reyes-Gavilán CG, Gueimonde M, González S. Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects. Nutrients. 2020; 12(12):3828. https://doi.org/10.3390/nu12123828
Chicago/Turabian StyleRuiz-Saavedra, Sergio, Nuria Salazar, Ana Suárez, Clara G. de los Reyes-Gavilán, Miguel Gueimonde, and Sonia González. 2020. "Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects" Nutrients 12, no. 12: 3828. https://doi.org/10.3390/nu12123828
APA StyleRuiz-Saavedra, S., Salazar, N., Suárez, A., de los Reyes-Gavilán, C. G., Gueimonde, M., & González, S. (2020). Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects. Nutrients, 12(12), 3828. https://doi.org/10.3390/nu12123828





