An Anthocyanin-Rich Extract Obtained from Portuguese Blueberries Maintains Its Efficacy in Reducing Microglia-Driven Neuroinflammation after Simulated Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Preparation of the Anthocyanin-Rich Extract and Its Digested Fraction
2.4. Quantification of Total Phenolic Content
2.5. Determination of the Phenolic Profile by HPLC-DAD-MS/MS
2.6. Cell Culture
2.7. Cell Viability
2.8. Measurement of Nitric Oxide Production
2.9. Assessment of Prostaglandin E2 Production
2.10. Quantitative Real-Time RT-PCR (qRT-PCR) for Evaluation of iNOS, COX-2, GCLC and GCLM mRNA Production
2.11. Assessment of TNF-Alpha Production
2.12. Evaluation of Intracellular Reactive Oxygen Species Production
2.13. Evaluation of GSH
2.14. Evaluation of NF-kB (p65) Activity
2.15. Evaluation of p-STAT1 (Tyr701) Levels
2.16. Statistical Analysis
3. Results
3.1. Chemical Characterisation of the Anthocyanin-Rich Extract Obtained from Portuguese Blueberries and of Its Digested Fraction
3.2. Neither the Original Anthocyanin Extract Nor Its Digested Fraction, at the Concentration Used, Affected the Viability of N9 MICROGLIAL Cells
3.3. Both the Original Anthocyanin Extract and Its Digested Fraction Reduced the Secretion of NO and PGE2, by Downregulating the mRNA Production of iNOS and COX-2, in Stimulated N9 Microglial Cells
3.4. The Anthocyanin-Rich Extract and Its Digested Fraction Reduced the Production of TNF-α in Stimulated N9 Microglial Cells
3.5. The Anthocyanin-Rich Extract and Its Digested Fraction Significantly Decreased the Intracellular Production of ROS in Stimulated N9 Microglial Cells
3.6. The Anthocyanin-Rich Extract Subjected or Not to In Vitro Digestion Significantly Increased the Levels of Reduced Glutathione (GSH), by Enhancing the mRNA Production of GCLC and GCLM, in Activated N9 Microglial Cells
3.7. Both the Original Anthocyanin-Rich Extract and the Digested Fraction Reduced NF-kB-p65 Activation without Interfering with JAK/STAT1 Pathway, in LPS- and IFN-γ-Exposed N9 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romier, B.; Schneider, Y.J.; Larondelle, Y.; During, A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofrumento, D.D.; Nicolardi, G.; Cianciulli, A.; Nuccio, F.D.; Pesa, V.L.; Carofiglio, V.; Dragone, T.; Calvello, R.; Panaro, M.A. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: Possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014, 20, 249–260. [Google Scholar] [CrossRef]
- Lofrumento, D.D.; Saponaro, C.; Cianciulli, A.; De Nuccio, F.; Mitolo, V.; Nicolardi, G.; Panaro, M.A. MPTP-Induced Neuroinflammation Increases the Expression of Pro-Inflammatory Cytokines and Their Receptors in Mouse Brain. Neuroimmunomodulation 2011, 18, 79–88. [Google Scholar] [CrossRef]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; Del Valle, J.; Pallàs, M. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixao, J.; Nunes, C.; Dinis, T.C.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, D.; Almeida, L.M.; Dinis, T.C. Anti-inflammatory protection afforded by cyanidin-3-glucoside and resveratrol in human intestinal cells via Nrf2 and PPAR-gamma: Comparison with 5-aminosalicylic acid. Chem. Biol. Interact. 2016, 260, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. The Impact of Chronic Intestinal Inflammation on Brain Disorders: The Microbiota-Gut-Brain Axis. Mol. Neurobiol. 2019, 56, 6941–6951. [Google Scholar] [CrossRef]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Miller-Fleming, L.; Pais, T.F. Microglia and inflammation: Conspiracy, controversy or control? Cell Mol. Life Sci. 2014, 71, 3969–3985. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-kappaB Function in the Nervous System. Front. Immunol. 2019, 10, 1043–1057. [Google Scholar] [CrossRef]
- Rodriguez, J.I.; Kern, J.K. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol. 2011, 7, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Young, A.M.; Campbell, E.; Lynch, S.; Suckling, J.; Powis, S.J. Aberrant NF-kappaB expression in autism spectrum condition: A mechanism for neuroinflammation. Front. Psychiatry 2011, 2, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauzour, D. Polyphenols and brain health. OCL 2017, 24, A202–A209. [Google Scholar] [CrossRef] [Green Version]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500–172510. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Ramos, T.; Bourzeix, M. Fractionation of Phenolic Compounds in Red Wine. Am. J. Enol. Vitic. 1988, 39, 259–262. [Google Scholar]
- Youdim, K.; McDonald, J.; Kalt, W.; Joseph, J. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Coates, E.M.; Popa, G.; Gill, C.I.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 4–17. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- McCallum, J.; Yang, R.; Young, J.; Strommer, J.; Tsao, R. Improved high performance liquid chromatographic separation of anthocyanin compounds from grapes using a novel mixed-mode ion-exchange reversed-phase column. J. Chromatogr. A 2007, 1148, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.K.; Maurya, A.; Singh, B.; Banerjee, K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2020, 13, 1355–1366. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Mass Bank. Available online: https://massbank.eu/MassBank/ (accessed on 29 October 2020).
