Dietary Ferulic Acid Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Treatment
2.3. Sample Collection
2.4. Western Blot
2.5. RNA Isolation, cDNA Synthesis, and Real-Time Quantitative PCR
2.6. The Antioxidant Capacity and Malondialdehyde Content Measurement
2.7. Biochemical Analysis of Serum and Liver Homogenate
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Indices in Serum, LD Muscle, and Liver
3.2. Expression of Antioxidant Enzyme Gene in LD Muscle and Liver
3.3. Effect of Ferulic Acid on Nrf2-ARE Signaling Pathways
3.4. Lipid Profiles of Serum, LD Muscle, and Liver
3.5. Liver Lipid-Metabolism-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Ingredients | Content | Nutrient Levels | Content |
|---|---|---|---|
| Corn (%) | 29.70 | Digestible energy (Mcal/kg) | 3.54 |
| Extruded corn (%) | 30.00 | Crude protein (%) | 20.0 |
| Soybean oil (%) | 1.39 | Calcium (%) | 0.75 |
| Sucrose (%) | 2.50 | Available P (%) | 0.37 |
| Whey power (%) | 5.00 | Digestible Lys (%) | 1.31 |
| Dehulled soybean meal (%) | 7.50 | Digestible Met (%) | 0.39 |
| Extruded soybean (%) | 10.00 | Digestible Thr (%) | 0.78 |
| Soybean protein concentrate (%) | 6.46 | Digestible Trp (%) | 0.21 |
| Fish meal (%) | 5.00 | ||
| L-Lysine·HCl (%) | 0.43 | ||
| DL-Methionine (%) | 0.09 | ||
| L-Threonine (%) | 0.16 | ||
| L-Tryptophan (%) | 0.02 | ||
| Choline chloride (%) | 0.10 | ||
| Calcium powder (%) | 0.66 | ||
| Dicalcium phosphate (%) | 0.34 | ||
| NaCl (%) | 0.30 | ||
| Vitamin premix a (%) | 0.05 | ||
| Mineral premix b (%) | 0.30 | ||
| Total | 100.00 |
| Genes | Primer | Sequence (5′-3′) | GenBank Accession No. | Size (bp) |
|---|---|---|---|---|
| SOD1 | Forward Reverse | AGACCTGGGCAATGTGACTG GTGCGGCCAATGATGGAATG | NM_001190422 | 102 |
| SOD2 | Forward Reverse | TGTAACTGAGCGATACGCCG GGTATTCGGCGCTCCTACAA | NM_214127 | 99 |
| GPX1 | Forward Reverse | GTGAATGGCGCAAATGCTCA ATTGCGACACACTGGAGACC | NM_214201 | 126 |
| GST | Forward Reverse | CCAACCCAGAAGACTGCTCA CATTCAGGTGGGCTCTTCGT | AB000884 | 102 |
