The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity
Abstract
:1. Introduction
2. Materials and Methods
3. Participants
4. Magnetic Resonance Imaging
5. Biochemical Measurements
6. Statistics
7. Results
7.1. Characteristics
7.2. Regional Adipose Tissue Deposition
7.3. Inflammatory Markers
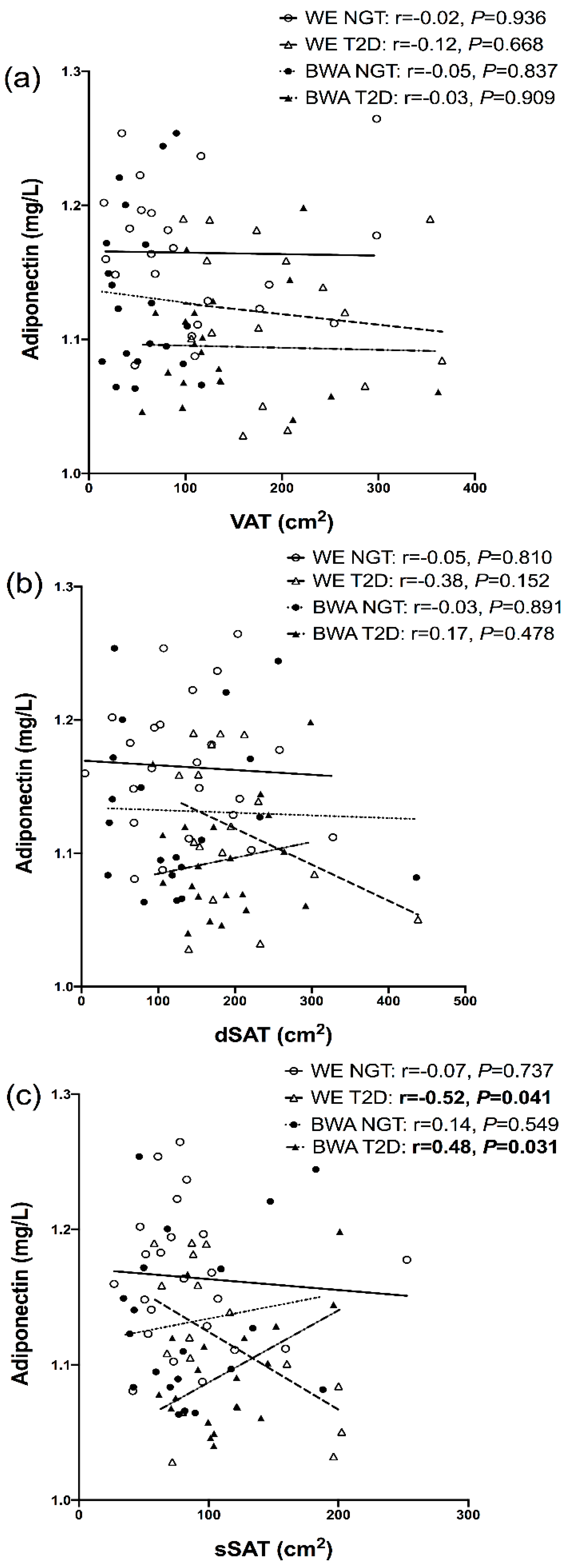
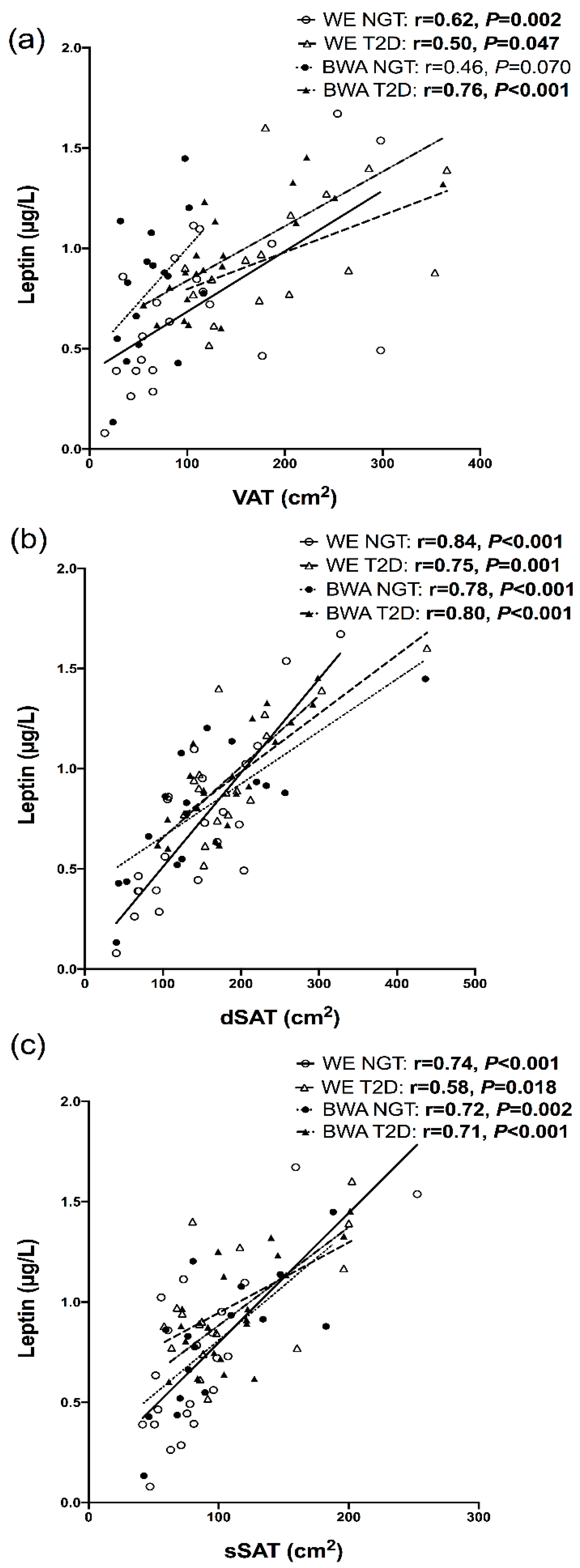
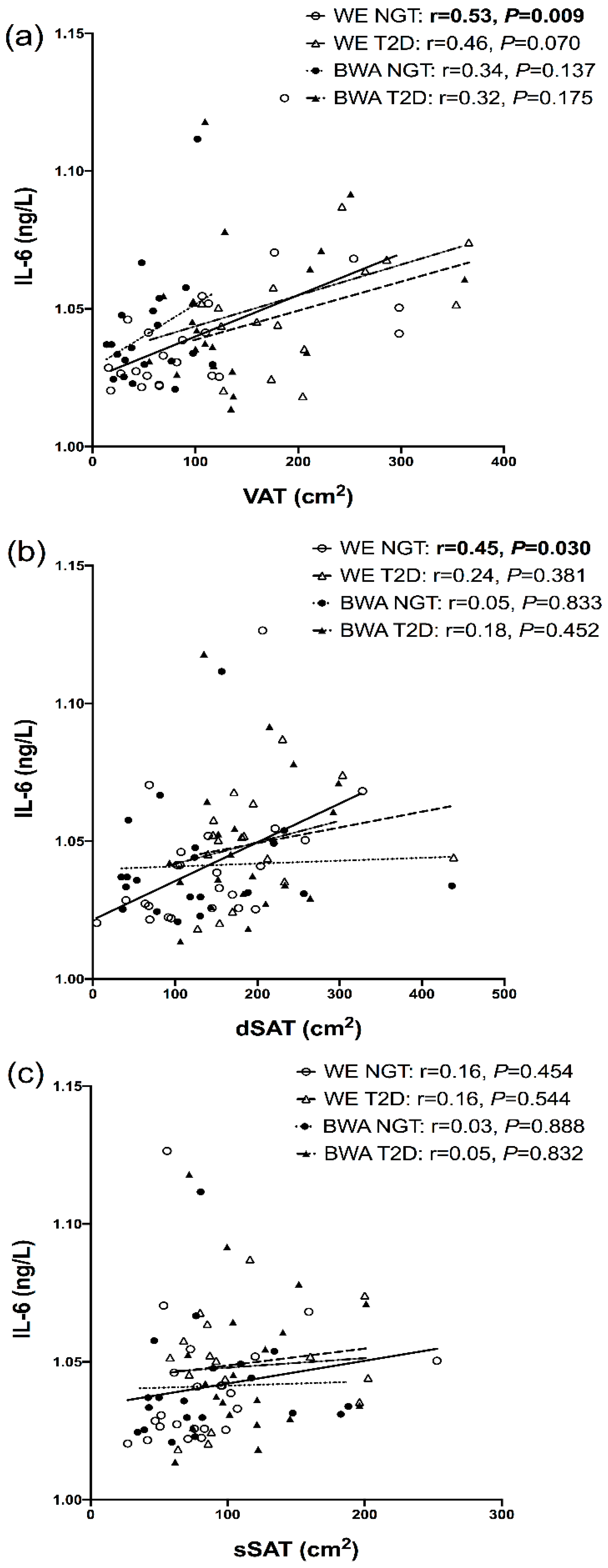
7.4. Relationships between Inflammatory Markers and Regional Adipose Depots
8. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BWA | black West African |
| BMI | body mass index |
| CV | coefficient of variation |
| dSAT | deep subcutaneous adipose tissue |
| HbA1c | glycated haemoglobin |
| IFN | Interferon |
| IL | Interleukin |
| MRI | magnetic resonance imaging |
| SAT | subcutaneous adipose tissue |
| sSAT | superficial subcutaneous adipose tissue |
| T2D | type 2 diabetes |
| TNF | tumour necrosis factor |
| VEGF | vascular endothelial growth factor |
| VAT | visceral adipose tissue |
| WE | white European |
| ASAT | abdominal subcutaneous adipose tissue |
| BP | blood pressure |
| dSAT | deep subcutaneous adipose tissue |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| NGT | normal glucose tolerance |
References
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.M. Ethnicity and Type 2 diabetes in the UK. Diabet. Med. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWilliams, J.M.; Meara, E.; Zaslavsky, A.M.; Ayanian, J.Z. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann. Intern. Med. 2009, 150, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.J.; Braun, W.; Enderle, J.; Bosy-Westphal, A. Beyond BMI: Conceptual Issues Related to Overweight and Obese Patients. Obes. Facts 2016, 9, 193–205. [Google Scholar] [CrossRef]
- Smith, J.D.; Borel, A.L.; Nazare, J.A.; Haffner, S.M.; Balkau, B.; Ross, R.; Massien, C.; Almeras, N.; Despres, J.P. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: Results from the INSPIRE ME IAA study. J. Clin. Endocrinol. Metab. 2012, 97, 1517–1525. [Google Scholar] [CrossRef] [Green Version]