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.; Brites, D.; et al. Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Phytohub. Available online: http://phytohub.eu/ (accessed on 29 October 2020).
- de Theije, C.G.; Wu, J.; da Silva, S.L.; Kamphuis, P.J.; Garssen, J.; Korte, S.M.; Kraneveld, A.D. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011, 66, S70–S80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234–6245. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.A.; Amin, F.U.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-kappaB and Akt/MAPKs signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J. Bioavailability of anthocyanins. Drug Metabol. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, B.; Zhang, Q.; Gao, N.; Zhang, X.; Meng, X. Effect of in vitro-simulated gastrointestinal digestion on the stability and antioxidant activity of blueberry polyphenols and their cellular antioxidant activity towards HepG2 cells. Int. J. Food Sci. Tech. 2017, 53, 61–71. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregorio, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Aleman, M.; Gironés-Vilaplana, A.; Álvarez, A.; López, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and In Vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5271–5278. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochem 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; Pascual-Teresa, S.d. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Nurmi, T.; Mursu, J.; Heinonen, M.; Nurmi, A.; Hiltunen, R.; Voutilainen, S. Metabolism of Berry Anthocyanins to Phenolic Acids in Humans. J. Agric. Food Chem. 2009, 57, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell Neurosci. 2018, 12, 215–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, C.; Gomes, C.; Vaz, A.R.; Brites, D. Exploring New Inflammatory Biomarkers and Pathways during LPS-Induced M1 Polarization. Mediators Inflamm. 2016, 2016, 6986175. [Google Scholar] [CrossRef]
- Hanisch, U.-K. Functional diversity of microglia—How heterogeneous are they to begin with? Front. Cell Neurosci. 2013, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tse, J.K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [Green Version]
- Dringen, R.; Hirrlinger, J. Glutathione pathways in the brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef]
