Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Patients
2.2. Diagnosis of Sarcopenia and Frailty
2.3. Clinical and Laboratory Assessments
2.4. Provisional Reclassification Based on the Serum 25-Hydroxyvitamin D Levels
2.5. Statistics
3. Results
3.1. Baseline Characteristics of Patients
3.2. Comparison of Clinical Characteristics among Patients with and without Sarcopenia
3.3. Significant Factors Related to Sarcopenia among Patients with Chronic Liver Disease
3.4. Comparison of Clinical Characteristics among Patients with and without Frailty
3.5. Significant Factors Related to Frailty among Patients with Chronic Liver Disease
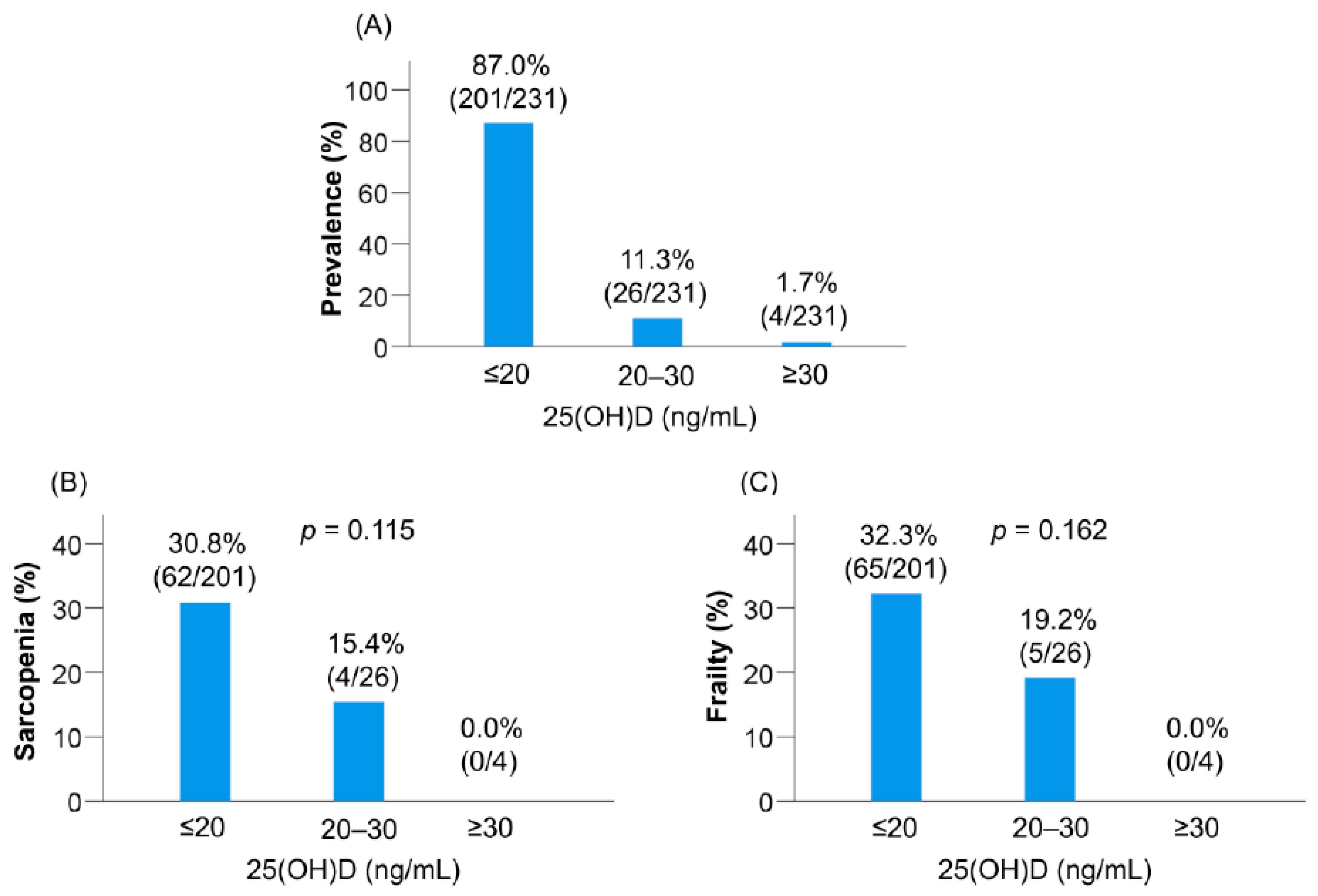
3.6. Prevalence of Sarcopenia and Frailty Defined according to the Conventional Classification of Vitamin D Status
3.7. Clinical Characteristics Based on the Provisional Reclassification of Vitamin D Status
3.8. Correlation between Serum 25(OH)D Levels and Baseline Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 615. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 38, 752–762. [Google Scholar] [CrossRef]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between Osteosarcopenia and Frailty in Patients with Chronic Liver Disease. J. Clin. Med. 2020, 26, 2381. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Moynagh, M.R.; Takahashi, N.; Angirekula, M.; Kennedy, C.C.; Mara, K.C.; Dierkhising, R.A.; Watt, K.D. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transpl. 2019, 25, 14–24. [Google Scholar] [CrossRef]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and Its Impact on Quality of Life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Dig. Dis. Sci. 2010, 55, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Nakchbandi, I.A. Osteoporosis and fractures in liver disease: Relevance, pathogenesis and therapeutic implications. World J. Gastroenterol. 2014, 20, 9427–9438. [Google Scholar] [PubMed]
- Konstantakis, C.; Tselekouni, P.; Kalafateli, M.; Triantos, C. Vitamin D deficiency in patients with liver cirrhosis. Ann. Gastroenterol. 2016, 29, 297–306. [Google Scholar] [CrossRef]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Yoshida, Y.; Arai, T.; Iwashita, A.N.; Itokawa, N.; Kondo, C.; Iwakiri, K. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol. Res. 2020, 50, 588–597. [Google Scholar] [CrossRef]