- Despres, J.P. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993, 9, 452–459. [Google Scholar]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Sniderman, A.D.; Bhopal, R.; Prabhakaran, D.; Sarrafzadegan, N.; Tchernof, A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int. J. Epidemiol. 2007, 36. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.E.; Thaete, F.L.; Troost, F.; Huwe, T.; Goodpaster, B.H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E941–E948. [Google Scholar] [CrossRef]
- Fujioka, S.; Matsuzawa, Y.; Tokunaga, K.; Tarui, S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metab. Clin. Exp. 1987, 36, 54–59. [Google Scholar] [CrossRef]
- Marinou, K.; Hodson, L.; Vasan, S.K.; Fielding, B.A.; Banerjee, R.; Brismar, K.; Koutsilieris, M.; Clark, A.; Neville, M.J.; Karpe, F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014, 37, 821–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markman, B.; Barton, F.E., Jr. Anatomy of the subcutaneous tissue of the trunk and lower extremity. Plast. Reconstr. Surg. 1987, 80, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Monzon, J.R.; Basile, R.; Heneghan, S.; Udupi, V.; Green, A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes. Res. 2002, 10, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, B.; Chiefari, E.; Foryst-Ludwig, A.; Currò, G.; Navarra, G.; Brunetti, F.S.; Mirabelli, M.; Corigliano, D.M.; Kintscher, U.; Britti, D.; et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine 2020, 59, 102912. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.H.; Song, S.W.; Jung, W.S.; Lee, Y.A.; Kim, H.N. Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: A case control study. Diabetol. Metab. Syndr. 2016, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Zulian, A.; Gentilini, D.; Maestrini, S.; Della Barba, A.; Invitti, C.; Corà, D.; Caselle, M.; Liuzzi, A.; Di Blasio, A.M. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity 2013, 21, 2562–2570. [Google Scholar] [CrossRef]
- Carroll, J.F.; Chiapa, A.L.; Rodriquez, M.; Phelps, D.R.; Cardarelli, K.M.; Vishwanatha, J.K.; Bae, S.; Cardarelli, R. Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity 2008, 16, 600–607. [Google Scholar] [CrossRef]