- Lau, F.C.; Joseph, J.A.; McDonald, J.E.; Kalt, W. Attenuation of iNOS and COX2 by blueberry polyphenols is mediated through the suppression of NF-κB activation. J. Funct. Foods 2009, 1, 274–283. [Google Scholar] [CrossRef]
- Ge, Y.T.; Zhong, A.Q.; Xu, G.F.; Lu, Y. Resveratrol protects BV2 mouse microglial cells against LPS-induced inflammatory injury by altering the miR-146a-5p/TRAF6/NF-kappaB axis. Immunopharmacol. Immunotoxicol. 2019, 41, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.J.; Levy, D.E.; Johnstone, R.W.; Clarke, C.J. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008, 19, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
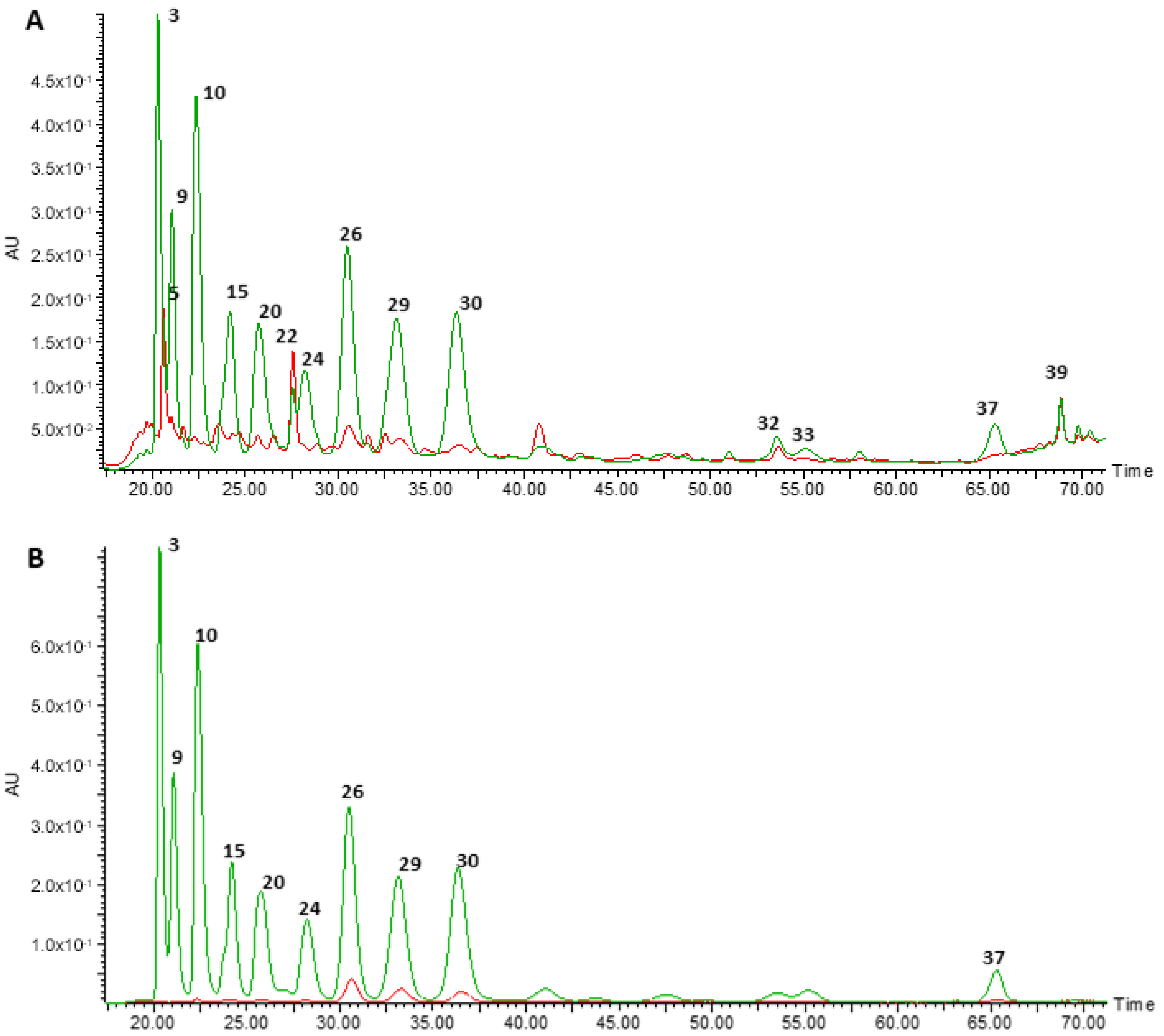





| Peak | RT (min) | λmax | m/z ([M+H]+) | MS/MS | Putative ID | Original Extract (ARE) | Digested Extract (DIG) | Recoveryears (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19.15 | - | 627 | 303; 285; 464; 333 | Delphinidin 3, 5-O-diglucoside | ARE | - | - | [31] |
| 2 | 20.00 | - | 597 | 303 | Delphinidin 3-O-sambubioside | ARE | - | - | [32] |
| 3 | 20.46 | 522 | 465 | 303; 257; 229; 153; 150 | Delphinidin 3-O-galactoside | ARE | - | - | [13,33] |
| 4 | 19.79 | 285 | 167 (-) | 152; 108; 36; 123 | Vanillic acid | ARE | DIG | 83 | [34] |
| 5 | 20.74 | 279 | 205 | 118; 146; 144; 132 | Tryptophan | ARE | DIG | 779 | [35] |
| 6 | 20.76 | 280 | 315 (-) | 153; 123 | Protocatechuic acid 4-O-glucoside | ARE | DIG | 136 | [36] |
| 7 | 21.03 | 287, 322 | 355 (-) | 192; 193 | Ferulic acid hexoside I | ARE | DIG | 473 | [36] |
| 8 | 21.16 | - | 515 (-) | 191 | Dicaffeoylquinic acid I | ARE | DIG | 184 | [34] |
| 9 | 21.20 | 522 | 465 | 303; 229; 257; 153 | Delphinidin 3-O-glucoside | ARE | - | - | [13,33] |
| 10 | 22.35 | 522 | 449 | 287; 137; 213; 241; 231; 269 | Cyanidin 3-galactoside | ARE | DIG | 8 | [13,33] |
| 11 | 22.50 | 284, 321 | 433 (-) | 300; 301 | Ellagic acid pentoside | ARE | - | [35] | |
| 12 | 22.87 | 287, 318 | 355 (-) | 192; 193 | Ferulic acid hexoside II | - | DIG | - | [36] |
| 13 | 23.85 | 517 | 449 | 287; 137; 213; 241; 231; 269 | Cyanidin 3-glucoside | ARE | DIG | 2 | [13,33] |
| 14 | 24.12 | 263, 324 | 325 (-) | 192; 193; 165 | Fertaric acid | - | DIG | - | [36] |
| 15 | 24.29 | 525 | 479 | 317; 302; 274; 257; 217; 203 | Petunidin 3-galactoside | ARE | DIG | 3 | [13,33] |
| 16 | 24.49 | 280 | 227 (-) | 135; 153 | Resveratrol | - | DIG | - | [36] |
| 17 | 25.40 | 522 | 595 | 287; 331; 247 | Cyanidin 3-rutinoside | ARE | - | - | [35] |
