The Dietary Replacement of Soybean Oil by Canola Oil Does Not Prevent Liver Fatty Acid Accumulation and Liver Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Analysis of Liver Fatty Acid Composition
2.3. Gene Expression Measurement
2.4. Statistical Analysis
3. Results
3.1. Food Intake, Body Weight, Liver Weight, Serum Glucose, Triacylglycerol, and Cholesterol
3.2. Saturated Fatty Acid (SFA) Composition
3.3. Monounsaturated Fatty Acid (MUFA) Composition
3.4. Polyunsaturated n-6 Fatty Acid (PUFA) Composition
3.5. Polyunsaturated n-3 Fatty Acid (PUFA) Composition
3.6. Analysis of Fatty Acids (FAs) Family Composition and n-6:n-3ratio
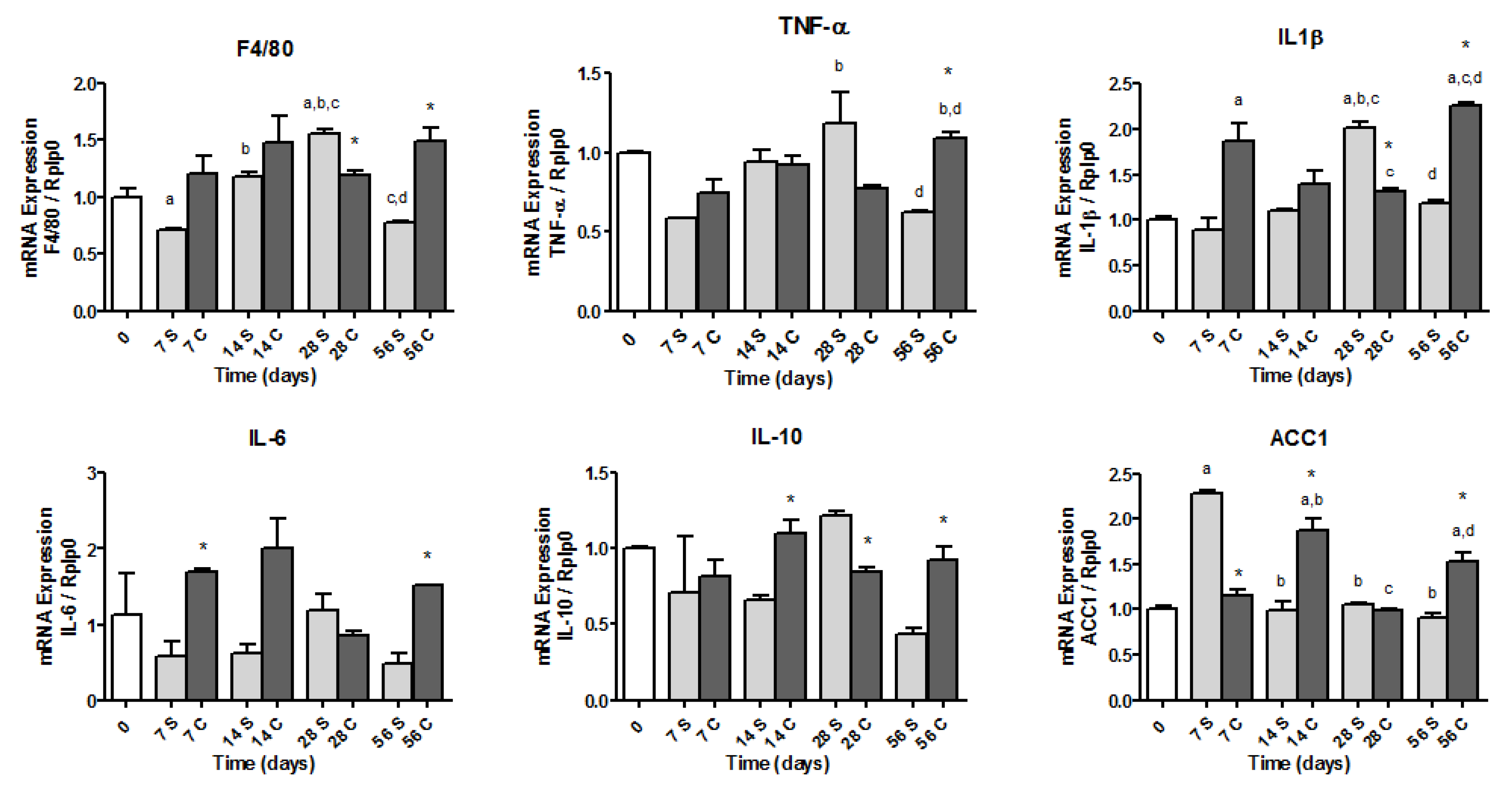
3.7. Gene Expressions
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The state of the disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Mae, A. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008, 19, 295–300. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Liver fatty acid composition and inflammation in mice fed with high-carbohydrate diet or high-fat diet. Nutrients 2016, 8, 682. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Bezerra, R.M.N.; Ueno, M.; Silva, M.S.; Tavares, D.Q.; Carvalho, C.R.O.; Saad, M.J.A.; Gontijo, J.A.R. A high-fructose diet induces insulin resistance but not blood pressure changes in normotensive rats. Braz. J. Med. Biol. Res. 2001, 34, 1155–1160. [Google Scholar] [CrossRef]
- da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Brain fatty acid composition and inflammation in mice fed with high-carbohydrate diet or high-fat diet. Nutrients 2018, 10, 1277. [Google Scholar] [CrossRef]
- Antunes, M.M.; Godoy, G.; de Almeida-Souza, C.B.; da Rocha, B.A.; da Silva-Santi, L.G.; Masi, L.N.; Carbonera, F.; Visentainer, J.V.; Curi, R.; Bazotte, R.B. A high-carbohydrate diet induces greater inflammation than a high-fat diet in mouse skeletal muscle. Braz. J. Med. Biol. Res. 2020, 53, 4–11. [Google Scholar] [CrossRef]
- Antunes, M.M.; Godoy, G.; Crisma, A.R.; Masi, L.N.; Curi, R. Adipose tissue is less responsive to food restriction anti-inflammatory effects than liver, muscle, and brain in mice. Braz. Arch. Biol. Technol. 2019, 52, 1–5. [Google Scholar] [CrossRef]