- Goff, L.M.; Ladwa, M.; Hakim, O.; Bello, O. Ethnic distinctions in the pathophysiology of type 2 diabetes: A focus on black African-Caribbean populations. Proc. Nutr. Soc. 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.F.; Fulda, K.G.; Chiapa, A.L.; Rodriquez, M.; Phelps, D.R.; Cardarelli, K.M.; Vishwanatha, J.K.; Cardarelli, R. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity 2009, 17, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Hyatt, T.C.; Hunter, G.R.; Oster, R.A.; Desmond, R.A.; Gower, B.A. Markers of inflammation and fat distribution following weight loss in African-American and white women. Obesity 2012, 20, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, L. Soul-Deep: The South London Diabetes and Ethnicity Phenotyping Study Protocol; King’s College London: London, UK, 2013. [Google Scholar]
- WHO. Screening for Type 2 Diabetes: Report of a World Health Organization and International Diabetes Federation Meeting; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Alderete, T.; Toledo-Corral, C.; Goran, M. Metabolic Basis of Ethnic Differences in Diabetes Risk in Overweight and Obese Youth. Curr. Diabetes Rep. 2014, 14, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedecke, J.H.; Mtintsilana, A.; Dlamini, S.N.; Kengne, A.P. Type 2 diabetes mellitus in African women. Diabetes Res. Clin. Pract. 2017, 123, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.; Dufour, S.; Zern, T.L.; Taksali, S.; Calí, A.M.G.; Dziura, J.; Shulman, G.I.; Pierpont, B.M.; Caprio, S. Interethnic Differences in Muscle, Liver and Abdominal Fat Partitioning in Obese Adolescents. PLoS ONE 2007, 2, e569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.; Goedecke, J.H.; Soderstrom, I.; Buren, J.; Alvehus, M.; Blomquist, C.; Jonsson, F.; Hayes, P.M.; Adams, K.; Dave, J.A.; et al. Depot- and ethnic-specific differences in the relationship between adipose tissue inflammation and insulin sensitivity. Clin. Endocrinol. 2011, 74, 51–59. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Evans, J.; Keswell, D.; Stimson, R.H.; Livingstone, D.E.; Hayes, P.; Adams, K.; Dave, J.A.; Victor, H.; Levitt, N.S.; et al. Reduced gluteal expression of adipogenic and lipogenic genes in Black South African women is associated with obesity-related insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E2029–E2033. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.D.; Borel, A.L.; Haffner, S.M.; Balkau, B.; Ross, R.; Massien, C.; Almeras, N.; Despres, J.P. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship with Cardiometabolic Risk/Intra-Abdominal Adiposity. Am. J. Clin. Nutr. 2012, 96, 714–726. [Google Scholar] [CrossRef] [Green Version]