| 18 | 25.70 | 522 | 419 | 286; 109; 149; 129 | Cyanidin 3-arabinoside | ARE | DIG | 3 | [13,33] |
| 19 | 25.90 | 524 | 705 (-) | 513 | Delphinidin hexoside dimmer I | ARE | DIG | 110 | - |
| 20 | 25.99 | 522 | 479 | 317; 302; 274; 203; 85; 245 | Petunidin 3-glucoside | ARE | DIG | 3 | [13,33] |
| 21 | 26.49 | - | 515 (-) | 191 | Dicaffeoylquinic acid II | ARE | DIG | 111 | [34] |
| 22 | 27.64 | 289, 315 | 355 (-) | 193; 134; 149 | Ferulic acid hexoside III | ARE | DIG | 138 | [36] |
| 23 | 28.06 | 526 | 463 | 301; 286; 203; 258 | Peonidin 3-galactoside | ARE | DIG | 17 | [13,33] |
| 24 | 28.29 | 526 | 449 | 317; 302; 274; 245 | Petunidin 3-arabinoside | ARE | - | - | [13,33] |
| 25 | 29.60 | - | 515 (-) | 191 | Dicaffeoylquinic acid III | ARE | DIG | 121 | [34] |
| 26 | 30.49 | 525 | 493 | 331; 315; 287; 270; 299; 150 | Malvidin 3-galactoside | ARE | DIG | 19 | [13,33] |
| 27 | 30.54 | 525 | 463 | 301; 286; 213; 258 | Peonidin 3-glucoside | ARE | DIG | 17 | [13] |
| 28 | 32.67 | 326, 295 | 353 (-) | 191; 85 | Caffeoyl quinic acid | ARE | DIG | 186 | [36] |
| 29 | 33.13 | 525 | 493 | 331; 315; 287; 270; 299; 242; 179 | Malvidin 3-glucoside | ARE | DIG | 18 | [13,33] |
| 30 | 36.59 | 526 | 463 | 331; 315; 287; 270; 179; 150 | Malvidin-3-arabinoside | ARE | DIG | 14 | [13,33] |
| 31 | 39.35 | 525 | 433 | 85; 86; 72; 301; 124; 182 | Peonidin 3-arabinoside | ARE | DIG | 85 | [13] |
| 32 | 51.25 | 522 | 705 (-) | 513; 339; 300 | Delphinidin hexoside dimmer II | ARE | DIG | 21 | - |
| 33 | 53.69 | 517 | 705 (-) | 513 | Delphinidin hexoside dimmer III | ARE | DIG | 21 | - |
| 34 | 55.35 | 524 | 535 | 331; 315; 287; 299; 270; 242 | Malvidin 3′-(6″-acetyl-galactoside) | ARE | DIG | 12 | [33] |
| 35 | 58.33 | 347, 525 | 319 | 153; 165; 111; 273; 245; 301 | Myricetin | ARE | DIG | 35 | [34] |
| 36 | 60.64 | - | 475 (-) | - | Ellagic acid 4-acetylpentoside | ARE | DIG | 146 | [35] |
| 37 | 65.42 | 529 | 535 | 331; 315; 287; 299; 179; 270; 242 | Malvidin 3′-(6″-acetyl-glucoside) | ARE | DIG | 11 | [33] |
| 38 | 68.27 | - | 611 | 303; 166; 71; 238; 350; 137; 153 | Rutin | ARE | DIG | 67 | - |
| 39 | 69.08 | 348 | 611 | 303; 129; 85; 71; 145; 137; 153; 229 | Hesperidin | ARE | DIG | 80 | - |
| 40 | 68.32 | - | 465 | 303; 85; 137; 153; 229; 257; 165 | Quercetin hexoside I | ARE | DIG | 72 | [33] |
| 41 | 68.99 | 354 | 465 | 303; 85; 137; 153; 229; 257; 165 | Quercetin hexoside II | ARE | DIG | 88 | [33] |
