Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review
Abstract
1. Introduction
2. Methods
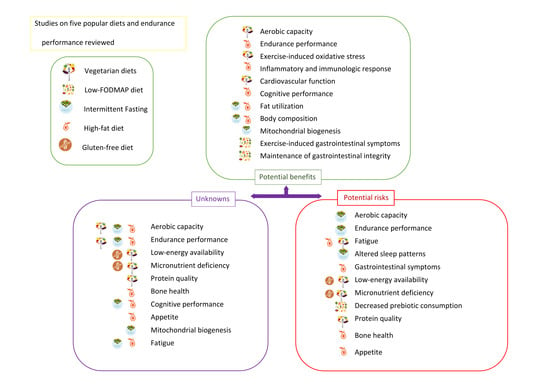
3. Popular Diets Applied to Improve Sports Performance in Endurance Athletes
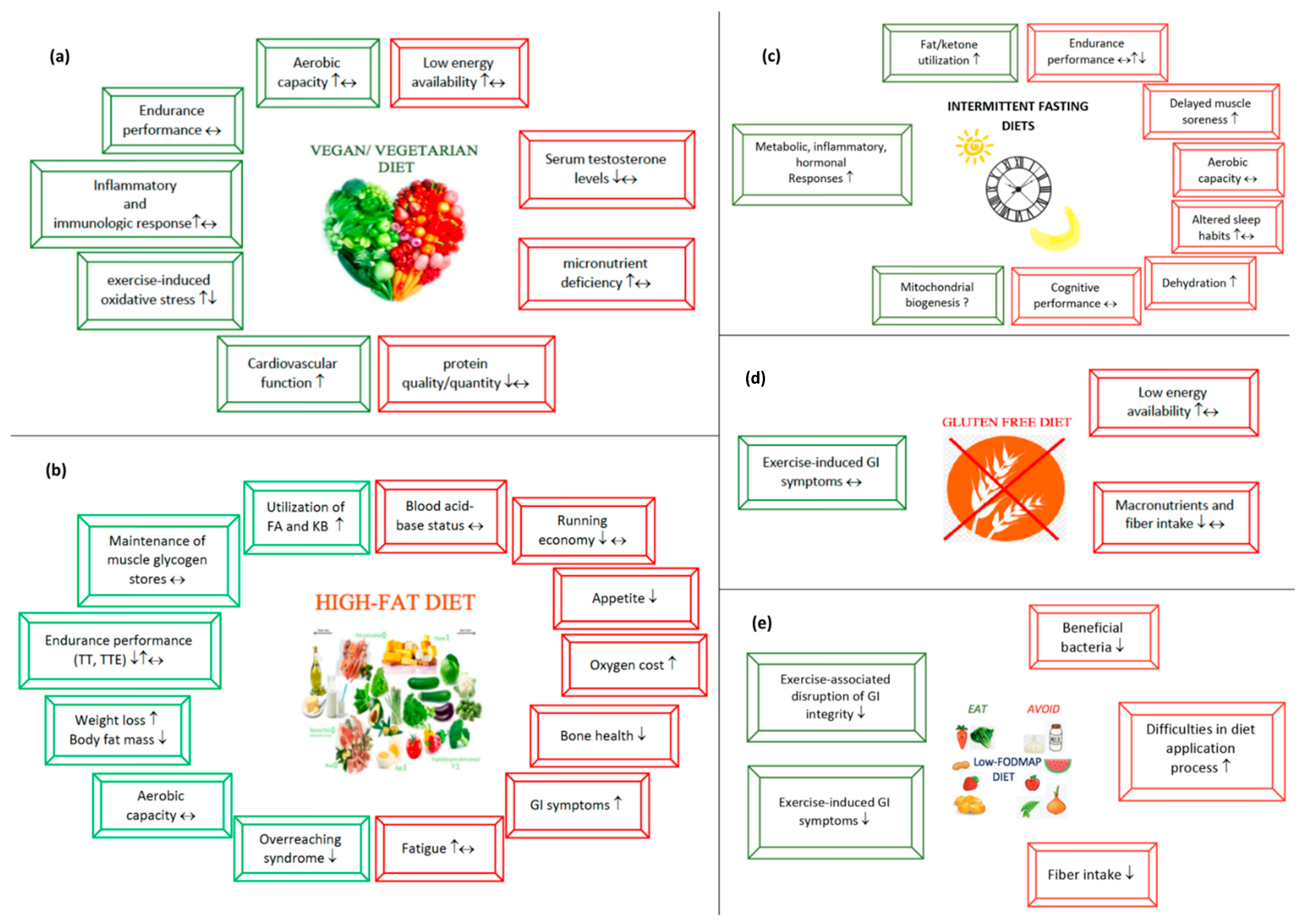
3.1. Vegetarian Diets
The Impact of Vegetarian Diets on Sports Performance
3.2. High-Fat Diets
3.2.1. Potential Beneficial Aspects of High-Fat Diets
3.2.2. Potential Risks Regarding High-Fat Diets
3.3. Intermittent Fasting
Intermittent Fasting and Sports Performance
Possible Benefits of Intermittent Fasting in Endurance Athletes
Risks to Be Considered When Applying Fasting Diets
3.4. Gluten-Free Diet
3.4.1. Why Do Endurance Athletes Consider a Gluten-Free Diet to Be Beneficial?
3.4.2. Possible Risks of a Gluten-Free Diet
3.5. Low-FODMAP Diet
3.5.1. Several Points Indicating That a Low-FODMAP Diet Is Advantageous
3.5.2. Potential Risks to Consider When Applying a Low-FODMAP Diet
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in ultra-endurance: State of the art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Kings, D.; Larson-Meyer, D.E. Dietary practices adopted by track-and-field athletes: Gluten-free, low FODMAP, vegetarian, and fasting. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Plant-based eating patterns and endurance performance: A focus on inflammation, oxidative stress and immune responses. Nutr. Bull. 2020, 45, 123–132. [Google Scholar] [CrossRef]
- Burke, L.M. Ketogenic low-CHO, high-fat diet: The future of elite endurance sport? J. Physiol. 2020. [Google Scholar] [CrossRef]
- Levy, E.; Chu, T. Intermittent fasting and its effects on athletic performance: A review. Curr. Sports Med. Rep. 2019, 18, 266–269. [Google Scholar] [CrossRef]
- Lis, D.M.; Stellingwerff, T.; Shing, C.M.; Ahuja, K.D.K.; Fell, J.W. Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 37–45. [Google Scholar] [CrossRef]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Larson-Meyer, E. Vegetarian and Vegan Diets for Athletic Training and Performance. Sports Sci. Exch. 2018, 29, 1–7. [Google Scholar]
- Wilson, P.B. Nutrition behaviors, perceptions, and beliefs of recent marathon finishers. Phys. Sportsmed. 2016, 44, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Wirnitzer, K.; Seyfart, T.; Leitzmann, C.; Keller, M.; Wirnitzer, G.; Lechleitner, C.; Rüst, C.A.; Rosemann, T.; Knechtle, B. Prevalence in running events and running performance of endurance runners following a vegetarian or vegan diet compared to non-vegetarian endurance runners: The NURMI Study. Springerplus 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef]
- Terink, R.; Witkamp, R.F.; Hopman, M.T.E.; Siebelink, E.; Savelkoul, H.F.J.; Mensink, M. A 2 week cross-over intervention with a low carbohydrate, high fat diet compared to a high carbohydrate diet attenuates exercise-induced cortisol response, but not the reduction of exercise capacity, in recreational athletes. Nutrients 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Carr, A.J.; Sharma, A.P.; Ross, M.L.; Welvaert, M.; Slater, G.J.; Burke, L.M. Chronic ketogenic low carbohydrate high fat diet has minimal effects on acid–base status in elite athletes. Nutrients 2018, 10, 236. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Maunder, E.D.; Dulson, D.K. Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med. Sci. Sports Exerc. 2019, 51, 2135–2146. [Google Scholar] [CrossRef]
- Heatherly, A.J.; Killen, L.G.; Smith, A.F.; Waldman, H.S.; Seltmann, C.L.; Hollingsworth, A.; O’Neal, E.K. Effects of Ad libitum Low-Carbohydrate High-Fat Dieting in Middle-Age Male Runners. Med. Sci. Sports Exerc. 2018, 50, 570–579. [Google Scholar] [CrossRef]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Keaney, L.; Dulson, D.K. Adaptation to a ketogenic diet modulates adaptive and mucosal immune markers in trained male endurance athletes. Scand. J. Med. Sci. Sports 2020, 31, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Sharma, A.P.; Heikura, I.A.; Forbes, S.F.; Holloway, M.; McKay, A.K.A.; Bone, J.L.; Leckey, J.J.; Welvaert, M.; Ross, M.L. Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS ONE 2020, 15, e0234027. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Prins, P.J.; Noakes, T.D.; Welton, G.L.; Haley, S.J.; Esbenshade, N.J.; Atwell, A.D.; Scott, K.E.; Abraham, J.; Raabe, A.S.; Buxton, J.D.; et al. High rates of fat oxidation induced by a low-carbohydrate, high-fat diet, do not impair 5-km running performance in competitive recreational athletes. J. Sports Sci. Med. 2019, 18, 738–750. [Google Scholar]
- Lambert, E.V.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef]
- Creighton, B.C.; Hyde, P.N.; Maresh, C.M.; Kraemer, W.J.; Phinney, S.D.; Volek, J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000429. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Christie, C.; Wilson, G.; Dennis, S.C.; Noakes, T.D.; Hopkins, W.G.; Lambert, E.V. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999, 48, 1509–1517. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Keaney, L.; Dulson, D.K. Acute hyperketonaemia alters T-cell-related cytokine gene expression within stimulated peripheral blood mononuclear cells following prolonged exercise. Eur. J. Appl. Physiol. 2020, 120, 191–202. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Faull, O.K.; Rolls, E.; Clarke, K.; Cox, P.J. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front. Physiol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Dearlove, D.J.; Harrison, O.K.; Hodson, L.; Jefferson, A.; Clarke, K.; Cox, P.J. The Effect of Blood Ketone Concentration and Exercise Intensity on Exogenous Ketone Oxidation Rates in Athletes. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Plews, D.; Laursen, P.; Dulson, D.K. The effect of 1,3-butanediol on cycling time-trial performance. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 466–473. [Google Scholar] [CrossRef]
- Leckey, J.J.; Ross, M.L.; Quod, M.; Hawley, J.A.; Burke, L.M. Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Front. Physiol. 2017, 8, 806. [Google Scholar] [CrossRef]
- Scott, B.E.; Laursen, P.B.; James, L.J.; Boxer, B.; Chandler, Z.; Lam, E.; Gascoyne, T.; Messenger, J.; Mears, S.A. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J. Sci. Med. Sport 2019, 22, 702–706. [Google Scholar] [CrossRef]
- Poffé, C.; Ramaekers, M.; Bogaerts, S.; Hespel, X.P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J. Appl. Physiol. 2020, 128, 1643–1653. [Google Scholar] [CrossRef]
- Prins, P.J.; D’Agostino, D.P.; Rogers, C.Q.; Ault, D.L.; Welton, G.L.; Jones, D.W.; Henson, S.R.; Rothfuss, T.J.; Aiken, K.G.; Hose, J.L.; et al. Dose response of a novel exogenous ketone supplement on physiological, perceptual and performance parameters. Nutr. Metab. 2020, 17, 81. [Google Scholar] [CrossRef]
- Evans, M.; McSwiney, F.T.; Brady, A.J.; Egan, B. No Benefit of Ingestion of a Ketone Monoester Supplement on 10-km Running Performance. Med. Sci. Sports Exerc. 2019, 51, 2506–2515. [Google Scholar] [CrossRef]
- Heikura, I.A.; Burke, L.M.; Hawley, J.A.; Ross, M.L.; Garvican-Lewis, L.; Sharma, A.P.; McKay, A.K.A.; Leckey, J.J.; Welvaert, M.; McCall, L.; et al. A Short-Term Ketogenic Diet Impairs Markers of Bone Health in Response to Exercise. Front. Endocrinol. 2020, 10, 880. [Google Scholar] [CrossRef]
- Carey, A.L.; Staudacher, H.M.; Cummings, N.K.; Stepto, N.K.; Nikolopoulos, V.; Burke, L.M.; Hawley, J.A. Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. J. Appl. Physiol. 2001, 91, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Angus, D.J.; Cox, G.R.; Cummings, N.K.; Febbraio, M.A.; Gawthorn, K.; Hawley, J.A.; Minehan, M.; Martin, D.T.; Hargreaves, M.; et al. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J. Appl. Physiol. 2000, 89, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Angus, D.J.; Cox, G.R.; Clark, S.A.; Cummings, N.K.; Desbrow, B.; Hargreaves, M. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med. Sci. Sports Exerc. 2002, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.K.; Lessard, S.J.; Chen, Z.P.; Garnham, A.P.; Burke, L.M.; Rivas, D.A.; Kemp, B.E.; Hawley, J.A. Fat adaptation followed by carbohydrate restoration increases AMPK activity in skeletal muscle from trained humans. J. Appl. Physiol. 2008, 105, 1519–1526. [Google Scholar] [CrossRef]