- de Almeida-Souza, C.; Antunes, M.M.; Godoy, G.; Schamber, C.; Silva, M.; Bazotte, R.B. Interleukin-12 as a biomarker of the bene fi cial effects of food restriction in mice receiving high fat diet or high carbohydrate diet. Braz. J. Med. Biol. Res. 2018, 51, 10–13. [Google Scholar] [CrossRef]
- Lee, C.H.; Fu, Y.; Yang, S.J.; Chi, C.C. Effects of omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: A systematic review and meta-analysis. Nutrients 2020, 12, 2769. [Google Scholar] [CrossRef]
- Tanaka, N.; Zhang, X.; Sugiyama, E.; Kono, H.; Horiuchi, A.; Nakajima, T.; Kanbe, H.; Tanaka, E.; Gonzalez, F.J.; Aoyama, T. Eicosapentaenoic acid improves hepatic steatosis independent of PPAR alpha activation through inhibition of SREBP-1 maturation in mice. Biochem. Pharmacol. 2010, 80, 1601–1612. [Google Scholar] [CrossRef]
- Alwayn, I.P.J.; Andersson, C.; Zauscher, B.; Gura, K.; Nosé, V.; Puder, M. Omega-3 fatty acids improve hepatic steatosis in a murine model: Potential implications for the marginal steatotic liver donor. Transplantation 2005, 79, 606–608. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 1–7. [Google Scholar] [CrossRef]
- Araya, J.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n − 6/n − 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Modulation of hepatic steatosis by dietary fatty acids. World J. Gastroenterol. 2014, 20, 1746–1755. [Google Scholar] [CrossRef]
- Misra, A.; Singhal, N.; Khurana, L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: Role of dietary fats and oils. J. Am. Coll. Nutr. 2010, 29, 289S–301S. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-tod, S.; Berger, A. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef]
- Gulesserian, T.; Widhalm, K. Effect of a rapeseed oil substituting diet on serum lipids and lipoproteins in children and adolescents with familial hypercholesterolemia. J. Am. Coll. Nutr. 2002, 21, 103–108. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Pedersen, A.; Sandström, B.; Marckmann, P.; Høy, C.E. Different effects of diets rich in olive oil, rapeseed oil and sunflower-seed oil on postprandial lipid and lipoprotein concentrations and on lipoprotein oxidation susceptibility. Br. J. Nut. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Junker, R.; Kratz, M.; Neufeld, M.; Erren, M.; Nofer, J.R.; Schulte, H.; Nowak-Göttl, U.; Assmann, G.; Wahrburg, U. Effects of diets containing olive oil, sunflower oil, or rapeseed oil on the hemostatic system. Thromb. Haemost. 2001, 85, 280–286. [Google Scholar]