| Nrf2 | Forward Reverse | GGAGCTGTTGATCTGTTGCG TCCATGTCCCTTGACAGCAA | XM_003133500 | 132 |
| GR CAT | Forward Reverse Forward Reverse | GTGAGCCGACTGAACACCAT CAGGATGTGAGGAGCTGTGT CAGATGAAGCATTGGAAGGAGC TTGTCTCCTATCGGATTCCCAG | AY368271 NM_214301 | 141 83 |
| ACC | Forward Reverse | ACCGAATTGGTTCCTTTGGAC CCAGTCCGATTCTTGCTCCA | AF175308 | 123 |
| HSL | Forward Reverse | CCCATCCTCTCCATCGACT CAGCAGTAGGCGTAGAAGCAC | NM_214315 | 83 |
| CPT1b | Forward Reverse | TGACTCGAATGTTCCGGGAG AGATCTTGCAGGTCTGCTTTCA | NM_001007191 | 118 |
| PPARα | Forward Reverse | GAGTTCGCCAAGTCCATCC CCGTCCTTGTTCATCACAGAG | NM_001044526 | 122 |
| GAPDH | Forward Reverse | ACACTGAGGACCAGGTTGTG GACGAAGTGGTCGTTGAGGG | NM_001206359 | 98 |
References
- Jonathan, C.C.; Jay, D.H.; Helen, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar]
- Toshiya, T.; Joji, Y.; Satoshi, I.; Hiroshi, A.; Hiroki, H.; Yukio, I.; Mitsuhiro, W.; Kenta, M.; Ryoichi, X.I.; Keisuke, T.; et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 2003, 100, 15924–15929. [Google Scholar]
- Borggreve, S.E.; Vries, R.D.; Dullaart, R.P.F. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: Role of lipolytic enzymes, lecithin: Cholesterol acyltransferase and lipid transfer proteins. Eur. J. Clin. Investig. 2003, 33, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Masafumi, F.; Yoichi, S.; Yasufumi, K.; Yusuke, M.; Atsushi, I.; Hideaki, K.; Hajime, Y.; Noriko, S.-A.; Maki, K.; Hiromichi, W.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2016, 11, 2029–2034. [Google Scholar]
- Jamal, S.R.; Maartje, E.V.; Benoit, J.A.; Jean-Pierre, D.; Erik, S.G.S.; John, J.P.K.; Nicholas, J.W.S.; Matthijs, B.; Kay-Tee, K. Metabolic dyslipidemia and risk of future coronary heart disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Int. J. Cardiol. 2010, 143, 399–404. [Google Scholar]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef]