- Buonomo, A.R.; Scotto, R.; Zappulo, E.; Nerilli, M.; Pinchera, B.; Perruolo, G.; Formisano, P.; Nappa, S.; Gentile, I. Severe Vitamin D Deficiency Increases Mortality Among Patients With Liver Cirrhosis Regardless of the Presence of HCC. In Vivo 2019, 33, 177–182. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Shimada, N.; Yoshizawa, K.; Abe, H.; Asano, T.; Ohkubo, Y.; Araki, M.; Ikegami, T.; Kondo, C.; et al. Influencing factors on serum 25-hydroxyvitamin D3 levels in Japanese chronic hepatitis C patients. BMC Infect. Dis. 2015, 15, 344. [Google Scholar] [CrossRef]
- Aspell, N.; Laird, E.; Healy, M.; Shannon, T.; Lawlor, B.; O’Sullivan, M. The Prevalence and Determinants of Vitamin D Status in Community-Dwelling Older Adults: Results from the English Longitudinal Study of Ageing (ELSA). Nutrients. 2019, 11, 1253. [Google Scholar] [CrossRef]
- Nakamura, K.; Tsugawa, N.; Saito, T.; Ishikawa, M.; Tsuchiya, Y.; Hyodo, K.; Maruyama, K.; Oshiki, R.; Kobayashi, R.; Nashimoto, M.; et al. Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study. Bone 2008, 42, 271–277. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Wagner, D.; Reiberger, T.; Mandorfer, M.; Schwarzer, R.; Ferlitsch, M.; Trauner, M.; Peck-Radosavljevic, M.; Ferlitsch, A. Low 25-OH-vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien. Klin. Wochenschr. 2017, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Kim, M.K.; Baek, K.H.; Song, K.H.; Il Kang, M.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: The Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab. 2011, 96, 3250–3256. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Davis, C.C.; Chaves, P.H.; Hirsch, C.H.; Robbins, J.A.; Arnold, A.M.; Newman, A.B.; Kritchevsky, S.B. Serum 25-hydroxyvitamin D and physical function in older adults: The Cardiovascular Health Study All Stars. J. Am. Geriatr Soc. 2011, 59, 1793–1801. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.; van Loon, L.J.; De Groot, L.C. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur. J. Clin. Nutr. 2013, 67, 1050–1055. [Google Scholar] [CrossRef]
- Wilhelm-Leen, E.R.; Hall, Y.N.; Deboer, I.H.; Chertow, G.M. Vitamin D deficiency and frailty in older Americans. J. Intern. Med. 2010, 268, 171–180. [Google Scholar] [CrossRef]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L.C. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur. J. Nutr. 2019, 58, 1173–1181. [Google Scholar] [CrossRef]