- Gillingham, L.G.; Gustafson, J.A.; Han, S.-Y.; Jassal, D.S.; Jones, P.J. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br. J. Nutr. 2011, 105, 417–427. [Google Scholar] [CrossRef]
- Bowen, K.J.; Kris-Etherton, P.; West, S.G.; A Fleming, J.; Connelly, P.W.; Lamarche, B.; Couture, P.; A Jenkins, D.J.; Taylor, C.G.; Zahradka, P.; et al. Diets Enriched with Conventional or High-Oleic Acid Canola Oils Lower Atherogenic Lipids and Lipoproteins Compared to a Diet with a Western Fatty Acid Profile in Adults with Central Adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Zare, M.; Faghih, S. Effects of canola oil consumption on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. J. Am. Coll. Nutr. 2019, 38, 185–196. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Faulkner, D.; Augustin, L.S.A.; Mitchell, S.; Ireland, C.; Srichaikul, K.; Mirrahimi, A.; Chiavaroli, L.; et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care 2014, 37, 1806–1814. [Google Scholar] [CrossRef]
- Kruse, M.; von Loeffelholz, C.; Hoffmann, D.; Pohlmann, A.; Seltmann, A.C.; Osterhoff, M.; Hornemann, S.; Pivovarova, O.; Rohn, S.; Jahreis, G.; et al. Dietary rapeseed/canola-oil supplementation reduces serum lipids and liver enzymes and alters postprandial inflammatory responses in adipose tissue compared to olive-oil supplementation in obese men. Mol. Nutr. Food Res. 2015, 59, 507–519. [Google Scholar] [CrossRef]
- Cano-Europa, E.; Ortiz-Butron, R.; Camargo, E.M.; Esteves-Carmona, M.M.; Oliart-Ros, R.M.; Blas-Valdivia, V.; Franco-Colin, M. A canola oil-supplemented diet prevents type 1 diabetes-caused lipotoxicity and renal dysfunction in a rat model. J. Med. Food 2016, 19, 1041–1047. [Google Scholar] [CrossRef]
- Chisholm, A.; Mc Auley, K.; Mann, J.; Williams, S.; Skeaff, M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 284–292. [Google Scholar] [CrossRef]