- Havemann, L.; West, S.J.; Goedecke, J.H.; Macdonald, I.A.; St Clair Gibson, A.; Noakes, T.D.; Lambert, E.V. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. J. Appl. Physiol. 2006, 100, 194–202. [Google Scholar] [CrossRef]
- Lambert, E.V.; Goedecke, J.H.; Van Zyl, C.; Murphy, K.; Hawley, J.A.; Dennis, S.C.; Noakes, T.D. High-fat diet versus habitual diet prior to carbohydrate loading: Effects on exercise metabolism and cycling performance. Int. J. Sport Nutr. 2001, 11, 209–225. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Spriet, L.L.; Watt, M.J.; Kimber, N.E.; Hargreaves, M.; Hawley, J.A.; Burke, L.M. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 380–388. [Google Scholar] [CrossRef]
- Mujika, I. Case study: Long-term low-carbohydrate, high-fat diet impairs performance and subjective well-being in a world-class vegetarian long-distance triathlete. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 339–344. [Google Scholar] [CrossRef]
- Zehnder, M.; Christ, E.R.; Ith, M.; Acheson, K.J.; Pouteau, E.; Kreis, R.; Trepp, R.; Diem, P.; Boesch, C.; Décombaz, J. Intramyocellular lipid stores increase markedly in athletes after 1.5 days lipid supplementation and are utilized during exercise in proportion to their content. Eur. J. Appl. Physiol. 2006, 98, 341–354. [Google Scholar] [CrossRef]
- Décombaz, J.; Grathwohl, D.; Pollien, P.; Schmitt, J.A.J.; Borrani, F.; Lecoultre, V. Effect of short-duration lipid supplementation on fat oxidation during exercise and cycling performance. Appl. Physiol. Nutr. Metab. 2013, 38, 766–772. [Google Scholar] [CrossRef]
- Murakami, I.; Sakuragi, T.; Uemura, H.; Menda, H.; Shindo, M.; Tanaka, H. Significant Effect of a Pre-Exercise High-Fat Meal after a 3-Day High-Carbohydrate Diet on Endurance Performance. Nutrients 2012, 4, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Haufe, S.; Eigendorf, J.; Wasserfurth, P.; Tegtbur, U.; Hahn, A. Exercise capacity of vegan, lacto-ovo-vegetarian and omnivorous recreational runners. J. Int. Soc. Sports Nutr. 2019, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Drabert, K.; Haufe, S.; Wasserfurth, P.; Eigendorf, J.; Tegtbur, U.; Hahn, A.; Tsikas, D. Exercise-Induced Oxidative Stress, Nitric Oxide and Plasma Amino Acid Profile in Recreational Runners with Vegetarian and Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1875. [Google Scholar] [CrossRef]
- Richter, E.A.; Kiens, B.; Raben, A.; Tvede, N.; Pedersen, B.K. Immune parameters in male atheletes after a lacto-ovo vegetarian diet and a mixed Western diet. Med. Sci. Sports Exerc. 1991, 23, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Potthast, A.B.; Nebl, J.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Hahn, A.; Das, A. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients 2020, 12, 1004. [Google Scholar] [CrossRef]
- Leischik, R.; Spelsberg, N. Vegan Triple-Ironman (Raw Vegetables/Fruits). Case Rep. Cardiol. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Kornexl, E. Energy and Macronutrient Intake of a Female Vegan Cyclist During an 8-Day Mountain Bike Stage Race. Baylor Univ. Med. Cent. Proc. 2014, 27, 42–45. [Google Scholar] [CrossRef]
- Lynch, H.M.; Wharton, C.M.; Johnston, C.S. Cardiorespiratory fitness and peak torque differences between vegetarian and omnivore endurance athletes: A cross-sectional study. Nutrients 2016, 8, 726. [Google Scholar] [CrossRef]
- Król, W.; Price, S.; Śliż, D.; Parol, D.; Konopka, M.; Mamcarz, A.; Wełnicki, M.; Braksator, W. A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography. Diagnostics 2020, 10, 477. [Google Scholar] [CrossRef]
- Brisswalter, J.; Bouhlel, E.; Falola, J.M.; Abbiss, C.R.; Vallier, J.M.; Hauswirth, C. Effects of ramadan intermittent fasting on middle-distance running performance in well-trained runners. Clin. J. Sport Med. 2011, 21, 422–427. [Google Scholar] [CrossRef]
- Chennaoui, M.; Desgorces, F.; Drogou, C.; Boudjemaa, B.; Tomaszewski, A.; Depiesse, F.; Burnat, P.; Chalabi, H.; Gomez-Merino, D. Effects of Ramadan fasting on physical performance and metabolic, hormonal, and inflammatory parameters in middle-distance runners. Appl. Physiol. Nutr. Metab. 2009, 34, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Chamari, K.; Briki, W.; Farooq, A.; Patrick, T.; Belfekih, T.; Herrera, C.P. Impact of Ramadan intermittent fasting on cognitive function in trained cyclists: A pilot study. Biol. Sport 2016, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Che Muhamed, A.M.; Mohamed, N.G.; Ismail, N.; Aziz, A.R.; Singh, R. Mouth rinsing improves cycling endurance performance during Ramadan fasting in a hot humid environment. Appl. Physiol. Nutr. Metab. 2014, 39, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.J.; Langton, H.M.; Mulligan, M.; Egan, B. Effects of Eight Weeks of 16:8 Time-restricted Eating in Male Middle- and Long-Distance Runners. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef]
- Lis, D.; Stellingwerff, T.; Kitic, C.M.; Ahuja, K.D.; Fell, J. No Effects of a Short-Term Gluten-free Diet on Performance in Nonceliac Athletes. Med. Sci. Sports Exerc. 2015, 47, 2563–2570. [Google Scholar] [CrossRef]
- Lis, D.; Stellingwerff, T.; Kitic, C.M.; Fell, J.W.; Ahuja, K.D.K. Low FODMAP: A Preliminary Strategy to Reduce Gastrointestinal Distress in Athletes. Med. Sci. Sports Exerc. 2018, 50, 116–123. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Costa, R.J.S. Applying a Low-FODMAP dietary intervention to a female ultraendurance runner with irritable bowel syndrome during a multistage ultramarathon. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 61–67. [Google Scholar] [CrossRef]
- Lis, D.; Ahuja, K.D.K.; Stellingwerff, T.; Kitic, C.M.; Fell, J. Case study: Utilizing a low FODMAP diet to combat exercise-induced gastrointestinal symptoms. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 481–487. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Taylor, B.; Muir, J.; Costa, R.J.S. Impact of 24-h high and low fermentable oligo-, di-, monosaccharide, and polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional heat stress. Appl. Physiol. Nutr. Metab. 2020, 45, 569–580. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-based diets for cardiovascular safety and performance in endurance sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Trapp, D.; Knez, W.; Sinclair, W. Could a vegetarian diet reduce exercise-induced oxidative stress? A review of the literature. J. Sports Sci. 2010, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of flavonoids on upper respiratory tract infections and immune function: A systematic review and meta-analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Borrione, P.; Grasso, L.; Quaranta, F.; Parisi, A. Vegetarian diet and athletes. Int. SportMed J. 2009, 10, 20–24. [Google Scholar] [CrossRef]
- Marquet, L.-A.; Brisswalter, J.; Louis, J.; Tiollier, E.; Burke, L.M.; Hawley, J.A.; Hausswirth, C. Enhanced Endurance Performance by Periodization of Carbohydrate Intake. Med. Sci. Sports Exerc. 2016, 48, 663–672. [Google Scholar] [CrossRef]
- Kennedy, D.O. Phytochemicals for Improving Aspects of Cognitive Function and Psychological State Potentially Relevant to Sports Performance. Sports Med. 2019, 49, 39–58. [Google Scholar] [CrossRef]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Cook, M.D.; Willems, M.E.T. Dietary anthocyanins: A review of the exercise performance effects and related physiological responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Morrow, J.D.; Utter, A.C.; Henson, D.A.; Proulx, W.R.; George, G.L. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr. Res. 2004, 24, 209–221. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a Polyphenol-Enriched Protein Powder on Exercise-Induced Inflammation and Oxidative Stress in Athletes: A Randomized Trial Using a Metabolomics Approach. PLoS ONE 2013, 8, 72215. [Google Scholar] [CrossRef]
