Iron Metabolism: Interactions with Energy and Carbohydrate Availability
Abstract
1. Introduction
2. Why Are Adequate Iron Stores Necessary for Athletes?
3. Hepcidin and Iron Regulation
4. Carbohydrate Availability and Iron Regulation
4.1. Post-Exercise Carbohydrate Intake
4.2. Carbohydrate Feeding during Exercise
4.3. Implications of Acute Carbohydrate Restriction
4.4. Implications of Long Term Carbohydrate Manipulation
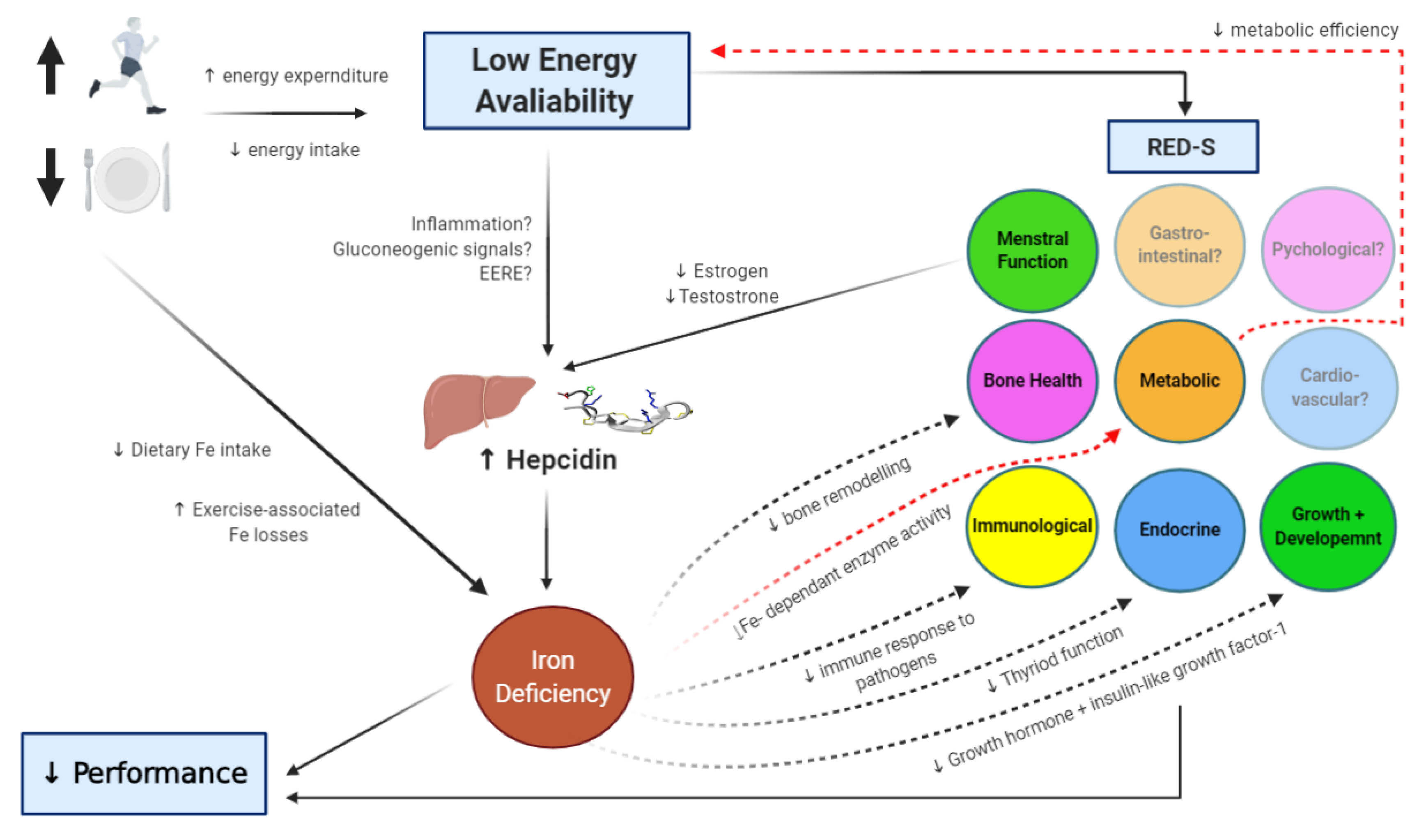
5. Energy Availability and Iron Regulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loucks, A.B. Energy balance and body composition in sports and exercise. J. Sports Sci. 2004, 22, 1–14. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; Mc Donnell, S.J.; Corish, C.A. Low energy availability in athletes: A review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED- S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) consensus statement on Relative Energy Deficiency in Sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Barrack, M.T.; Nattiv, A.; Fredericson, M. Parallels with the female athlete triad in male athletes. Sports Med. 2016, 46, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Jeukendrup, A.; Morton, J.P.; Stellingwerff, T.; Maughan, R.J. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 451–463. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Sharma, A.P.; Heikura, I.A.; Forbes, S.F.; Holloway, M.; McKay, A.K.A.; Bone, J.L.; Leckey, J.J.; Welvaert, M.; Ross, M.L. Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS ONE 2020, 15, e0234027. [Google Scholar]
- Burke, L.M.; Whitfield, J.; Heikura, I.A.; Ross, M.L.R.; Tee, N.; Forbes, S.F.; Hall, R.; McKay, A.K.A.; Wallett, A.M.; Sharma, A.P. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J. Physiol. 2020. [Google Scholar] [CrossRef]
- Hawley, J.A.; Leckey, J.J. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015, 45 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]
- Burke, L.M. Ketogenic low CHO, high fat diet: The future of elite endurance sport? J. Physiol. 2020. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Holtzman, B.; Cooper, K.M.; Flynn, E.F.; Bruinvels, G.; Tenforde, A.S.; Popp, K.L.; Simpkin, A.J.; Parziale, A.L. Low energy availability surrogates correlate with health and performance consequences of Relative Energy Deficiency in Sport. Br. J. Sports Med. 2019, 53, 628–633. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131 (Suppl. S2), 568S–580S. [Google Scholar] [CrossRef]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594S–597S. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Myhre, K.E.; Webber, B.J.; Cropper, T.L.; Tchandja, J.N.; Ahrendt, D.M.; Dillon, C.A.; Haas, R.W.; Guy, S.L.; Pawlak, M.T.; Federinko, S.P. Prevalence and impact of anemia on basic trainees in the US air force. Sports Med. Open 2016, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, B.; Weller, E.; Mairbaurl, H.; Bartsch, P. Effects of iron repletion on blood volume and performance capacity in young athletes. Med. Sci. Sports Exerc. 2001, 33, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Giordano, C.; Brownlie, T.; Haas, J.D. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J. Appl. Physiol. 2000, 88, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Sinclair, L.M. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur. J. Clin. Nutr. 2007, 61, 30–39. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Haas, J.D. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215. [Google Scholar] [CrossRef]
- Peeling, P.; Blee, T.; Goodman, C.; Dawson, B.; Claydon, G.; Beilby, J.; Prins, A. Effect of iron injections on aerobic-exercise performance of iron-depleted female athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 221–231. [Google Scholar] [CrossRef]