- Papazzo, A.; Conlan, X.A.; Lexis, L.; Lewandowski, P.A. Differential effects of dietary canola and soybean oil intake on oxidative stress in stroke-prone spontaneously hypertensive rats. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Miyazaki, M.; Takemura, N.; Watanabe, S.; Hata, N.; Misawa, Y.; Okuyama, H. Dietary docosahexaenoic acid ameliorates, but rapeseed oil and safflower oil accelerate renal injury in stroke-prone spontaneously hypertensive rats as compared with soybean oil, which is associated with expression for renal transforming growth factor-β. Biochim. Biophys. Acta 2000, 1483, 101–110. [Google Scholar] [CrossRef]
- Ratnayake, W.M.N.; Plouffe, L.; Hollywood, R.; L’Abbé, M.R.; Hidiroglou, N.; Sarwar, G.; Mueller, R. Influence of sources of dietary oils on the life span of stroke-prone spontaneously hypertensive rats. Lipids 2000, 35, 409–420. [Google Scholar] [CrossRef]
- Huang, M.Z.; Watanabe, S.; Kobayashi, T.; Nagatsu, A.; Sakakibara, J.; Okuyama, H. Unusual effects of some vegetable oils on the survival time of stroke- prone spontaneously hypertensive rats. Lipids 1997, 32, 745–751. [Google Scholar] [CrossRef]
- Naito, Y.; Nagata, T.; Takano, Y.; Nagatsu, T.; Ohara, N. Rapeseed oil ingestion and exacerbation of hypertension-related conditions in stroke prone spontaneously hypertensive rats. Toxicology 2003, 187, 205–216. [Google Scholar] [CrossRef]
- Lauretti, E.; Praticò, D. Effect of canola oil consumption on memory, synapse and neuropathology in the triple transgenic mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Naito, Y.; Kasama, K.; Yoshida, H.; Ohara, N. Thirteen-week dietary intake of rapeseed oil or soybean oil as the only dietary fat in Wistar Kyoto rats change in blood pressure. Food Chem. Toxicol. 2000, 38, 811–816. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Damiano, F.; Siculella, L.; Zara, V. Differential effects of high-carbohydrate and high-fat diets on hepatic lipogenesis in rats. Eur. J. Nutr. 2014, 53, 1103–1114. [Google Scholar] [CrossRef]
- Ishii, S.; Iizuka, K.; Miller, B.C.; Uyeda, K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc. Natl. Acad. Sci. USA 2004, 101, 15597–15602. [Google Scholar] [CrossRef]
- Figueiredo, I.L.; Claus, T.; Oliveira Santos Júnior, O.; Almeida, V.C.; Magon, T.; Visentainer, J.V. Fast derivatization of fatty acids in different meat samples for gas chromatography analysis. J. Chromatogr. A 2016, 1456, 235–241. [Google Scholar] [CrossRef]