- Park, C.H.; Kwak, Y.S.; Seo, H.K.; Kim, H.Y. Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors. Iran. J. Public Health 2018, 47, 27–32. [Google Scholar] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.-Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and/or Banana Consumption Mitigate Arachidonic, Cytochrome P450 Oxylipin Generation during Recovery from 75-km Cycling: A Randomized Trial. Front. Nutr. 2020, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Somerville, V.X.; Hurst, R.D. The effect of New Zealand blackcurrant on sport performance and related biomarkers: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.C.; Hueglin, S.; Broad, E. Tart Cherry Juice in Athletes: A Literature Review and Commentary. Curr. Sports Med. Rep. 2017, 16, 230–239. [Google Scholar] [CrossRef]
- Alba, C.M.-A.; Daya, M.; Franck, C. Tart Cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2019, 59, 626–638. [Google Scholar] [CrossRef]
- Torregrosa-García, A.; Ávila-Gandía, V.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; López-Román, F.J. Pomegranate extract improves maximal performance of trained cyclists after an exhausting endurance trial: A randomised controlled trial. Nutrients 2019, 11, 721. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Smith, K.A.; Kisiolek, J.N.; Willingham, B.D.; Morrissey, M.C.; Leyh, S.M.; Saracino, P.G.; Baur, D.A.; Cook, M.D.; Ormsbee, M.J. Ultra-endurance triathlon performance and markers of whole-body and gut-specific inflammation. Eur. J. Appl. Physiol. 2020, 120, 349–357. [Google Scholar] [CrossRef]
- Welc, S.S.; Clanton, T.L. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp. Physiol. 2013, 98, 359–371. [Google Scholar] [CrossRef]
- Neubauer, O.; König, D.; Wagner, K.H. Recovery after an Ironman triathlon: Sustained inflammatory responses and muscular stress. Eur. J. Appl. Physiol. 2008, 104, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Steensberg, A.; Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Pedersen, B.K. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J. Appl. Physiol. 2005, 99, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Risk of upper respiratory tract infection in athletes: An epidemiologic and immunologic perspective. J. Athl. Train. 1997, 32, 344–349. [Google Scholar] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñó, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Rideout, C.A. Nutritional considerations for vegetarian athletes. Nutrition 2004, 20, 696–703. [Google Scholar] [CrossRef]
- Cialdella-Kam, L.; Kulpins, D.; Manore, M. Vegetarian, Gluten-Free, and Energy Restricted Diets in Female Athletes. Sports 2016, 4, 50. [Google Scholar] [CrossRef]
- Howie, B.J.; Shultz, T.D. Dietary and hormonal interrelationships among vegetarian Seventh-Day Adventists and nonvegetarian men. Am. J. Clin. Nutr. 1985, 42, 127–134. [Google Scholar] [CrossRef]
- Allen, N.E.; Appleby, P.N.; Davey, G.K.; Key, T.J. Hormones and diet: Low insulin-like growth factor-1 but normal bioavailable androgens in vegan men. Br. J. Cancer 2000, 83, 95–97. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; de Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Ciuris, C.; Lynch, H.M.; Wharton, C.; Johnston, C.S. A comparison of dietary protein digestibility, based on diaas scoring, in vegetarian and non-vegetarian athletes. Nutrients 2019, 11, 3016. [Google Scholar] [CrossRef]
- Fuhrman, J.; Ferreri, D.M. Fueling the vegetarian (vegan) athlete. Curr. Sports Med. Rep. 2010, 9, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Schuchardt, J.P.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Characterization, dietary habits and nutritional intake of omnivorous, lacto-ovo vegetarian and vegan runners—A pilot study. BMC Nutr. 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Schuchardt, J.P.; Ströhle, A.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Micronutrient status of recreational runners with vegetarian or non-vegetarian dietary patterns. Nutrients 2019, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Williamson, E. Nutritional implications for ultra-endurance walking and running events. Extrem. Physiol. Med. 2016, 5, 13. [Google Scholar] [CrossRef]
- Black, K.; Slater, J.; Brown, R.C.; Cooke, R. Low energy availability, plasma lipids, and hormonal profiles of recreational athletes. J. Strength Cond. Res. 2018, 32, 2816–2824. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Torstveit, M.K.; et al. International Olympic Committee (IOC) Consensus statement on relative energy deficiency in sport (red-s): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- Brytek-Matera, A.; Czepczor-Bernat, K.; Jurzak, H.; Kornacka, M.; Kołodziejczyk, N. Strict health-oriented eating patterns (orthorexic eating behaviours) and their connection with a vegetarian and vegan diet. Eat. Weight Disord. 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.; Skouby, S.; Møller, S.S.; Faber, J.; Sundgot-Borgen, J.; Sjödin, A. Low-energy density and high fiber intake are dietary concerns in female endurance athletes. Scand. J. Med. Sci. Sports 2016, 26, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Hough, P.A.; Earle, J. Energy Balance during a Self-Sufficient, Multistage Ultramarathon. J. Hum. Perform. Extrem. Environ. 2017, 13, 5. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Raben, A.; Kiens, B.; Richter, E.A.; Rasmussen, L.B.; Svenstrup, B.; Micic, S.; Benett, P. Serum sex hormones and endurance performance after a lacto-ovo vegetarian and a mixed diet—Publications. Med. Sci. Sports Exerc. 1992, 24, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.; Ferrando, A.; Sheffield-Moore, M.; Urban, R. Testosterone and muscle protein metabolism. Mayo Clin. Proc. 2000, 75, S55–S60. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Nackeeran, S.; Blachman-Braun, R.; Ramasamy, R. The association between plant-based content in diet and testosterone levels in US adults. World J. Urol. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets—A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Visioli, F. Polyphenols in Sport: Facts or Fads? CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; ISBN 9781466567573. [Google Scholar]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef]
- D’angelo, S. Polyphenols and Athletic Performance: A Review on Human Data. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone bodies in neurological diseases: Focus on neuroprotection and underlying mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.M.; Churchward-Venne, T.A.; Bailey, D.; Van Loon, L.J.C. Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Med. 2017, 47, 383–391. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Lis, D. From Celiac Disease, Gluten-Sensitivity vs Gluten Sensationalism, to Fodmap Reduction as a Tool to Manage Gastrointestinal Symptoms in Athletes. Exercise-Induced Gastrointestinal Syndrome and Diet. Sports Sci. Exch. 2018, 29, 1–6. [Google Scholar]
- Poffé, C.; Ramaekers, M.; van Thienen, R.; Hespel, P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J. Physiol. 2019, 597, 3009–3027. [Google Scholar] [CrossRef]
- Webster, C.C.; Swart, J.; Noakes, T.D.; Smith, J.A. A carbohydrate ingestion intervention in an elite athlete who follows a low-carbohydrate high-fat diet. Int. J. Sports Physiol. Perform. 2018, 13, 957–960. [Google Scholar] [CrossRef]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast growth factor 21 and the adaptive response to nutritional challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef]
- Yeo, W.K.; Carey, A.L.; Burke, L.; Spriet, L.L.; Hawley, J.A. Fat adaptation in well-trained athletes: Effects on cell metabolism. Appl. Physiol. Nutr. Metab. 2011, 36, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Kysel, P.; Haluzíková, D.; Doležalová, R.P.; Laňková, I.; Lacinová, Z.; Kasperová, B.J.; Trnovská, J.; Hrádková, V.; Mráz, M.; Vilikus, Z.; et al. The influence of cyclical ketogenic reduction diet vs. Nutritionally balanced reduction diet on body composition, strength, and endurance performance in healthy young males: A randomized controlled trial. Nutrients 2020, 12, 2832. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Chau, M.D.L.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of Ketone Bodies on Endurance Exercise. Curr. Sports Med. Rep. 2018, 17, 444–453. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Burke, L.M.; Whitfield, J.; Heikura, I.A.; Ross, M.L.R.; Tee, N.; Forbes, S.F.; Hall, R.; McKay, A.K.A.; Wallett, A.M.; Sharma, A.P. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J. Physiol. 2020. [Google Scholar] [CrossRef]