- Fang, D.; Wan, Y.; Shen, W.; Cao, J.; Sun, Z.; Yu, H.; Zhang, Q.; Cheng, W.; Chen, J.; Ning, B. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol. Cell. Biochem. 2013, 381, 127–137. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Li, X.; Zhou, H. Inflammation: A novel therapeutic target/direction in atherosclerosis. Curr. Pharm. Des. 2017, 23, 1226–1227. [Google Scholar] [CrossRef]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.U.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.Y.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Xiong, S.Q.; Zhu, Z.M. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients 2016, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Shea, J.W. Dietary choline and betaine and risk of CVD: A systematic review and meta-analysis of prospective studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, R.S.; Enns, C.W.; Goldman, J.D.; Moshfegh, A.J. Dietary flavonoid intake is inversely associated with cardiovascular disease risk as assessed by body mass index and waist circumference among adults in the united states. Nutrients 2017, 9, 827. [Google Scholar] [CrossRef] [Green Version]
- Mattera, R.; Benvenuto, M.; Giganti, M.; Tresoldi, I.; Pluchinotta, F.; Bergante, S.; Guido, T.; Laura, M.; Vittorio, M.; Andrea, M.; et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients 2017, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, M.T.; Emilia, A. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar]
- Vittorio, C.; Stella, C.; Eduardo, P.; Carolin, C.; Maria, S.; Pierfrancesco, M.; Cesare, M. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar]
- Sanjeev, S.; Rema, M.B.; Meesala, K.M.; Guruswami, G.; Vikas, K.R. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 20631–20653. [Google Scholar] [CrossRef]
- Kuldeep, D.; Kumaragurubaran, K.; Rekha, K.; Ashok, M.; Ruchi, T.; Rajneesh, R.; Sandip, K.K.; Sana, U.; Rifat, U.K.; Mahmoud, A.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar]
- Li, H.; Mo, C.; Mahmoud, A.; Liu, J.; Sun, C. Health benefits and potential applications of anthocyanins in poultry feed industry. World Poult. Sci. J. 2018, 74, 251–264. [Google Scholar]