- Visentainer, J.V. Aspectos analíticos da resposta do detector de ionização em chama para ésteres de ácidos graxos em biodiesel e alimentos. Quim. Nova 2012, 35, 274–279. [Google Scholar] [CrossRef]
- Dentin, R.; Pégorier, J.P.; Benhamed, F.; Foufelle, F.; Ferré, P.; Fauveau, V.; Magnuson, M.A.; Girard, J.; Postic, C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 2004, 279, 20314–20326. [Google Scholar] [CrossRef] [PubMed]
- Howell, G., 3rd; Deng, X.; Yellaturu, C.; Park, E.A.; Wilcox, H.G.; Raghow, R.; Elam, M.B. N-3 polyunsaturated fatty acids suppress insulin-induced SREBP-1c transcription via reduced trans-activating capacity of LXRalpha. Biochim. Biophys Acta 2009, 1791, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.W.; Li, Y. DHA regulates lipogenesis and lipolysis genes in mice adipose and liver. Mol. Biol. Rep. 2011, 38, 731–737. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Amin, A.M.; Siao, K.; Her, C.; Fitch, M.; Beysen, C.; Turner, S.M.; Goodsell, A.; Baron, J.L.; Grenert, J.P.; et al. Specific macronutrients exert unique influences on the adipose-liver axis to promote hepatic steatosis in mice. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 223–236. [Google Scholar] [CrossRef]
- Liao, C.C.; Ou, T.T.; Huang, H.P.; Wang, C.J. The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up-regulation of AMP-activated kinase. J. Sci. Food Agric. 2014, 94, 1154–1162. [Google Scholar] [CrossRef]
- Gu, L.Y.; Qiu, L.W.; Chen, X.F.; Lü, L.; Mei, Z.C. Oleic acid-induced hepatic steatosis is coupled with downregulation of aquaporin 3 and upregulation of aquaporin 9 via activation of p38 signaling. Horm. Metab. Res. 2015, 47, 259–264. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Rosso, N.; Tiribelli, C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12, 20. [Google Scholar] [CrossRef]
- Sijben, J.W.C.; Calder, P.C. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc. Nutr. Soc. 2007, 66, 237–259. [Google Scholar] [CrossRef]