- Chang, C.K.; Borer, K.; Lin, P.J. Low-Carbohydrate-High-Fat Diet: Can it Help Exercise Performance? J. Hum. Kinet. 2017, 56, 81–92. [Google Scholar] [CrossRef]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Samsonienė, L.; Stukas, R. Dietary Acid-Base Balance in High-Performance Athletes. Int. J. Environ. Res. Public Health 2020, 17, 5332. [Google Scholar] [CrossRef] [PubMed]
- Hietavala, E.M.; Stout, J.R.; Hulmi, J.J.; Suominen, H.; Pitkänen, H.; Puurtinen, R.; Selänne, H.; Kainulainen, H.; Mero, A.A. Effect of diet composition on acid-base balance in adolescents, young adults and elderly at rest and during exercise. Eur. J. Clin. Nutr. 2015, 69, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Todd King, M.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; Revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43–64. [Google Scholar] [CrossRef]
- Fromentin, C.; Tomé, D.; Nau, F.; Flet, L.; Luengo, C.; Azzout-Marniche, D.; Sanders, P.; Fromentin, G.; Gaudichon, C. Dietary proteins contribute little to glucose production, even under optimal gluconeogenic conditions in healthy humans. Diabetes 2013, 62, 1435–1442. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- White, A.M.; Johnston, C.S.; Swan, P.D.; Tjonn, S.L.; Sears, B. Blood Ketones Are Directly Related to Fatigue and Perceived Effort during Exercise in Overweight Adults Adhering to Low-Carbohydrate Diets for Weight Loss: A Pilot Study. J. Am. Diet. Assoc. 2007, 107, 1792–1796. [Google Scholar] [CrossRef]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef]
- Margolis, L.M.; O’Fallon, K.S. Utility of Ketone Supplementation to Enhance Physical Performance: A Systematic Review. Adv. Nutr. 2020, 2, 412–419. [Google Scholar] [CrossRef]
- Puchalska, P.; Martin, S.E.; Huang, X.; Nagy, L.; Patti, G.J.; Correspondence, P.A.C.; Lengfeld, J.E.; Daniel, B.; Graham, M.J.; Han, X.; et al. Hepatocyte-Macrophage Acetoacetate Shuttle Protects against Tissue Fibrosis Article Hepatocyte-Macrophage Acetoacetate Shuttle Protects against Tissue Fibrosis. Cell Metab. 2019, 29, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Carrero, K. A Literature Review on Intermittent Fasting. Senior Honors Thesis, Liberty University, Lynchburg, Virginia, 2020. [Google Scholar]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Fallah, J.S.; Coyle, E.F. The effects of fasting on metabolism and performance. Br. J. Sports Med. 2010, 44, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Baum, O.; Lurman, G.; Mueller, M. Molecular Mechanisms of Muscle Plasticity with Exercise. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 1, pp. 1383–1412. [Google Scholar]
- Marosi, K.; Moehl, K.; Navas-Enamorado, I.; Mitchell, S.J.; Zhang, Y.; Lehrmann, E.; Aon, M.A.; Cortassa, S.; Becker, K.G.; Mattson, M.P. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. FASEB J. 2018, 32, 3844–3858. [Google Scholar] [CrossRef] [PubMed]
- Chaouachl, A.; Leiper, J.B.; Souissi, N.; Coutts, A.J.; Chamari, K. Effects of ramadan intermittent fasting on sports performance and training: A review. Int. J. Sports Physiol. Perform. 2009, 4, 419–434. [Google Scholar]
- Chaouachi, A.; Leiper, J.B.; Chtourou, H.; Aziz, A.R.; Chamari, K. The effects of Ramadan intermittent fasting on athletic performance: Recommendations for the maintenance of physical fitness. J. Sports Sci. 2012, 30, S53–S73. [Google Scholar] [CrossRef]
- Loy, S.F.; Conlee, R.K.; Winder, W.W.; Nelson, A.G.; Arnall, D.A.; Fisher, A.G. Effects of 24-h fast on cycling endurance time at two different intensities. J. Appl. Physiol. 1986, 61, 654–659. [Google Scholar] [CrossRef]
- Nieman, D.C.; Carlson, K.A.; Brandstater, M.E.; Naegele, R.T.; Blankenship, J.W. Running endurance in 27-h-fasted humans. J. Appl. Physiol. 1987, 63, 2502–2509. [Google Scholar] [CrossRef]
- Zinker, B.A.; Britz, K.; Brooks, G.A. Effects of a 36-h fast on human endurance and substrate utilization. J. Appl. Physiol. 1990, 69, 1849–1855. [Google Scholar] [CrossRef]
- Dohm, G.L.; Beeker, R.T.; Israel, R.G.; Tapscott, E.B. Metabolic responses to exercise after fasting. J. Appl. Physiol. 1986, 61, 1363–1368. [Google Scholar] [CrossRef]
- Watson, A.M. Sleep and Athletic Performance. Curr. Sports Med. Rep. 2017, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Rehrer, N.J. Fluid and Electrolyte Balance in Ultra-Endurance Sport. Sports Med. 2001, 31, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. The impact of Ramadan observance upon athletic performance. Nutrients 2012, 4, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Wagner-Jones, K.; Madden, R.F.; Erdman, K.A. Dietary restrictions in endurance runners to mitigate exercise-induced gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2020, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Lis, D.; Ahuja, K.D.K.; Stellingwerff, T.; Kitic, C.M.; Fell, J. Food avoidance in athletes: FODMAP foods on the list. Appl. Physiol. Nutr. Metab. 2016, 41, 1002–1004. [Google Scholar] [CrossRef]
- Ciacci, C.; Ciclitira, P.; Hadjivassiliou, M.; Kaukinen, K.; Ludvigsson, J.F.; McGough, N.; Sanders, D.S.; Woodward, J.; Leonard, J.N.; Swift, G.L. The gluten-Free diet and its current application in coeliac disease and dermatitis Herpetiformis. United Eur. Gastroenterol. J. 2015, 3, 121–135. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Osorio, C.E.; Mejías, J.H.; Rustgi, S. Gluten detection methods and their critical role in assuring safe diets for celiac patients. Nutrients 2019, 11, 2920. [Google Scholar] [CrossRef]
- Leone, J.E.; Wise, K.A.; Mullin, E.M.; Gray, K.A.; Szlosek, P.A.; Griffin, M.F.; Jordan, C.A. Celiac Disease Symptoms in Athletes: Prevalence Indicators of Perceived Quality of Life. Sports Health 2020, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, S.; Cusano, P.; Di Palma, D. Gluten-free diets in athletes. J. Phys. Educ. Sport 2020, 20, 2330–2336. [Google Scholar] [CrossRef]
- Halson, S.L.; Martin, D.T. Lying to win—Placebos and sport science. Int. J. Sports Physiol. Perform. 2013, 8, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Zopf, Y. Gluten and FODMAPS—Sense of a Restriction/When Is Restriction Necessary? Nutrients 2019, 11, 1957. [Google Scholar] [CrossRef]
- Peters, H.P.F.; Bos, M.; Seebregts, L.; Akkermans, L.M.A.; Van Berge Henegouwen, G.P.; Bol, E.; Mosterd, W.L.; de Vries, W.R. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: Prevalence, medication, and etiology. Am. J. Gastroenterol. 1999, 94, 1570–1581. [Google Scholar] [CrossRef]
- Tuck, C.J.; Muir, J.G.; Barrett, J.S.; Gibson, P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: Role in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 819–834. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and recent developments of the low-FODMAP diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Noriega Fernández, E.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nutr. 2018, 57, 25–49. [Google Scholar] [CrossRef]
- Yan, Y.L.; Hu, Y.; Gänzle, M.G. Prebiotics, FODMAPs and dietary fiber—Conflicting concepts in development of functional food products? Curr. Opin. Food Sci. 2018, 20, 30–37. [Google Scholar] [CrossRef]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Killian, L.; Lee, S.-Y. Nutritional Habits and FODMAPs in Relation to Gastrointestinal Issues of Endurance Athletes. Gastroenterology 2017, 152, S751. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.P.; Jeukendrup, A. Nutritional Recommendations to Avoid Gastrointestinal Distress during Exercise. Available online: https://www.gssiweb.org/sports-science-exchange/article/sse-114-nutritional-recommendations-to-avoid-gastrointestinal-distress-during-exercise (accessed on 28 October 2019).