- Lovejoy, J.C.; Smith, S.R.; Rood, J.C. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes. Res. 2001, 9, 10–16. [Google Scholar] [CrossRef]
- Bódis, K.; Jelenik, T.; Lundbom, J.; Markgraf, D.F.; Strom, A.; Zaharia, O.-P.; Karusheva, Y.; Burkart, V.; Müssig, K.; Kupriyanova, Y.; et al. Expansion and Impaired Mitochondrial Efficiency of Deep Subcutaneous Adipose Tissue in Recent-Onset Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, e1331–e1343. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, J.; Divoux, A.; Prifti, E.; Poitou, C.; Pelloux, V.; Hugol, D.; Basdevant, A.; Bouillot, J.L.; Chevallier, J.M.; Bedossa, P.; et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: Relation with liver histopathology in morbid obesity. J. Hepatol. 2012, 56, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Saad, R.; Gungor, N.; Arslanian, S.A. Does adiponectin explain the lower insulin sensitivity and hyperinsulinemia of African-American children? Pediatric Diabetes 2005, 6, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Degawa-Yamauchi, M.; Dilts, J.R.; Bovenkerk, J.E.; Saha, C.; Pratt, J.H.; Considine, R.V. Lower serum adiponectin levels in African-American boys. Obes. Res. 2003, 11, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, T.C.; Phadke, R.P.; Hunter, G.R.; Bush, N.C.; Muñoz, A.J.; Gower, B.A. Insulin sensitivity in African-American and white women: Association with inflammation. Obesity 2009, 17, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Conroy, S.M.; Ollberding, N.J.; Kim, Y.; Lim, U.; Cooney, R.V.; Franke, A.A.; Wilkens, L.R.; Hernandez, B.Y.; Goodman, M.T.; et al. Ethnic differences in serum adipokine and C-reactive protein levels: The multiethnic cohort. Int. J. Obes. 2014, 38, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin Levels and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Bello, O.; Ladwa, M.; Hakim, O.; Marathe, C.; Shoajee-Moradie, F.; Charles-Edwards, G.; Peacock, J.L.; Umpleby, M.A.; Amiel, S.; Goff, L. Differences in the link between insulin sensitivity and ectopic fat in men of Black African and White European ethnicity. Eur. J. Endocrinol. 2019, 182, 91–101. [Google Scholar] [CrossRef]