- Mirea, A.M.; Tack, C.J.; Chavakis, T.; Joosten, L.A.B.; Toonen, E.J.M. IL-1 family cytokine pathways underlying NAFLD: Towards new treatment strategies. Trends Mol. Med. 2018, 24, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Carpino, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The role of tissue macrophage mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat. Inflamm. 2017, 2017, 8162421. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- Hanke, D.; Zahradka, P.; Mohankumar, S.K.; Clark, J.L.; Taylor, C.G. A diet high in α-linolenic acid and monounsaturated fatty acids attenuates hepatic steatosis and alters hepatic phospholipid fatty acid profile in diet-induced obese rats. Prostaglandins Leukot. Essent. Fat Acids 2013, 89, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Lambelet, P.; Grandgirard, A.; Gregoire, S.; Juaneda, P.; Sebedio, J.L.; Bertoli, C. Formation of modified fatty acids and oxyphytosterols during refining of low erucic acid rapeseed oil. J. Agric. Food Chem. 2003, 51, 4284–4290. [Google Scholar] [CrossRef] [PubMed]
- Mboma, J.; Leblanc, N.; Angers, P.; Rocher, A.; Vigor, C.; Oger, C.; Reversat, G.; Vercauteren, J.; Galano, J.M.; Durand, T.; et al. Effects of cyclic fatty acid monomers from heated vegetable oil on markers of inflammation and oxidative stress in male Wistar rats. J. Agric. Food Chem. 2018, 66, 7172–7180. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Gao, H.; Chen, C.; Deng, Q.; Huang, Q.; Ma, Z.; Huang, F.; Huang, F. Optimized rapeseed oils rich in endogenous micronutrients protect high fat diet fed rats from hepatic lipid accumulation and oxidative stress. Nutrients 2015, 7, 8491–8502. [Google Scholar] [CrossRef]
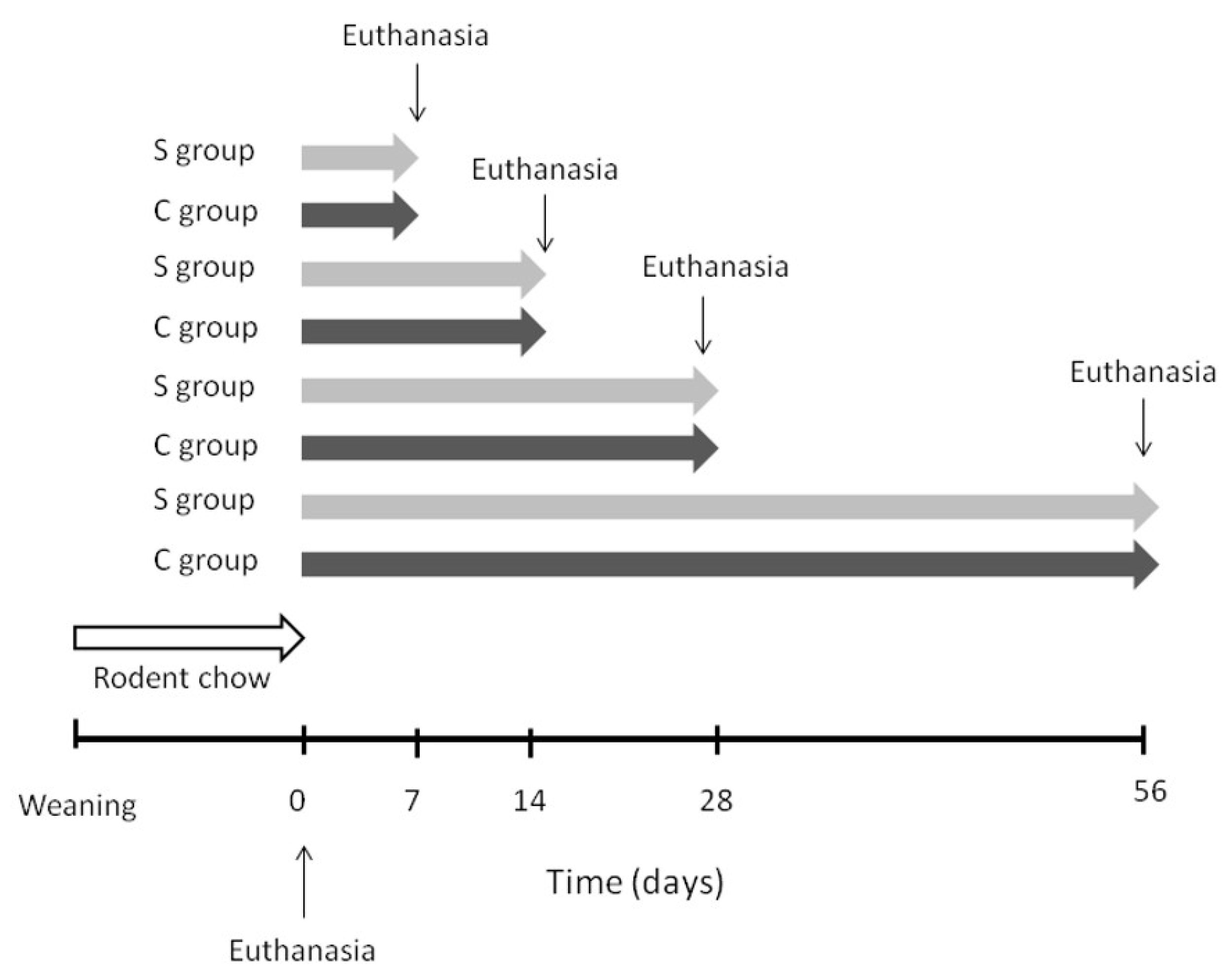
| Fatty Acids | S Group | C Group |
|---|---|---|
| Palmitic acid (16:0) | 70.01 ± 1.12 | 40.24 ± 0.37 * |
| Stearic acid (18:0) | 26.25 ± 0.40 | 20.19 ± 0.13 * |
| Oleic acid (18:1n-9) | 150.88 ± 2.24 | 420.41 ± 3.85 * |
| Vaccenic acid (18:1n-7) | 8.34 ± 0.16 | 21.52 ± 0.23 * |
| Linoleic acid (18:2n-6) | 303.97 ± 4.46 | 136.98 ± 1.19 * |
| α-Linolenic acid (18:3n-3) | 31.27 ± 0.46 | 61.43 ± 0.72 * |
| SFA | 96.27 ± 1.53 | 60.43 ± 0.51 * |
| MUFA | 159.23 ± 2.40 | 441.93 ± 4.09 * |
| PUFA | 336.79 ± 4.94 | 198.41 ± 1.92 * |
| n-6 | 305.52 ± 4.47 | 136.98 ± 1.19 * |
| n-3 | 31.27 ± 0.46 | 61.43 ± 0.72 * |
| n-6/n-3 | 9.76 ± 0.00 | 2.58 ± 0.00 * |
| Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | ||
|---|---|---|---|---|---|---|---|
| Food intake (g/day) | S group | - | 9.54 ± 0.97 | 10.10 ± 0.57 | 9.10 ± 0.40 | 7.71 ± 0.13 | - |
| C group | - | 10.46 ± 0.74 | 8.83 ± 0.62 | 9.04 ± 0.60 | 8.35 ± 0.14 * | - | |
| Body weight (g) | S group | 34.78 ± 0.42 | 36.38 ± 1.16 | 40.55 ± 1.05 | 51.58 ± 2.41 a,b,c | 47.70 ± 2.03 a,b,c | 37.1 |
| C group | 37.28 ± 0.93 | 44.46 ± 1.23 * | 52.66 ± 3.92 a,b | 46.27 ± 3.42 a | 33.0 | ||
| Liver weight (g) | S group | 1.42 ± 0.02 | 1.33 ± 0.16 | 1.45 ± 0.03 | 1.74 ± 0.07 a,b | 1.51 ± 0.04 | 6.3 |
| C group | 1.36 ± 0.10 | 1.68 ± 0.09 | 1.97 ± 0.22 a,b | 1.73 ± 0.03 * | 21.8 | ||
| Relative liver weight (g/100g) | S group | 4.10 ± 0.06 | 3.67 ± 0.51 | 3.58 ± 0.03 | 3.40 ± 0.11 | 3.24 ± 0.05 a | −20.9 |
| C group | 3.64 ± 0.22 | 3.63 ± 0.06 | 3.72 ± 0.21 | 3.49 ± 0.09 * | −14.8 | ||
| Glucose (mg/dL) | S group | 108.70 ± 6.43 | 104.60 ± 11.80 | 92.26 ± 4.71 | 91.96 ± 8.15 | 116.16 ± 7.09 | 6.8 |
| C group | 96.26 ± 4.13 | 100.23 ± 7.48 | 101.20 ± 13.41 | 126.30 ± 9.06 | 16.2 | ||
| Triacylglycerol (mg/dL) | S group | 90.18 ± 11.24 | 173.16 ± 14.22 a | 212.08 ± 39.13 a | 167.33 ± 13.47 | 160.99 ± 19.02 a | 78.5 |
| C group | 248.22 ± 21.37 a,* | 315.15 ± 57.84 a | 237.90 ± 18.22 a,* | 170.55 ± 11.04 c | 89.1 | ||
| Cholesterol (mg/dL) | S group | 132.38 ± 5.87 | 157.00 ± 8.34 | 124.60 ± 11.40 | 163.20 ± 11.32 | 182.86 ± 9.22 a,c | 38.1 |
| C group | 151.60 ± 13.81 | 131.83 ± 20.55 | 199.20 ± 15.87 a,c | 191.13 ± 9.69 a,c | 44.3 |
| SFA | Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | |
|---|---|---|---|---|---|---|---|
| Lauric acid (12:0) | S group | 1.37 ± 0.07 | 4.23 ± 0.81 a | 4.27 ± 0.24 a | 2.21 ± 0.08 b,c | 2.88 ± 0.43 a | 110.2 |
| C group | 5.53 ± 0.37 a | 2.33 ± 0.12 a,b,* | 2.06 ± 0.06 b | 2.40 ± 0.30 a,b | 75.1 | ||
| Myristic acid (14:0) | S group | 6.36 ± 0.39 | 52.58 ± 12.98 a | 26.65 ± 1.48 a,b | 22.49 ± 0.57 a,b | 33.53 ± 0.61 a,b | 427.2 |
| C group | 36.02 ± 2.38 a | 26.58 ± 2.53 a | 34.58 ± 1.13 a,* | 37.85 ± 3.08 a,c | 495.1 | ||
| Palmitic acid (16:0) | S group | 451.55 ± 31.74 | 1176.55 ± 68.73 a | 1267.90 ± 48.82 a | 1245.10 ± 13.58 a | 1567.95 ± 44.03 a,b,c,d | 247.2 |