- Costa, R.J.S.; Gaskell, S.K.; McCubbin, A.J.; Snipe, R.M.J. Exertional-heat stress-associated gastrointestinal perturbations during Olympic sports: Management strategies for athletes preparing and competing in the 2020 Tokyo Olympic Games. Temperature 2020, 7, 58–88. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Costa, R.J.S. Does the temperature of water ingested during exertional-heat stress influence gastrointestinal injury, symptoms, and systemic inflammatory profile? J. Sci. Med. Sport 2018, 21, 771–776. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Camões-Costa, V.; Snipe, R.M.J.; Dixon, D.; Russo, I.; Huschtscha, Z. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile. J. Appl. Physiol. 2019, 126, 1281–1291. [Google Scholar] [CrossRef]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Knechtle, B. Nutrition in ultra-endurance racing—aspects of energy balance, fluid balance and exercise-associated hyponatremia. Med. Sport. 2013, 17, 200–210. [Google Scholar] [CrossRef]
- Barrett, J.S. Extending Our Knowledge of Fermentable, Short-Chain Carbohydrates for Managing Gastrointestinal Symptoms. Nutr. Clin. Pract. 2013, 28, 300–306. [Google Scholar] [CrossRef]
- Valeur, J.; Småstuen, M.C.; Knudsen, T.; Lied, G.A.; Røseth, A.G. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig. Dis. Sci. 2018, 63, 429–436. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; de Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S. How to institute the low-FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Gabbard, S.L.; Crowell, M.D. Pathophysiology, evaluation, and treatment of bloating: Hope, hype, or hot air? Gastroenterol. Hepatol. 2011, 7, 729–739. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Study Design | Diet/Application | Duration | Exercise Protocol(s) | Main Findings | Ref. |
|---|---|---|---|---|---|---|
| High-Fat Diets | ||||||
| Endurance-trained male athletes (n = 20) | A non-randomized control trial | K-LCHF diet (n = 9; %CHO:fat:protein = 6:77:17) or HCD (n = 11; 65:20:14) | 12 weeks | A 100-km TT performance, a 6-s sprint, and a CPT | ↓ Body mass ↓ Body fat percentage ↑ Average relative power during the 6 s sprint sprint and CPT ↑ Fat oxidation during exercise ↔ 100 km TT endurance performance | [14] |
| Recreational male athletes (n = 14) | A randomized, crossover design | K-LCHF diet (<10%CHO, 75% fat) and 2 week HCD (>50% CHO), >2 weeks washout period in between | 2 weeks | A 90-min bicycle ergometer exercise test at 60%Wmax | ↓ Exercise-induced cortisol response; however, better results observed in HCD ↓ Exercise capacity ↑ Fat oxidation during exercise ↑ Perceived exertion after exercise ↔ Post-exercise s-IgA levels at week 2 | [15] |
| Professional male race walkers (n = 25) | A mix of repeated-measures and parallel-group design | K-LCHF diet (n = 10; 75–80% FAT, <50 g CHO, 17% protein), HCD (n = 8; 60–65% CHO, 20% FAT, 15–20% protein), or PCD, (n = 7; 60–65% CHO, 20% FAT, 15–20% protein) | 3 weeks |
| ↔ VO2peak ↓ 10 km race walk performance ↑ Perceived exertion after exercise ↑ Oxygen cost ↑ Fat oxidation during exercise | [16] |
| Male and female elite race walkers (n = 24) | A mix of repeated-measures and parallel-group design | K-LCHF diet (n = 9; 75–80% FAT, <50 g CHO, 15–20% protein), HCD (n = 8; 60–65% CHO, 20% FAT, 15–20% protein), or PCD, (n = 7; 60–65% CHO, 20% FAT, 15–20% protein) | 3 weeks |
| ↔ VO2peak ↔ Blood acid-base status | [17] |
| Endurance-trained male athletes (n = 8) | A randomized repeated-measures crossover study | K-LCHF diet (75–80% FAT, <50 g CHO, 15–20% protein), HCD (43% CHO, 38% FAT, 19% protein) | 4.5 weeks |
| ↔ TTE performance ↔ Perceived exertion after exercise ↓ Exercise efficiency above 70% VO2max ↔Exercise efficiency above 70% VO2max | [18] |
| Recreationally competitive male runners (n = 8) | A pre–post-test | K-LCHF diet (<50 g CHO, 70% FAT (ad libitum), or HCD (habitual diet defined as moderate to high CHO) | 3 weeks |
| ↔ 5 km TT performance ↔ Perceived exertion after exercise ↑ Fat oxidation during exercise ↓ Body mass ↓ Skinfold thickness ↔ Exercise-induced cardiorespiratory, thermoregulatory, or perceptual responses | [19] |
| Elite male cyclists (n = 5) | A pre–post-test | K-LCHF diet (<20 g CHO, 85% FAT, 15% protein) for 3 weeks immediately after a 1 week HCD (66% CHO, 33%FAT, 1.75 g protein/kg BW/d) | 4 weeks (3 weeks LCKD after 1 week HCD) |
| ↔ VO2max ↔ TTE performance ↑ Fat oxidation ↔ Blood glucose levels during TTE performance | [20] |
| Recreational athletes (n = 5) | Case study | K-LCHF diet (ad libitum FAT, <50 g CHO, 1.75 g protein/kg BW/d) | 10 weeks |
| ↓ TTE performance ↑ Fat oxidation during exercise even at higher intensities ↓ Body mass ↓ Skinfold thickness | [21] |
| Endurance-trained male athletes (n = 8) | A randomized, repeated-measures, crossover study | K-LCHF diet (75–80% FAT, <50 g CHO, 15–20% protein), HCD (43% CHO, 38% FAT, 19% protein) | 4.5 weeks |
| Preservation of mucosal immunity ↑ Both pro- and anti-inflammatory T-cell-related cytokine responses to a multiantigen in vitro | [22] |
| Elite race walkers (n = 25) | A mix of repeated-measures and parallel-group design | K-LCHF diet (n = 10; 75–80% FAT, <50 g CHO, 17% protein), HCD (n = 8; 60–65% CHO, 2% FAT, 15–20% protein), or PCD, (n = 7; 60–65% CHO, 20% FAT, 15–20% protein) | 3.5 weeks |
| ↔ VO2peak ↓ 10 km race walk performance ↑ Perceived exertion after exercise ↑ Oxygen cost ↑ Whole-body fat oxidation | [23] |
| Male ultra-endurance runners (n = 20) | A cross-sectional study design | K-LCHF diet (n = 10, 10:19:70) diet or Habitual high-CHO (n = 10, %CHO:protein:fat = 59:14:25) diet | An average of 20 months (range 9–36 months) |
| ↑ Fat oxidation ↔ Muscle glycogen utilization and repletion after 180 min of running and 120 min of recovery | [24] |
| Male competitive recreational distance runners (n = 7) | A randomized counterbalanced, crossover design | K-LCHF diet (n = 10; 75–80% FAT, <50 g CHO, 17% protein), or HCD (n = 8; 60–65% CHO, 20% FAT, 15–20% protein) | 6 weeks |
| ↔ VO2max ↔ TT performance ↑ Fat oxidation | [25] |
| Endurance-trained male cyclists (n = 5) | Crossover design | A high-fat diet (70% FAT) or an equal-energy, high-carbohydrate diet (70% CHO) | 2×2 weeks, 2 week washout period in between (ad libitum diet during washout period) |
| ↑ TTE performance during MIE ↔ Endurance performance during HIE ↑ Fat oxidation | [26] |
| Highly trained male ultra-endurance runners (n = 20) | A cross-sectional study design | Habitual low CHO (n = 10; <20% CHO, >60% FAT) or high CHO (n = 10; >55% CHO) | At least 6 months | ↑ Circulating total cholesterol, LDL-C, and HDL-C concentrations ↑ Fewer small, dense LDL-C particles | [27] | |
| Trained male off-road cyclists (n = 8) | A crossover design | A mixed diet (%CHO:fat:protein = 50:30:20) or a NK-LCHF diet (15:70:15) | 4 weeks | A continuous exercise protocol on a cycling ergometer with varied intensity (90 min at 85% LT, then 15 min at 115% LT) | ↑ VO2max ↓ Body mass ↓ Body fat percentage ↑ Fat oxidation ↓ Post-exercise muscle damage ↓ CK and LDH concentration at rest and during the 105 min exercise protocol in the NK-LCHF diet trial | [28] |
| Endurance trained cyclists (n = 16) | A randomized, controlled study design | A NK-LCHF diet (19:69:10) or a habitual diet (%CHO:fat:protein = 53:30:13) | 15 days | a 2.5-h constant-load ride at 70% VO2peak followed by a simulated 40-km cycling TT while ingesting a 10% 14C-glucose + 3.44% MCT emulsion at a rate of 600 mL/h | ↑ Fat oxidation ↔ TT performance | [29] |