- Hakim, O.; Bello, O.; Bonadonna, R.C.; Mohandas, C.; Shojaee-Moradie, F.; Jackson, N.; Boselli, L.; Whitcher, B.; Shuaib, H.; Alberti, K.; et al. Ethnic differences in intrahepatic lipid and its association with hepatic insulin sensitivity and insulin clearance between men of black and white ethnicity with early type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 2163–2168. [Google Scholar] [CrossRef]
- Hakim, O.; Bello, O.; Ladwa, M.; Christodoulou, D.; Bulut, E.; Shuaib, H.; Peacock, J.L.; Umpleby, A.M.; Charles-Edwards, G.; Amiel, S.A.; et al. Ethnic differences in hepatic, pancreatic, muscular and visceral fat deposition in healthy men of white European and black west African ethnicity. Diabetes Res. Clin. Pract. 2019, 156, 107866. [Google Scholar] [CrossRef]
- Bays, H.E.; Gonzalez-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef] [Green Version]
- Bluher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. 2016, 130, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Ladwa, M.; Bello, O.; Hakim, O.; Shojaee-Moradie, F.; Boselli, L.; Charles-Edwards, G.; Stadler, M.; Peacock, J.L.; Umpleby, A.M.; Amiel, S.A.; et al. Insulin clearance as the major player in the hyperinsulinaemia of black African men without diabetes. Diabetes Obes. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Piccinini, F.; Kabir, M.; Ader, M. Novel aspects of the role of the liver in carbohydrate metabolism. Metab. Clin. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Shen, W.; Lee, M.; Choh, A.C.; Czerwinski, S.A.; Siervogel, R.M.; Towne, B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am. J. Clin. Nutr. 2007, 85, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, D.; Kelley, D.E.; Yim, J.E.; Spence, N.; Albu, J.; Boxt, L.; Pi-Sunyer, F.X.; Heshka, S. Adipose tissue distribution is different in type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorello, L.B.; Schlundt, D.G.; Cohen, S.S.; Steinwandel, M.D.; Buchowski, M.S.; McLaughlin, J.K.; Hargreaves, M.K.; Blot, W.J. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am. J. Public Health 2007, 97, 2260–2267. [Google Scholar] [CrossRef]