- Takatoshi, M.; Koichi, M.; Yoshihiko, M.; Masafumi, A.; Hideo, O.; Yasuto, S.; Yusuke, S.; Tadashi, H. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar]
- Chao, J.; Huo, T.-I.; Cheng, H.-Y.; Jen-Chieh, T.; Liao, J.-W.; Lee, M.-S.; Qin, X.-M.; Ming-Tsuen, H.; Pao, L.-H.; Peng, W-H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myoung, J.S.; Catherine, W.R.; Seok, H.N.; Mi, Y.K. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar]
- Ketmanee, S.; Upa, K.; Weerapon, S.; Poungrat, P.; Patchareewan, P.; Parichat, P.; Stephen, E.G.; Veerapol, K. Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients 2015, 7, 6446–6464. [Google Scholar]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloid Surf. B. 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Venugopal, P.M. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef]
- Fang, X.; Mou, Y.; Huang, Z.; Li, Y.; Han, L.; Zhang, Y.; Feng, Y.; Chen, Y.; Jiang, X.; Zhao, W.; et al. The sequence and analysis of a Chinese pig genome. GigaScience 2012, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Martien, A.M.G.; Alan, L.A.; Hirohide, U.; Christopher, K.T.; Yasuhiro, T.; Max, F.R.; Claire, R.G.; Chankyu, P.; Denis, M.; Hendrik-Jan, M.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar]
- Chen, L.Z.; Xin, Y.N.; Geng, N.; Jiang, M.; Zhang, D.D.; Xuan, S.Y. PNPLA3 I148M variant in nonalcoholic fatty liver disease: Demographic and ethnic characteristics and the role of the variant in nonalcoholic fatty liver fibrosis. World J. Gastroenterol. 2015, 21, 794–802. [Google Scholar] [CrossRef]
- Péter, F.; Mariann, H.; Ildikó, S.; Paragh, G. Paraoxonase-1 and adipokines: Potential links between obesity and atherosclerosis. Chem. Biol. Interact. 2016, 259, 388–393. [Google Scholar]
- Jessica, E.C.J.; William, P.E.; Rushi, P.; Adhiraj, L.; Nicholas, B.V.; Jeffrey, A.P.; Cecile, V. Inhibition of acetyl-CoA carboxylase 1 (ACC1) and 2 (ACC2) reduces proliferation and de novo lipogenesis of EGFRvIII human glioblastoma cells. PLoS ONE 2017, 12, e0169566. [Google Scholar]