| Trained male cyclists (n = 9) | A repeated-measures, randomized, crossover study | 2 × 0.35 g/kg KE or placebo (30 min before and 60 min after exercise) | Acute ingestion | A 85-min steady state exercise at 73% VO2max, followed by a 7 kJ/kg TT (~ 30 min) | ↑ Transient type-I T-cell immunity at the gen level | [30] |
| Endurance-trained male and female athletes (male/female, 9/3) | A single-blind, randomized and counterbalanced, crossover design | KE (330 mg/kg BW of βHB containing beverage, or bitter-flavored placebo drink before exercise | Acute ingestion | An incremental bicycle ergometer exercise test to exhaustion | ↔ Blood pH and HCO3 levels ↔ TTE performance | [31] |
| Endurance-trained athletes (male/female:5/1) | A single-blind, random order controlled, crossover design | A 400 mL, low-dose β-HB KME 252 mg/kg BW, “low ketosis”; a high-dose βHB KME (752 mg/kg BW, “high ketosis”, or a bitter-flavored water (placebo) | Acute ingestion, 60 min prior to exercise | A 60-min continuous cycling exercise, consisting of 20 min intervals at 25%, 50% and 75% Wmax | ↓ Contribution of exogenous βHB to overall energy expenditure ↑ Exercise efficiency when blood βHB levels above 2 mmol/L ↑ Nausea | [32] |
| High-performance athletes | Study 1: A randomized crossover design Study 2, 3 and 5: A randomized, single-blind, crossover design Study 4: A two-way crossover study | Study 1 (n = 6): A KE (573 mg/kg BW) drink at rest, and during 45 min of cycling exercise 40% and 75% of WMax; with 1 week washout period in between Study 2 (n = 10):
| Acute ingestion | Study 1:
| ↑ TT performance following 1 h of high-intensity exercise ↑ Fat oxidation ↓ Plasma lactate levels during exercise ↑ D-βHB oxidation according to exercise intensity (from 0.35 g/min at 40% WMax to 0.5 g/min at 75% WMax) ↔ Blood glucose levels | [33] |
| Trained male cyclists (n = 9) | A repeated-measures, randomized, crossover study | A drink containing 0.35 g/kg BW BD or placebo | Acute ingestion (30 min before and 60 min during 85 min of steady state exercise) | A steady state cycling at the power output eliciting 85% of their VT followed by a TT performance equivalent to 7 kJ/kg (~25–35 min) | ↔ TT performance and average power output ↔ Blood glucose and lactate levels ↑ Fat oxidation ↑ GI symptoms | [34] |
| Elite male cyclists (n = 10) | A randomized crossover design | A 1,3-butanediol AcAc diester (2×250 mg/kg BW) or a viscosity and color-matched plasebo drink | Acute ingestion, ~30 min before and immediately prior to commencing the warm up | ~A 31-km laboratory-based TT performance on a cycling ergometer | ↓ TT performance ↑ GI symptoms (nausea and reflux) ↑ Fat oxidation | [35] |
| Male runners (n = 11) | A randomized crossover design | An energy matched ∼650 mL drink containing 60 g CHO + 0.5 g/kg BW 1.3-butanediol (CHO-BD) or 110 g ± 5 g CHO alone | Acute ingestion (50% after baseline measurements + 25% after 30 min of seated rest, + 25% after 10 min rest period after completing submaximal running) | A 60-min submaximal running, followed by a 5-km running time trial | ↔ TT performance ↔ Overall lactate concentration ↑ Blood glucose levels after TT performance ↑ Fat oxidation | [36] |
| Highly trained male cyclists (n = 12) | A randomized crossover design | A KE drink (65 g (918,102 mg/kg, range: 722–1072 mg/kg) of KE [ 96% βHB] or a viscosity- and taste-matched placebo | Acute ingestion (at 60 and 20 min before and at 30 min during race) | A simulated cycling race, which consisted of a 3-h intermittent cycling, a 15-min time trial, and a maximal sprint | ↔ High-intensity exercise performance in the final stage of the event ↑ Upper-abdominal discomfort ↓ Appetite after exercise ↔ Net muscle glycogen breakdown | [37] |
| Recreational male distance runners (n = 13) | A randomized, double-blind, placebo-controlled, cross- over design | Either one (KS1: 22.1 g) or two (KS2: 44.2 g) servings of the ketone supplement (βHB + MCT) or a flavor-matched placebo drink | Acute ingestion (60 min prior to exercise) | A 5-km running TT on a treadmill | ↔ Post-exercise glucose concentration ↔ TT performance ↔ Perceived exertion after exercise Dose–response impact on cognitive function | [38] |
| Eight trained, middle- and long-distance runners (male/female, 7/1) | A double-blind, randomized crossover design | An 8% carbohydrate-electrolyte solution before and during exercise, either alone (CHO + PLA), or with 573 mg/kg of a ketone monoester supplement (CHO + KME) | Acute ingestion | A 60-min submaximal exercise at 65%VO2max immediately followed by a 10-km TT | ↔ TT performance ↔ VO2max, running economy, RER, HR, perceived exertion ↔ Cognitive performance ↔ Plasma glucose and lactate levels ↑ Fat oxidation | [39] |
| Male and female elite race walkers | A non-randomized clinical trial | A K-LCHF diet (n = 18; 75–80% FAT, <50 g CHO, 15–20% PRO) followed by an acute CHO restoration, or HCD (n = 14; 60–65% CHO, 20% FAT, 15–20% PRO) | 3.5 weeks | A hybrid laboratory/field test of 25 km (males) or 19 km (females) at around 50 km race pace at 75% VO2max | ↓ Bone resorption markers at rest and post-exercise ↑ Bone formation markers at rest and throughout exercise Partial recovery of these effects following CHO restoration | [40] |
| Well-trained competitive male cyclists or triathletes (n = 7) | A randomized, crossover design | Day 1: a standard CHO diet (%CHO:fat:protein = 58:27:15) Day 2–7: either an HFD (16:69:15) or HCD (70:15:15) for 6 days Day 8: HCD (70:15:15) | 6 day fat adaptation followed by 1 day CHO restoration, a 18 day washout period between | Day 9: A 4-h cycling ergometer at 65% VO2peak, followed by a 60-min TT | ↔ TT performance ↑ Fat oxidation | [41] |
| Well-trained competitive male cyclists or triathletes (n = 8) | A randomized, crossover design | Day 1–5: either an HFD (%CHO:fat:protein = 19:68:13) or an HCD (74:13:13) Day 6: HCD (74:13:13) | 5 day fat adaptation followed by 1 day CHO restoration, a 2 week washout period between | A 2-h cycling at 70% VO2max; followed by 7 kJ/kg TT | ↔ TT performance ↑ Fat oxidation ↔ Muscle glycogen utilization ↔ Plasma glucose uptake | [42] |
| Well-trained competitive male cyclists or triathletes (n = 8) | A randomized, double-blind crossover design | Day 1–5: either an HFD (%CHO:fat:protein = 19:68:13) or an HCD (74:13:13) Day 6: HCD (74:13:13) Pre-exercise: a CHO breakfast (CHO 2 g/kg). During exercise: CHO intake (0.8 g/kg/h) | 5 day fat adaptation followed by 1 day CHO restoration, a 2 week washout period between | A 2-h cycling at 70% VO2max; followed by 7 kJ/kg TT | ↔ TT performance ↑ Fat oxidation | [43] |
| Well-trained competitive male cyclists or triathletes (n = 8) | A randomized, double-blind crossover design | Day 1–5: either an HFD (%CHO:fat:protein = 19:68:13) or an HCD (74:13:13) Day 6: HCD (74:13:13) | 5 day fat adaptation followed by 1 day CHO restoration, a 2 week washout period between | A 60-min steady state ride at 70% VO2max | ↓ Muscle glycogen utilization ↑ Fat oxidation ↑ Pre-exercise AMPK-1 and AMPK-2 activity ↓ Exercise-induced AMPK-1 and AMPK-2 activity | [44] |
| Endurance-trained male cyclists (n = 8) | A randomized, single-blind, crossover design | Day 1–6: either a NK- LCHF diet (%CHO:fat:protein = 16.8:68.2:15.0) or an HCD (67.8:17.1:15.1) Day 6: HCD (16.8:68.2:15.0) | 6 day fat adaptation followed by 1 day CHO restoration, a 2 week washout period between | A 100-km TT on their bicycles; five 1 km sprint distances after 10, 32, 52, 72, and 99 km, four 4 km sprint distances after 20, 40, 60, and 80 km | ↔ TT performance ↑ Fat oxidation ↓ 1 km sprint power ↔ Perceived exertion | [45] |