| 42 | 69.86 | - | 465 | 303; 85; 137; 153; 229; 257; 165 | Quercetin hexoside III | ARE | DIG | 35 | [33] |
| 43 | 72.00 | 272 | 477 (-) | 301 | Quercetin 3-glucuronide | ARE | DIG | 49 | [35] |
| 44 | 72.76 | - | 287 | 121; 241; 145; 153 | Cyanidin | ARE | DIG | 71 | [34] |
| 45 | 72.96 | 348 | 625 | 317; 302; 153; 139; 285; 274 | Isorhamnetin-3-O-rutinoside/Isorhamnetin-3-O-galactoside-6”-rhamnoside/Myricetin 3-O-rutinoside | ARE | DIG | 79 | - |
| 46 | 73.37 | 353 | 625 | 317; 85; 129; 243; 75; 111; 302; 285; 153; 274 | Isorhamnetin-3-O-rutinoside/Isorhamnetin-3-O-galactoside-6″-rhamnoside/Myricetin 3-O-rutinoside | ARE | DIG | 83 | - |
| 47 | 73.78 | 350 | 549 (-) | 505; 300; 301; 355; 429; 63 | Quercetin 3-O-(6″-malonyl-glucoside) | ARE | DIG | 42 | [36] |
| 48 | 74.52 | - | 509 | 347; 103; 314; 85; 287; 286; 286; 153; 331; 139 | Syringetin-3-O-galactoside | ARE | - | - | - |
| 49 | 75.72 | - | 509 | 347; 287; 229; 291; 165; 286; 153; 331; 139 | Syringetin-3-O-glucoside | ARE | - | - | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, D.; Henriques, J.F.; Serra, T.; Bento Silva, A.; Bronze, M.R.; Dinis, T.C.P.; Almeida, L.M. An Anthocyanin-Rich Extract Obtained from Portuguese Blueberries Maintains Its Efficacy in Reducing Microglia-Driven Neuroinflammation after Simulated Digestion. Nutrients 2020, 12, 3670. https://doi.org/10.3390/nu12123670
Serra D, Henriques JF, Serra T, Bento Silva A, Bronze MR, Dinis TCP, Almeida LM. An Anthocyanin-Rich Extract Obtained from Portuguese Blueberries Maintains Its Efficacy in Reducing Microglia-Driven Neuroinflammation after Simulated Digestion. Nutrients. 2020; 12(12):3670. https://doi.org/10.3390/nu12123670
Chicago/Turabian StyleSerra, Diana, Joana F. Henriques, Teresa Serra, Andreia Bento Silva, Maria Rosário Bronze, Teresa C. P. Dinis, and Leonor M. Almeida. 2020. "An Anthocyanin-Rich Extract Obtained from Portuguese Blueberries Maintains Its Efficacy in Reducing Microglia-Driven Neuroinflammation after Simulated Digestion" Nutrients 12, no. 12: 3670. https://doi.org/10.3390/nu12123670
APA StyleSerra, D., Henriques, J. F., Serra, T., Bento Silva, A., Bronze, M. R., Dinis, T. C. P., & Almeida, L. M. (2020). An Anthocyanin-Rich Extract Obtained from Portuguese Blueberries Maintains Its Efficacy in Reducing Microglia-Driven Neuroinflammation after Simulated Digestion. Nutrients, 12(12), 3670. https://doi.org/10.3390/nu12123670