- Morten, D.; Jozef, L.; Cecilia, H.; Thorkil, P.; Henrik, G. Regulation and role of hormone-sensitive lipase in rat skeletal muscle. Proc. Nutr. Soc. 2004, 63, 309–314. [Google Scholar]
- Zachary, G.H.; Joseph, T.R.; Olivia, B.; Carles, L.; Seung-Hee, K.; Raul, M.; Frederick, W.A.; Wu, Z.; Pere, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1. EMBO J. 2007, 26, 1913–1923. [Google Scholar]
- Huang, Q.C.; Han, X.Y.; Xu, Z.R.; Yang, X.Y.; Chen, T.; Zheng, X.T. Betaine suppresses carnitine palmitoyl transferase I in skeletal muscle but not in liver of finishing pigs. Livest. Sci. 2009, 126, 130–135. [Google Scholar] [CrossRef]
- Li, J.; Tomonaga, I.; Joseph, S.J.; Cui, T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin. Targets 2009, 13, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Tambi, J.; Anupam, A. Heme oxygenase and renal disease. Curr. Hypertens. Rep. 2009, 11, 56–62. [Google Scholar]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [Green Version]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: The first twenty years (1968–1988). Free Radic. Biol. Med. 1988, 5, 363–369. [Google Scholar] [CrossRef]
- Antje, B.; Stefanie, D.; Dirk, K.; Zhou, Z.; Regina, B. The GI-GPx gene is a target for Nrf2. Mol. Cell. Biol. 2005, 25, 4914–4923. [Google Scholar]
- Li, W.; Kong, A. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2010, 48, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayman, M.M.; Omnia, E.H.; Walaa, G.H.; May, B.J.; Sanaa, M.A.E. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar]
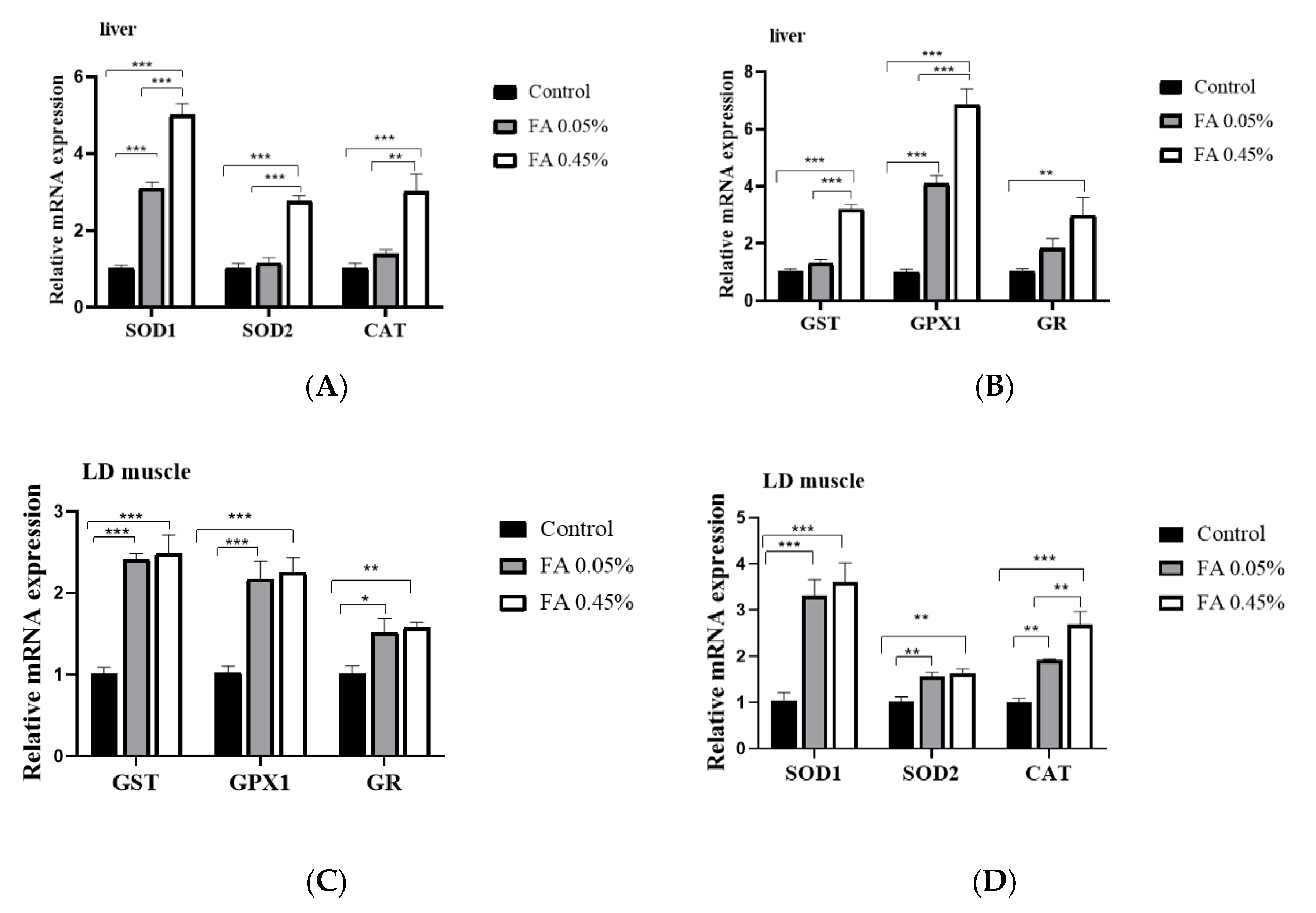

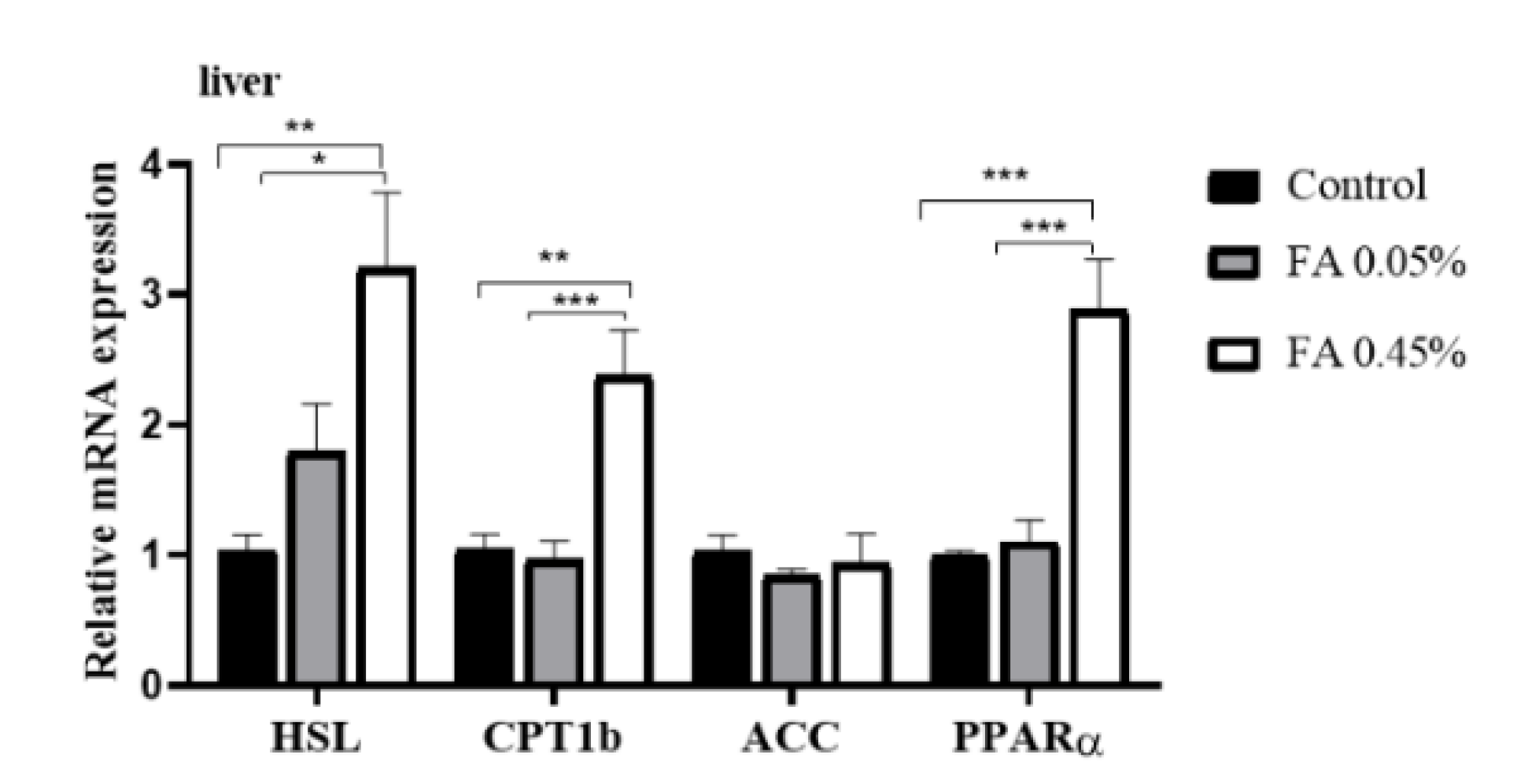
| Items | Control | FA 0.05% | FA 0.45% |
|---|---|---|---|
| Serum | |||
| MDA, nmol/mL | 3.02 ± 0.11 A | 2.31 ± 0.19 B | 2.29 ± 0.11 B |
| CAT, U/mL | 2.64 ± 0.35 A | 4.79 ± 0.60 B | 4.66 ± 0.43 B |
| T-SOD, U/mL | 297.21 ± 2.33 a | 316.03 ± 8.88 ab | 334.77 ± 14.35 b |
| LD muscle | |||
| MDA, nmol/mg prot | 1.99 ± 0.19 A | 1.33 ± 0.07 Bb | 0.82 ± 0.12 Cc |
| CAT, U/mg prot | 3.39 ± 0.25 | 3.52 ± 0.35 | 3.53 ± 0.12 |
| T-SOD, U/mg prot | 30.94 ± 0.93 A | 40.33 ± 0.65 B | 50.42 ± 2.09 C |