- Wong, Y.Y.; McCaul, K.A.; Yeap, B.B.; Hankey, G.J.; Flicker, L. Low vitamin D status is an independent predictor of increased frailty and all-cause mortality in older men: The Health in Men Study. J. Clin. Endocrinol. Metab. 2013, 98, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.; Crespo, C.J.; Michael, Y.; Ramirez-Marrero, F.A.; Brodowicz, G.R.; Bartlett, S.; Andersen, R.E. The effect of vitamin D and frailty on mortality among non-institutionalized US older adults. Eur. J. Clin. Nutr. 2012, 66, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001; 5, M146–M156. [Google Scholar]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Aging-related anorexia and its association with disability and frailty. J. Cachexia Sarcopenia Muscle 2018, 9, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Pojednic, R.M.; Ceglia, L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Pojednic, R.M.; Ceglia, L.; Olsson, K.; Gustafsson, T.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif. Tissue Int. 2015, 96, 256–263. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Low-dose vitamin D prevents muscular atrophy and reduced falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc. Dis. 2005, 20, 187–192, Retracted in Cerebrovasc. Dis. 2017, 44, 240. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D₃ supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Association between Sarcopenia and Depression in Patients with Chronic Liver Diseases. J. Clin. Med. 2019, 8, 634. [Google Scholar] [CrossRef]
- An, R.; Lu, L. Antidepressant use and functional limitations in U.S. older adults. J. Psychosom. Res. 2016, 80, 31–36. [Google Scholar] [CrossRef]
- Stokes, C.S.; Grünhage, F.; Baus, C.; Volmer, D.A.; Wagenpfeil, S.; Riemenschneider, M.; Lammert, F. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin. Nutr. 2016, 35, 950–957. [Google Scholar] [CrossRef]


| Variable | All Patients | Sarcopenia Group | Non-Sarcopenia Group | p Value |
|---|---|---|---|---|
| Patients, n (%) | 231 | 66 (28.6) | 165 (71.4) | |
| Man, n (%) | 95 (41.1) | 23 (34.8) | 72 (43.6) | 0.220 |
| Age (years) | 70.0 (60.0–76.0) | 76.0 (72.8–80.3) | 68.0 (58.0–73.0) | <0.001 |
| BMI (kg/m2) | 23.1 (20.7–26.0) | 20.5 (19.2–22.6) | 24.2 (21.8–26.6) | <0.001 |
| Liver cirrhosis, n (%) | 98 (42.4) | 40 (60.6) | 58 (35.2) | <0.001 |
| Etiology | ||||
| HBV/HCV/PBC/other, n | 42/90/60/39) | 5/36/19/6 | 37/54/41/33) | 0.002 |
| Total bilirubin (mg/dL) | 0.7 (0.5–0.9) | 0.6 (0.5–0.9) | 0.7 (0.5–0.9) | 0.610 |
| Albumin (g/dL) | 4.1 (3.9–4.4) | 3.9 (3.6–4.3) | 4.2 (3.9–4.4) | 0.003 |