| Endurance-trained male cyclists (n = 5) | Randomized, crossover design | Either 10 day habitual diet (~30% fat), followed with 3 day HCD or 10 day high-fat diet (> 65% fat), followed by 3 day HCD 1 h prior to each trial: −400 mL 3.44% MCT (C8–10) solution During trial: 600 mL/h 10% glucose (14C) + 3.44% MCT solution | 10 day HFD + 3 day HCD vs. 10 day habitual diet + 3 day HCD
| A 150-min cycling at 70% VO2peak, followed immediately by a 20-km TT | ↑ TT performance ↑ Fat oxidation ↓ Muscle glycogen utilization ↔ Body fat, BW | [46] |
| Endurance-trained male cyclists or triathletes (n = 7) | A randomized, double-blind crossover design | Day 1–5: either an HFD (%CHO:fat:protein = 19:68:13) or an HCD (74:13:13) Day 6: HCD (74:13:13) | 5 day fat adaptation, a 2 week washout period between | A 20-min steady state cycling at 70% VO2peak, 1 min rest, a 1 min all-out sprint at 150% PPO, and followed by 4 kJ/kg TT | ↑ Fat oxidation ↓ Glycogenolysis and PDH activation ↔ Muscle glycogen contents at rest | [47] |
| A lacto-ovo vegetarian athlete who adhered to an LCHF diet for 32 weeks | Case study | An LCHF diet for 32 weeks | 32 weeks | Three professional races while on the LCHF diet in week 21, 24, and 32 (consumption of CHO before and during the race as advised) | ↓ Half-ironman performance at week 21 ↓ Ironman performance at week 24 and 32 ↔ Exercise-induced GI symptoms | [48] |
| Trained male cyclists (n = 11) | A reference-controlled crossover (two treatment, two period), balanced, masked, single-center outpatient metabolic trial | HCD (% CHO:protein:fat = 73/14/12) for 2.5 days or HCD for first day and followed by the last 1.5 days with fat-enriched feeding (43/9/48) | 2.5 days (1 day HCD, followed by lipid supplementation for 1.5 day), or 2.5 day HCD | Pre- and post-intervention;
| ↔ Perceived exertion after exercise ↔ Fat oxidation during prolonged exercise ↑ Replenishment of both glycogen content and IMCL stores ↔ TT performance | [49] |
| Trained male cyclists (n = 22) | A single-blind (clinical trial staff were blinded), 2-treatment crossover randomized clinical trial | An HCD, (CHO 7.4 g/kg BW, FAT 0.5 g/kg BW) for 2.5 days or a high-CHO fat-supplemented (HCF) diet ((first day similar with HCD, followed by 1.5 days with a replication of the HC diet with 240 g surplus fat (30% saturation)) distributed over the last 4 meals of the diet period | 2.5 days (1 day HCD, followed by lipid supplementation for 1.5 day), or 2.5 day HCD | A fixed-task simulated TT lasting approximately 1-h A VO2peak test | ↔ TT performance ↔ Fat oxidation during submaximal or 1 h TT exercise ↔ Reaction time throughout TT | [50] |
| Male collegiate long-distance athletes (n = 8) | A double-blind, placebo- controlled, crossover study design | 3 days before the trial: an HCD (% CHO:fat:protein = 71:19:10) 4 h before exercise: HF meal (% CHO:fat:protein = 30:55:15) or HC meal (% CHO:fat:protein = 70:21:9) Immediately before exercise:
| Acute ingestion (either HF meal or HC meal 4 h before exercise) | An 80-min fixed-load test on a treadmill at ~70 VO2max, followed with continuous endurance running to exhaustion at ~80% VO2max | ↑ TTE performance in pre-exercise HF meal plus M consumption after CHO-loading ↑ Fat oxidation | [51] |
| Vegetarian Diets | ||||||
| Vegan (n = 24), LOV (n = 26) and omnivorous (n = 26) recreational runners | A cross-sectional study design | Omnivorous, LOV or vegan diet for at least half a year | At least 6 months | An incremental exercise test on a bicycle ergometer | ↔ maximum power output ↔ Exercise capacity ↔ Blood lactate and glucose concentration during incremental exercise | [52] |
| Vegan (n = 23), LOV (n = 25) and omnivorous (n = 25) recreational runners | A cross-sectional study design | Omnivorous, LOV or vegan diet for at least half a year | At least 6 months | An incremental exercise test on a bicycle ergometer | ↑ exercise-induced MDA concentration in the vegan (+15% rise) and LOV (+24% rise) groups ↔ NO metabolism | [53] |
| Male endurance athletes (n = 8) | A crossover design | A mixed meat-rich diet (69% animal protein sources) or a LOV diet (82% vegetable protein sources) | 2 ×6 weeks, 4 week washout period in between (ad libitum diet during washout period) |
| ↔ Immunological parameters ↑ Fiber intake ↑ P/S ratio of fatty acids ↔ VO2max capacity | [54] |
| Omnivorous, lacto-ovo vegetarian, and vegan recreational runners (21–25 subjects, respectively) | A cross-sectional study | Omnivorous, lacto-ovo-vegetarian or vegan diet for at least half a year | At least 6 months | An incremental exercise test on a bicycle ergometer | ↑ exercise-induced MDA concentration ↓ Sirtuin activities in vegans | [55] |
| A male vegan ultra-triathlete and a control group of 10 Ironman triathletes | Case report | A vegan ultra-triathlete adhered to a raw vegan diet and a control group of 10 Ironman triathletes adhered to a mixed diet | Vegan athlete living on a raw vegan diet for 6 years, vegan for 9 years and a vegetarian for 13 years | A Triple-Ironman distance (11.4 km swimming, 540 km cycling, and 126 km running) | ↑ VO2max ↔ Exercise performance ↔ Exercise capacity ↔ Systolic and diastolic functions | [56] |
| A female vegan mountain biker | Case report | A vegan athlete living on a vegan diet for approximately 15 years | A vegan diet for approximately 15 years | The Transalp Challenge 2004 (altitude climbed, 22.500 m; total distance, 662 km, lasts approximately 8 days) | Successfully completing ultra-endurance mountain biking with a well-planned and implemented vegan diet | [57] |
| Vegetarian (n = 27) and omnivore (n = 43) elite endurance athletes | Cross-sectional study design | Vegetarian and omnivore endurance athletes who adhered to their respective diets for at least three months | At least three months | A VO2max test on the treadmill | ↔ Exercise performance ↔ Protein intake (kg BW/day) ↑ VO2max (in females) ↔ VO2max (in males) | [58] |
| Vegan (n = 22) and omnivorous (n = 30) amateur runners | Cross-sectional study design | Vegan and omnivore athletes; diet adherence time not reported | - | VO2max and peak power output test on the treadmill | Better systolic and diastolic function ↑ VO2max | [59] |
| Intermittent Fasting Diets | ||||||
| Well-trained, middle-distance runners (n = 18) | A non-randomized, controlled study | RIF vs. control | 1 month | Beginning and at the end of Ramadan:
| ↓ TT exercise performance ↔ VO2max ↔ Running efficiency | [60] |
| Middle-distance athletes (n = 8) | Pre–post-test | RIF | 1 month | 5 days before, 7 and 21 days after Ramadan:
| ↓ Nocturnal sleep time ↓ Energy intake ↔ BW and body fat percentage ↔ Testosterone/cortisol ratio ↑ Fatigue ↑ Transient alteration in circulating IL-6, adrenaline, noradrenaline levels | [61] |
| Elite under 23 cyclists (n = 16) | Parallel randomized trial | Time-restrictive eating (TRE) (16 h fasting, 8 h eating periods) or normal diet; both the same energy and macronutrient composition | 4 weeks | Pre- and post-diet:
| ↔ VO2max ↔ endurance performance ↑ PPO/BW ratio ↓ BW and body fat percentage ↔ Fat-free mass | [62] |