- Clénin, G.; Cordes, M.; Huber, A.; Schumacher, Y.O.; Noack, P.; Scales, J.; Kriemler, S. Iron deficiency in sports-definition, influence on performance and therapy. Swiss Med. Wkly. 2015, 145, w14196. [Google Scholar] [CrossRef]
- Burden, R.J.; Pollock, N.; Whyte, G.P.; Richards, T.; Moore, B.; Busbridge, M.; Srai, S.K.; Otto, J.; Pedlar, C.R. Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Med. Sci. Sports Exerc. 2015, 47, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T. Iron deficiency in athletes: An update. Am. J. Lifestyle Med. 2012, 6, 319–327. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Gillen, Z.M.; McKay, B.D.; Koehler, K.; Cramer, J.T. High prevalence of poor iron status among 8-to 16-year-old youth athletes: Interactions among biomarkers of iron, dietary intakes, and biological maturity. J. Am. Coll. Nutr. 2020, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.P. Iron status and the female athlete. J. Trace Elem. Med. Biol. 2012, 26, 124–126. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Hintze, K.J.; McClung, J.P. Hepcidin: A critical regulator of iron metabolism during hypoxia. Adv. Hematol. 2011, 2011, 510304. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.; Pickkers, P.; Nemeth, E.; van der Hoeven, H.; Swinkels, D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 2005, 106, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Effects of exercise on hepcidin response and iron metabolism during recovery. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 583–597. [Google Scholar] [CrossRef]
- Peeling, P.; McKay, A.K.A.; Pyne, D.B.; Guelfi, K.J.; McCormick, R.H.; Laarakkers, C.M.; Swinkels, D.W.; Garvican-Lewis, L.A.; Ross, M.L.R.; Sharma, A.P.; et al. Factors influencing the post-exercise hepcidin-25 response in elite athletes. Eur. J. Appl. Physiol. 2017, 117, 1233–1239. [Google Scholar] [CrossRef]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef]
- Li, T.L.; Gleeson, M. The effects of carbohydrate supplementation during the second of two prolonged cycling bouts on immunoendocrine responses. Eur. J. Appl. Physiol. 2005, 95, 391–399. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur. J. Appl. Physiol. 2015, 115, 2521–2530. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the academy of nutrition and dietetics, dietitians of Canada, and the American college of sports medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.C.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Timing of post-exercise carbohydrate ingestion: Influence on IL-6 and hepcidin responses. Eur. J. Appl. Physiol. 2015, 115, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar] [PubMed]
- Dahlquist, D.T.; Stellingwerff, T.; Dieter, B.P.; McKenzie, D.C.; Koehle, M.S. Effects of macro- and micronutrients on exercise-induced hepcidin response in highly trained endurance athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Robson-Ansley, P.; Walshe, I.; Ward, D. The effect of carbohydrate ingestion on plasma interleukin-6, hepcidin and iron concentrations following prolonged exercise. Cytokine 2011, 53, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Townsend, M.A.; Trinder, D.; Peeling, P. The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur. J. Appl. Physiol. 2012, 112, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Sherman, W.M.; Fink, W.J.; Maresh, C.; Witten, M.; Miller, J.M. The role of dietary carbohydrates in muscle glycogen resynthesis after strenuous running. Am. J. Clin. Nutr. 1981, 34, 1831–1836. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Burke, L.M. Self-reported periodization of nutrition in elite female and male runners and race walkers. Front. Physiol. 2018, 9, 1732. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized nutrition for athletes. Sports Med. 2017, 47 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Mckay, A.K.A.; Heikura, I.A.; Burke, L.M.; Peeling, P.; Pyne, D.B.; van Swelm, R.P.L.; Laarakkers, C.M.; Cox, G.R. Influence of periodizing dietary carbohydrate on iron regulation and immune function in elite triathletes. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Sim, M.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Seven days of high carbohydrate ingestion does not attenuate post-exercise IL-6 and hepcidin levels. Eur. J. Appl. Physiol. 2016, 116, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Noakes, T.; Phinney, S.D. Rethinking fat as a fuel for endurance exercise. Eur. J. Sport Sci. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction—Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Whitfield, J.; Burke, L.M.; McKay, A.K.A.; Heikura, I.A.; Hall, R.; Fensham, N.; Sharma, A.P. Acute ketogenic diet and ketone ester supplementation impairs race wak performance. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef]
- Webster, C.C.; Noakes, T.D.; Chacko, S.K.; Swart, J.; Kohn, T.A.; Smith, J.A. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J. Physiol. 2016, 594, 4389–4405. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Peeling, P.; Pyne, D.B.; Welvaert, M.; Tee, N.; Leckey, J.J.; Sharma, A.P.; Ross, M.L.R.; Garvican-Lewis, L.A.; Swinkels, D.W.; et al. Chronic adherence to a ketogenic diet modifies iron metabolism in elite athletes. Med. Sci. Sports Exerc. 2019, 51, 548–555. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Doyle, L. Low-carbohydrate ketogenic diets in male endurance athletes demonstrate different micronutrient contents and changes in corpuscular haemoglobin over 12 weeks. Sports 2019, 7, 201. [Google Scholar] [CrossRef]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Peeling, P.; Pyne, D.B.; Welvaert, M.; Tee, N.; Leckey, J.J.; Sharma, A.P.; Ross, M.L.R.; Garvican-Lewis, L.A.; van Swelm, R.P.L.; et al. Acute carbohydrate ingestion does not influence the post-exercise iron-regulatory response in elite keto-adapted race walkers. J. Sci. Med. Sport 2019, 22, 635–640. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Peeling, P.; Pyne, D.B.; Tee, N.; Welveart, M.; Heikura, I.A.; Sharma, A.P.; Whitfield, J.; Ross, M.L.; van Swelm, R.P.L.; et al. Sustained exposure to high carbohydrate availability does not influence iron regulatory responses in elite endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2020, in press. [Google Scholar]
- Jeukendrup, A.E. Training the gut for athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Camões-Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Petkus, D.L.; Murray-Kolb, L.E.; de Souza, M.J. The unexplored crossroads of the female athlete triad and iron deficiency: A narrative review. Sports Med. 2017, 47, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Nachtigall, D. Iron supplementation in athletes. Current recommendations. Sports Med. 1998, 26, 207–216. [Google Scholar] [CrossRef]
- Wells, K.R.; Jeacocke, N.A.; Appaneal, R.; Smith, H.D.; Vlahovich, N.; Burke, L.M.; Hughes, D. The Australian Institute of Sport (AIS) and National Eating Disorders Collaboration (NEDC) position statement on disordered eating in high performance sport. Br. J. Sports Med. 2020, 54, 1247–1258. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Teien, H.K.; Madslien, E.H.; et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef]