| Prothrombin time INR | 1.02 (0.96–1.11) | 1.05 (0.96–1.13) | 1.02 (0.96–1.10) | 0.130 |
| BCAA (μmol/L) | 408 (357–475) | 364 (302–425) | 432 (381–488) | <0.001 |
| 25(OH)D (ng/mL) | 14.0 (10.5–18.1) | 12.2 (8.8–15.7) | 14.7 (11.4–18.4) | 0.001 |
| Vitamin D insufficiency, n (%) | 26 (11.3) | 4 (6.1) | 22 (13.3) | 0.114 |
| Vitamin D deficiency, n (%) | 201 (87.0) | 62 (93.9) | 139 (84.2) | 0.048 |
| SMI (kg/m2) | ||||
| All patients | 6.30 (5.54–7.12) | 5.20 (4.82–5.71) | 6.75 (5.98–7.44) | <0.001 |
| Man | 7.13 (6.48–7.87) | 6.18 (5.49–6.84) | 7.49 (7.05–8.15) | <0.001 |
| Woman | 5.86 (5.20–6.44) | 5.09 (4.67–5.30) | 6.05 (5.83–6.59) | <0.001 |
| Handgrip strength (kg) | ||||
| All patients | 22.1 (17.4–29.4) | 16.8 (13.8–18.4) | 24.5 (19.9–32.6) | <0.001 |
| Man | 30.5 (24.1–37.5) | 22.8 (18.3–24.2) | 33.4 (28.5–38.9) | <0.001 |
| Woman | 18.8 (15.1–22.5) | 15.0 (12.8–17.2) | 21.3 (18.5–24.0) | <0.001 |
| Gait speed (m/s) | 1.10 (0.91–1.25) | 0.90 (0.68–1.07) | 1.15 (1.02–1.28) | <0.001 |
| Slow gait speed, n (%) | 81 (35.1) | 49 (74.2) | 32 (19.4) | <0.001 |
| Frailty, n (%) | 70 (30.3) | 51 (77.3) | 19 (11.5) | <0.001 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p Value | |
| Age (years) | 1.083 (1.047–1.121) | <0.001 | 1.087 (1.044–1.130) | <0.001 |
| BMI (kg/m2) | 0.726 (0.649–0.813) | <0.001 | 0.720 (0.635–0.817) | <0.001 |
| Liver cirrhosis | 2.838 (1.576–5.111) | 0.001 | 2.493 (1.180–5.266) | 0.017 |
| Albumin (g/dL) | 0.334 (0.184–0.605) | <0.001 | ||
| Prothrombin time INR | 11.004 (1.104–109.651) | 0.041 | ||
| BCAA (μmol/L) | 0.992 (0.988–0.995) | <0.001 | ||
| 25(OH)D (ng/mL) | 0.896 (0.841–0.953) | 0.001 | 0.863 (0.794–0.937) | <0.001 |
| Vitamin D deficiency | 2.899 (0.971–8.661) | 0.057 | ||
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p Value | |
| Age (years) | 1.091 (1.054–1.129) | <0.001 | 1.094 (1.052–1.138) | <0.001 |
| BMI (kg/m2) | 0.869 (0.801–0.943) | 0.001 | ||
| Liver cirrhosis | 4.844 (2.645–8.870) | <0.001 | 3.749 (1.843–7.626) | <0.001 |
| Albumin (g/dL) | 0.292 (0.159–0.534) | <0.001 | ||
| Prothrombin time INR | 18.162 (1.821–181.168) | 0.013 | ||
| BCAA (μmol/L) | 0.991 (0.988–0.995) | <0.001 | 0.994 (0.990–0.998) | 0.008 |
| 25(OH)D (ng/mL) | 0.893 (0.840–0.950) | 0.001 | 0.887 (0.822–0.957) | 0.002 |
| Vitamin D deficiency | 2.390 (0.875–6.526) | 0.089 | ||
| Variable | L-VD | M-VD | H-VD | p Value |
|---|---|---|---|---|
| Patients, n (%) | 57 (24.7) | 115 (49.8) | 59 (25.5) | |