- Brancati, F.L.; Kao, W.H.; Folsom, A.R.; Watson, R.L.; Szklo, M. Incident type 2 diabetes mellitus in African American and white adults: The Atherosclerosis Risk in Communities Study. JAMA 2000, 283, 2253–2259. [Google Scholar] [CrossRef]
| NGT | T2D | ||||||
|---|---|---|---|---|---|---|---|
| WE (n = 23) | BWA (n = 23) | WE (n = 19) | BWA (n = 20) | P eth | P gly | P eth*gly | |
| Age (years) * | 33.5 (28.4–39.5) | 28.8 (24.6–33.7) | 55.7 (52.3–59.3) | 53.8 (50.2–57.8) | 0.15 | <0.001 | 0.36 |
| Weight (kg) | 86.5 ± 16.5 | 84.1 ± 14.6 | 100.6 ± 16.7 | 92.4 ± 11.8 | 0.11 | 0.001 | 0.38 |
| BMI (kg/m2) | 26.5 ± 4.5 | 26.7 ± 3.6 | 31.9 ± 4.3 | 30.0 ± 3.5 | 0.34 | <0.001 | 0.24 |
| Waist circumference (cm) | 93.8 ± 14.6 | 87.5 ± 9.2 | 112.3 ± 12.7 | 104.9 ± 9.7 | 0.009 | <0.001 | 0.83 |
| HbA1c (%) | 5.44 ± 0.24 | 5.54 ± 0.48 | 6.68 ± 0.74 | 6.76 ± 0.70 | 0.46 | <0.001 | 0.94 |
| HbA1c (mmol/mol) | 35.9 ± 2.9 | 37.0 ± 5.27 | 49.5 ± 8.19 | 50.4 ± 7.80 | 0.45 | <0.001 | 0.94 |
| Fasting plasma glucose (mmol/l) | 5.20 ± 0.39 | 5.13 ± 0.44 | 6.95 ± 1.35 | 6.71 ± 0.96 | 0.39 | <0.001 | 0.63 |
| Systolic BP (mm Hg) | 121.9 ± 9.1 | 123.1 ± 12.3 | 132.1 ± 13.0 | 137.5 ± 13.7 | 0.21 | <0.001 | 0.42 |
| Diastolic BP (mm Hg) | 71.1 ± 8.2 | 70.7 ± 11.5 | 82.6 ± 9.1 | 86.1 ± 7.4 | 0.45 | <0.001 | 0.34 |
| Total cholesterol (mmol/l) | 4.76 ± 1.1 | 4.27 ± 1.1 | 4.41 ± 0.83 | 4.09 ± 0.70 | 0.051 | 0.19 | 0.68 |
| LDL cholesterol (mmol/l) | 2.99 ± 0.82 | 2.66 ± 0.87 | 2.34 ± 0.69 | 2.32 ± 0.53 | 0.27 | 0.003 | 0.35 |
| HDL cholesterol (mmol/l) | 1.27 ± 0.31 | 1.30 ± 0.42 | 1.22 ± 0.25 | 1.18 ± 0.37 | 0.97 | 0.23 | 0.62 |
| Triglyceride (mmol/l) * | 1.09 (0.86–1.33) | 0.68 (0.57–0.79) | 1.83 (1.41–2.26) | 1.27 (0.94–1.61) | <0.001 | <0.001 | 0.66 |
| Regional adipose tissue | |||||||
| VAT (cm2) *,a | 79.0 (55–112) | 46.1 (34–61) | 184.4 (148–229) | 128.1 (103–159) | 0.002 | <0.001 | 0.54 |
| ASAT (cm2) *,a | 193.2 (149–249) | 181.9 (136–243) | 291.0 (243–348) | 285.6 (247–330) | 0.73 | <0.001 | 0.85 |
| VAT:ASAT *,a | 0.46 (0.34–0.59) | 0.30 (0.20–0.39) | 0.70 (0.52–0.87) | 0.48 (0.39–0.58) | 0.002 | <0.001 | 0.70 |
| dSAT (cm2) *,a | 109.8 (76–159) | 102.6 (73–145) | 188.6 (159–223) | 175.3 (150–205) | 0.63 | <0.001 | 0.99 |