| Male trained cyclists (n = 11) | A non-randomized repeated-measures experimental study design | Ramadan fasting (15 h 15 min fasting period) | 29 days | A slow progressively increasing training load period (endurance training at first, and then intensity training included progressively) | ↑ Perceived exertion ↑ DOMS ↔ Total sleep time ↓ duration of deep and REM sleep stages ↔ Cognitive performance | [63] |
| Adolescent male cyclists (n = 9) | A partially double-blind, placebo-controlled, randomized design | A CHO mouth rinse (with 25 mL of the solution) (CMR), a placebo mouth rinse (PMR), and a no-rinse (NOR) trial during Ramadan fasting state (fasting period ~13.5 h) | The last two weeks of Ramadan | A cycling exercise at 65% VO2peak for 30 min followed by a 10 km TT under hot (32 °C) humid (75%) condition | ↑ TT performance in the CMR and PMR groups ↓ Perceived exertion in the CMR compared to the NOR ↔ Total sleep time | [64] |
| Trained male middle- and long-distance runners (n = 17) | A randomized, parallel-group, pre-and post-experimental design | A TRE (fasting: 16 h, ad libitum eating: 8 h) (n = 10) or normal diet (n = 7) | 8 weeks | An incremental test until exhaustion | ↓ BW ↔ VO2max ↔ Running economy ↔ Blood lactate, glucose, and insulin ↓ Daily energy intake | [65] |
| Gluten-Free Diet | ||||||
| Non-coeliac or non-IBS competitive endurance cyclists (n = 13) | A controlled, randomized, double-blind, crossover study design | GFD or gluten-containing diet plus additional 2 gluten-free or gluten-containing food bars (total 16 g wheat gluten per day) | 2 × 7 days, a 10 day washout period in between | A steady state cycling at 70% Wmax for 45 min followed by a 15 min TT | ↔ TT performance ↔ GI symptoms ↔ Intestinal damage ↔ Well-being | [66] |
| Low-FODMAP Diet | ||||||
| Recreationally competitive runners with non-clinical GI symptoms (5 males, 6 females) | A single-blind, crossover design | Either a high-FODMAP or a low-FODMAP (<0.5 g FODMAP/meal) diet | 2×6 days, 1 day washout period in between |
| In the low-FODMAP group; ↔ Well-being ↓ GI symptoms | [67] |
| A female ultra- endurance runner | Case study | A 4 week low-FODMAP diet, (3.9 g FODMAP/day) | 4 week low-FODMAP diet + 6 week reintroduction of high-FODMAP foods | A 6-day 186.7 km multistage ultra-marathon | Minimal GI symptoms ↑ Nausea ↓ Energy, protein, CHO, and water intake compared to the recommended guidelines | [68] |
| A recreationally competitive multisport athlete | Case study; a single-blind approach | A 6 day low-FODMAP diet (7.2 ± 5.7g FODMAPs/day) vs. habitual diet (81 ± 5 g FODMAPs/day) | 6 days | Same training period both diet trial (Swim 60 min (day 1); cycle 60 min (day 2); rest (day 3); run intervals 70 min (day 4); cycle 180 min and steady state run 65 min (day 5) and; run intervals 65 min (day 6)) | ↓ Exercise-induced GI symptoms | [69] |
| Endurance runners (n = 18) | A double-blind randomized crossover design | A high- (46.9 ± 26.2 g FODMAP/day) or low- (2.0 ± 0.7 FODMAP/day) FODMAP diet | 2 × 1 day; before each experimental trial | A 2-h running at 60% VO2max in 35 °C ambient temperature | In the low-FODMAP group; ↓ Exercise-associated disruption of GI integrity ↓ Exercise-associated GI symptoms ↓ Breath H2 concentration | [70] |
| Type | Other Terms Mentioned in Endurance Sport Research | Definition/Application | Ref. |
|---|---|---|---|
| Vegetarian diets | |||
| Vegetarian diet | Vegetarian diet | Excludes all meats but may allow some animal products. | [99] |
| Ovo-vegetarian diet | Not detected | Excludes all meat and dairy products from the diet, but allows eggs. | [99] |
| Lacto-vegetarian diet | Not detected | Excludes all meat and eggs from the diet, but allows dairy products. | [99] |
| Lacto-ovo vegetarian diet | Lacto-ovo vegetarian diet | Excludes all types of meat from the diet, but allows the consumption of eggs and dairy products. | [99] |
| Pesco-vegetarian diet | Not detected | Excludes all animal products from the diet except fish. | [99] |
| Flexitarian diet | Not detected | A diet that flexible in terms of the consumption of animal products and allow to consume them occasionally. | [99] |
| Vegan diet | |||
| Vegan diet | Vegan diet | Excludes all animal products from the diet. | [99] |
| High-fat diets | |||
| Ketogenic low-CHO high-fat diet | Ketogenic diet; low-CHO ketogenic diet; ketogenic low-carbohydrate diet; keto-adaptation; high-fat diet; low-carbohydrate diet; low-carbohydrate, high-fat ketogenic diet | Consists of very low-CHO (20–50·g−1 day) and high-fat (75–80% of total energy) content with sufficient (15–20%) protein intake, resulting in increased ketone concentrations in blood named ketosis. | [5] |
| Non-ketogenic low-CHO high-fat diet | Non-ketogenic low-CHO high-fat diet, high-fat diet; low-carbohydrate diet | Consists of low-CHO (15–20% of total energy) and high-fat (60–65% of total energy) content with sufficient (15–20%) protein intake. | [5] |
| Acute ketone body supplementation | Ketone ester supplementation, ketone salt supplementation, a ketone monoester supplement, ketone diester ingestion, an exogenous ketone supplement | Creates exogenous ketosis, is applied in forms of either ketone salts or ketone esters. | [126] |
| CHO restoration following fat adaptation | Fat adaptation followed by CHO loading, keto-adaptation and glycogen restoration | A diet that is consumed a high-CHO diet for 1–3 days, and followed by a ketogenic or non-ketogenic high-fat diet for 5 to 14 days. | [5] |
| Intermittent fasting diets | |||
| Complete alternate-day fasting | Intermittent fasting | Includes alternate fasting days (does not allow foods and drink consumption), and eating days (allow food and drink consumption ad libitum). | [127] |
| Modified fasting | Not detected | Includes a nocturnal fasting period of 16/18/20 h and an ad libitum-eating period of 8/6/4 h, (e.g., 5:2 diet, which includes 5 days (allows for food and drink consumption ad libitum) and 2 non-consecutive days (allows the consumption of 20–25% of energy needs ad libitum)). | [127] |
| Time-restricted eating | Time-restrictive eating (16/8) | Allows food or beverages at certain time periods, including regular, extended intervals (e.g., 16:8 diet with 16 h of fasting without energy intake and 8 h of food intake ad libitum). | [127] |
| Religious fasting | Ramadan intermittent fasting, Ramadan fast, Ramadan fasting | Comprises several fasting regimens based on specific religious and spiritual purposes (e.g., Ramadan fasting involving a fasting period from sunrise to sunset). | [127] |
| Gluten-free diet | Complete exclusion of gluten and gluten-containing products. | [128] | |
| Low-FODMAP diet | |||
| Long-term FODMAP elimination | A low-FODMAP diet, low-FODMAP foods |
| [129] |
| Short-term FODMAP elimination | 24 h low-FODMAP diet | A strict FODMAP diet for 1 to 3 days before intensive training or races. | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devrim-Lanpir, A.; Hill, L.; Knechtle, B. Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review. Nutrients 2021, 13, 491. https://doi.org/10.3390/nu13020491
Devrim-Lanpir A, Hill L, Knechtle B. Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review. Nutrients. 2021; 13(2):491. https://doi.org/10.3390/nu13020491
Chicago/Turabian StyleDevrim-Lanpir, Aslı, Lee Hill, and Beat Knechtle. 2021. "Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review" Nutrients 13, no. 2: 491. https://doi.org/10.3390/nu13020491
APA StyleDevrim-Lanpir, A., Hill, L., & Knechtle, B. (2021). Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review. Nutrients, 13(2), 491. https://doi.org/10.3390/nu13020491