| Man, n (%) | 20 (35.1) | 41 (35.7) | 34 (57.6) | 0.012 |
| Age (years) | 68.0 (55.0–77.0) | 70.0 (61.0–76.0) | 72.0 (64.0–77.0) | 0.317 |
| BMI (kg/m2) | 22.2 (20.2–26.0) | 22.8 (20.4–26.1) | 23.6 (22.3–25.8) | 0.489 |
| Liver cirrhosis, n (%) | 30 (52.6) | 48 (41.7) | 20 (33.9) | 0.122 |
| Etiology | ||||
| HBV/HCV/PBC/other, n | 8/21/15/13 | 20/50/27/18 | 14/19/18/8 | 0.499 |
| Total bilirubin (mg/dL) | 0.7 (0.5–1.3) | 0.6 (0.5–0.8) | 0.7 (0.5–0.9) | 0.238 |
| Albumin (g/dL) | 4.1 (3.7–4.5) | 4.1 (3.8–4.3) | 4.2 (4.0–4.4) | 0.209 |
| Prothrombin time INR | 1.06 (0.96–1.16) | 1.01 (0.95–1.11) | 1.00 (0.97–1.07) | 0.139 |
| BCAA (µmol/L) | 382 (312–432) | 420 (364–478) | 438 (379–501) | <0.001 |
| 25(OH)D (ng/mL) | 8.4 (6.9–9.7) | 14.0 (12.4–15.9) | 20.1 (18.7–23.5) | <0.001 |
| SMI (kg/m2) | ||||
| ALL patients | 5.87 (5.08–6.96) | 6.16 (5.66–6.95) | 7.04 (5.97–7.43) | 0.002 |
| Man | 7.07 (6.44–8.27) | 6.98 (6.17–7.76) | 7.23 (7.01–8.11) | 0.152 |
| Woman | 5.39 (4.87–5.94) | 5.97 (5.44–6.49) | 5.90 (5.16–6.70) | 0.014 |
| Handgrip strength (kg) | ||||
| ALL patients | 18.6 (14.8–25.1) | 22.1 (17.8–27.1) | 26.9 (20.6–33.2) | <0.001 |
| Man | 26.3 (18.3–33.5) | 30.2 (23.9–37.0) | 32.5 (27.0–38.9) | 0.036 |
| Woman | 17.4 (14.1–19.9) | 19.1 (16.5–22.5) | 19.4 (16.7–24.0) | 0.082 |
| Sarcopenia, n (%) | 28 (49.1) | 27 (23.5) | 11 (18.6) | <0.001 |
| Gait speed (m/s) | 1.00 (0.69–1.16) | 1.11 (0.92–1.25) | 1.17 (1.00–1.38) | 0.003 |
| Slow gait speed, n (%) | 29 (50.9) | 38 (33.0) | 14 (23.7) | 0.007 |
| Frailty, n (%) | 28 (49.1) | 33 (28.7) | 9 (15.3) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Saruta, M.; Tsubota, A. Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease. Nutrients 2020, 12, 3810. https://doi.org/10.3390/nu12123810
Saeki C, Kanai T, Nakano M, Oikawa T, Torisu Y, Saruta M, Tsubota A. Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease. Nutrients. 2020; 12(12):3810. https://doi.org/10.3390/nu12123810
Chicago/Turabian StyleSaeki, Chisato, Tomoya Kanai, Masanori Nakano, Tsunekazu Oikawa, Yuichi Torisu, Masayuki Saruta, and Akihito Tsubota. 2020. "Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease" Nutrients 12, no. 12: 3810. https://doi.org/10.3390/nu12123810
APA StyleSaeki, C., Kanai, T., Nakano, M., Oikawa, T., Torisu, Y., Saruta, M., & Tsubota, A. (2020). Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease. Nutrients, 12(12), 3810. https://doi.org/10.3390/nu12123810