- Ishibashi, A.; Kojima, C.; Tanabe, Y.; Iwayama, K.; Hiroyama, T.; Tsuji, T.; Kamei, A.; Goto, K.; Takahashi, H. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol. Rep. 2020, 8, e14494. [Google Scholar] [CrossRef]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Erythroferrone: An erythroid regulator of hepcidin and iron metabolism. HemaSphere 2018, 2, e35. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Black, K.E.; O’Brien, W.J. Hepcidin as a prospective individualized biomarker for individuals at risk of low energy availability. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Berryman, C.E.; Harris, M.N.; Karl, J.P.; Lieberman, H.R.; McClung, J.P.; Rood, J.C.; Pasiakos, S.M. Testosterone administration during energy deficit suppresses hepcidin and increases iron availability for erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.L.; Zhang, S.P.; Wang, L.; Li, J.P.; Qu, G.B.; He, J.Y.; Rong, H.Q.; Ji, H.; Liu, S.J. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. The role of energy availability in reproductive function in the female athlete triad and extension of its effects to men: An initial working model of a similar syndrome in male athletes. Sports Med. 2019, 49 (Suppl. S2), 125–137. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.; Shao, M.; Tesfaye, G.C.; Yang, S. Associations of iron intake, serum iron and serum ferritin with bone mineral density in women: The national health and nutrition examination survey, 2005–2010. Calcif. Tissue Int. 2020, 106, 232–238. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M.P. Chronic iron deficiency as an emerging risk factor for osteoporosis: A hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.Y.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef]
- Drew, M.; Vlahovich, N.; Hughes, D.; Appaneal, R.; Burke, L.M.; Lundy, B.; Rogers, M.; Toomey, M.; Watts, D.; Lovell, G.; et al. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br. J. Sports Med. 2017, 52, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P. Nutrition and athlete immune health: New perspectives on an old paradigm. Sports Med. 2019, 49 (Suppl. S2), 153–168. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Crichton, R.R.; Taylor, D.L.; Della Corte, L.; Srai, S.K.; Dexter, D.T. Iron and the immune system. J. Neural Transm. 2011, 118, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tyrrell, D.A.; Smith, A.P. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991, 325, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Kohrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, T.S.; De Sanctis, V.; Yassin, M.; Adel, A. Growth and growth hormone-insulin like growth factor-I (GH-IGF-I) axis in chronic anemias. Acta Bio Med. Atenei Parm. 2017, 88, 101. [Google Scholar]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine effects of relative energy deficiency in sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, A.K.A.; Pyne, D.B.; Burke, L.M.; Peeling, P. Iron Metabolism: Interactions with Energy and Carbohydrate Availability. Nutrients 2020, 12, 3692. https://doi.org/10.3390/nu12123692
McKay AKA, Pyne DB, Burke LM, Peeling P. Iron Metabolism: Interactions with Energy and Carbohydrate Availability. Nutrients. 2020; 12(12):3692. https://doi.org/10.3390/nu12123692
Chicago/Turabian StyleMcKay, Alannah K. A., David B. Pyne, Louise M. Burke, and Peter Peeling. 2020. "Iron Metabolism: Interactions with Energy and Carbohydrate Availability" Nutrients 12, no. 12: 3692. https://doi.org/10.3390/nu12123692
APA StyleMcKay, A. K. A., Pyne, D. B., Burke, L. M., & Peeling, P. (2020). Iron Metabolism: Interactions with Energy and Carbohydrate Availability. Nutrients, 12(12), 3692. https://doi.org/10.3390/nu12123692