| Liver | |||
| MDA, nmol/mg prot | 6.31 ± 0.24 A | 4.39 ± 0.17 B | 3.10 ± 0.23 C |
| CAT, U/mg prot | 12.38 ± 0.04 a | 11.56 ± 0.09 Aa | 13.44 ± 0.47 Bb |
| T-SOD, U/mg prot | 837.33 ± 13.90 A | 916.59 ± 15.00 A | 1121.71 ± 46.57 B |
| GSH-PX, U/mg prot | 66.35 ± 2.13 A | 71.14 ± 1.20 A | 84.92 ± 4.40 B |
| T-AOC, U/mg prot | 1.30 ± 0.04 | 1.31 ± 0.11 | 1.36 ± 0.06 |
| Items | Control | FA 0.05% | FA 0.45% |
|---|---|---|---|
| Serum | |||
| TG, mmol/L | 0.515 ± 0.062 | 0.461 ± 0.052 | 0.504 ± 0.027 |
| T-CHO, mmol/L | 3.865 ± 0.347 Aa | 2.806 ± 0.236 b | 2.703 ± 0.215 B |
| LDL-C, mmol/L | 1.819 ± 0.165 A | 1.288 ± 0.069 B | 1.320 ± 0.070 B |
| HDL-C, mmol/L | 2.623 ± 0.106 A | 4.293 ± 0.171 B | 4.031 ± 0.123 B |
| LD muscle | |||
| TG, mmol/mg prot | 0.121 ± 0.010 | 0.146 ± 0.026 | 0.198 ± 0.041 |
| T-CHO, mmol/mg prot | 0.132 ± 0.017 A | 0.040 ± 0.007 B | 0.046 ± 0.004 B |
| Liver | |||
| TG, mmol/mg prot | 0.149 ± 0.007 a | 0.147 ± 0.006 a | 0.128 ± 0.003 b |
| T-CHO, mmol/mg prot | 0.051 ± 0.006 | 0.047 ± 0.005 | 0.045 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. Dietary Ferulic Acid Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Weaned Piglets. Nutrients 2020, 12, 3811. https://doi.org/10.3390/nu12123811
Wang Y, Chen X, Huang Z, Chen D, Yu B, Yu J, Chen H, He J, Luo Y, Zheng P. Dietary Ferulic Acid Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Weaned Piglets. Nutrients. 2020; 12(12):3811. https://doi.org/10.3390/nu12123811
Chicago/Turabian StyleWang, Youxia, Xiaoling Chen, Zhiqing Huang, Daiwen Chen, Bing Yu, Jie Yu, Hong Chen, Jun He, Yuheng Luo, and Ping Zheng. 2020. "Dietary Ferulic Acid Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Weaned Piglets" Nutrients 12, no. 12: 3811. https://doi.org/10.3390/nu12123811
APA StyleWang, Y., Chen, X., Huang, Z., Chen, D., Yu, B., Yu, J., Chen, H., He, J., Luo, Y., & Zheng, P. (2020). Dietary Ferulic Acid Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Weaned Piglets. Nutrients, 12(12), 3811. https://doi.org/10.3390/nu12123811