| sSAT (cm2) *,a | 75.5 (62–93) | 76.6 (60–97) | 100.5 (81–125) | 108.8 (93–127) | 0.64 | 0.002 | 0.74 |
| dSAT:sSAT a | 1.68 ± 0.84 | 1.42 ± 0.49 | 1.93 ± 0.47 | 1.65 ± 0.35 | 0.047 | 0.075 | 0.92 |
| Inflammatory markers | |||||||
| Adiponectin (mg/L) * | 4.61 (3.89–5.37) | 3.41 (2.64–4.22) | 3.33 (2.46–4.26) | 2.44 (1.88–3.02) | 0.005 | 0.003 | 0.76 |
| Leptin (µg/L) *,b | 5.19 (3.44–7.83) | 6.55 (4.42–9.70) | 10.9 (7.7–15.5) | 9.0 (6.8–12.0) | 0.92 | 0.003 | 0.23 |
| IL-6 (ng/L) * | 0.99 (0.73–1.25) | 0.95 (0.73–1.17) | 1.32 (1.05–1.59) | 1.18 (0.87–1.50) | 0.49 | 0.030 | 0.71 |
| TNF-α (ng/L) c | 2.48 ± 0.54 | 2.39 ± 0.75 | 3.03 ± 0.80 | 2.49 ± 0.55 | 0.034 | 0.033 | 0.13 |
| IL-10 (ng/L) *,d | 0.57 (0.44–0.70) | 0.75 (0.62–0.88) | 0.51 (0.41–0.61) | 0.63 (0.49–0.80) | 0.019 | 0.16 | 0.60 |
| Resistin (µg/L) *,e | 4.07 (3.57–4.63) | 4.38 (3.68–5.21) | 3.51 (3.00–4.11) | 3.81 (3.13–4.64) | 0.32 | 0.073 | 0.95 |
| IFN-γ (ng/L) *,f | 4.29 (3.62–5.07) | 5.03 (4.04–6.26) | 5.88 (4.7–7.3) | 4.08 (2.6–6.4) | 0.38 | 0.65 | 0.027 |
| IL-8 (ng/L) g | 8.44 ± 3.62 | 8.15 ± 2.16 | 9.86 ± 4.50 | 11.0 ± 3.96 | 0.59 | 0.008 | 0.37 |
| VEGF (ng/L) * | 51.3 (37.6–70.0) | 60.1 (43.4–83.2) | 83.2 (62.5–110.7) | 90.7 (65.5–125.5) | 0.43 | 0.004 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, O.; Bello, O.; Ladwa, M.; Peacock, J.L.; Umpleby, A.M.; Charles-Edwards, G.; Amiel, S.A.; Goff, L.M. The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity. Nutrients 2020, 12, 3796. https://doi.org/10.3390/nu12123796
Hakim O, Bello O, Ladwa M, Peacock JL, Umpleby AM, Charles-Edwards G, Amiel SA, Goff LM. The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity. Nutrients. 2020; 12(12):3796. https://doi.org/10.3390/nu12123796
Chicago/Turabian StyleHakim, Olah, Oluwatoyosi Bello, Meera Ladwa, Janet L. Peacock, A. Margot Umpleby, Geoffrey Charles-Edwards, Stephanie A. Amiel, and Louise M. Goff. 2020. "The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity" Nutrients 12, no. 12: 3796. https://doi.org/10.3390/nu12123796
APA StyleHakim, O., Bello, O., Ladwa, M., Peacock, J. L., Umpleby, A. M., Charles-Edwards, G., Amiel, S. A., & Goff, L. M. (2020). The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity. Nutrients, 12(12), 3796. https://doi.org/10.3390/nu12123796