| C group | 1453.63 ± 4.10 a,* | 1312.49 ± 62.84 a | 1735.27 ± 12.6 a,b,c,* | 1644.88 ± 37.52 a,b,c | 264.2 | ||
| Stearic acid (18:0) | S group | 219.57 ± 16.4 | 447.56 ± 25.02 a | 453.21 ± 11.55 a | 404.55 ± 9.46 a | 411.70 ± 12.85 a | 87.5 |
| C group | 469.20 ± 0.59 a | 464.50 ± 16.42 a | 469.79 ± 2.88 a,* | 391.29 ± 8.58 a,b,c,d | 78.2 | ||
| Arachidic acid (20:0) | S group | 3.19 ± 0.19 | 10.30 ± 0.98 a | 6.70 ± 0.72 a,b | 7.64 ± 0.21 a | 12.15 ± 0.28 a,c,d | 280.8 |
| C group | 17.37 ± 0.60 a,* | 10.37 ± 0.48 a,b,* | 9.88 ± 0.39 a,b,* | 16.84 ± 1.31 a,c,d,* | 427.8 | ||
| Heneicosanoic acid (21:0) | S group | 6.35 ± 0.41 | 9.28 ± 0.17 a | 3.77 ± 0.59 a,b | 5.95 ± 0.09 b,c | 8.09 ± 0.45 a,c,d | 27.4 |
| C group | 10.79 ± 0.52 a | 4.64 ± 0.14 a,b | 3.56 ± 0.15 a,b,* | 4.17 ± 0.17 a,b,* | −34.3 |
| MUFA | Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | |
|---|---|---|---|---|---|---|---|
| Palmitoleic acid (16:1n-7) | S group | 20.82 ± 1.45 | 121.40 ± 6.32 a | 186.67 ± 10.21 a | 205.95 ± 4.01 a,b,c | 308.54 ± 6.63 a,b,c,d | 1381.9 |
| C group | 168.55 ± 1.01 a,* | 183.85 ± 12.39 a | 265.35 ± 9.80 a,b,c,* | 355.62 ± 10.61 a,b,c,d,* | 1608.0 | ||
| 7-hexadecanoic acid (16:1n-9) | S group | 7.84 ± 0.42 | 21.42 ± 1.00 a | 21.63 ± 1.50 a | 26.53 ± 0.72 a,b,c | 42.50 ± 1.18 a,b,c,d | 442.0 |
| C group | 40.48 ± 0.62 a,* | 48.13 ± 1.35 a,* | 157.33 ± 8.77 a,b,c,* | 69.90 ± 2.18 a,b,c,d,* | 791.5 | ||
| Vaccenic acid (18:1n-7) | S group | 31.72 ± 2.24 | 92.76 ± 3.17 a | 116.16 ± 4.76 a | 140.88 ± 1.90 a,b,c | 225.60 ± 9.28 a,b,c,d | 611.2 |
| C group | 133.74 ± 0.59 a,* | 144.52 ± 6.14 a,* | 250.94 ± 1.55 a,b,c,* | 271.54 ± 6.02 a,b,c,d,* | 756.0 | ||
| Oleic acid (18:1n-9) | S group | 225.55 ± 13.84 | 1283.73 ± 157.30 a | 1197.56 ± 71.24 a | 1222.64 ± 18.93 a | 1789.55 ± 49.59 a,b,c,d | 693.4 |
| C group | 1633.75 ± 12.65 a | 1766.14 ± 77. 38 a,* | 3402.46 ± 5.38 a,b,c,* | 2814.65 ± 80.90 a,b,c,d,* | 1147.9 | ||
| Gondoic acid (20:1n-9) | S group | 3.31 ± 0.22 | 9.00 ± 0.79 a | 12.70 ± 0.78 a,b | 12.98 ± 0.21 a,b | 23.78 ± 1.01 a,b,c,d | 618.4 |
| C group | 16.37 ± 0.13 a,* | 16.30 ± 0.92 a,* | 27.06 ± 0.19 a,b,c,* | 35.66 ± 2.48 a,b,c,d,* | 977.3 | ||
| Nervonic acid (24:1n-9) | S group | 14.23 ± 0.72 | 12.11 ± 0.08 | 12.70 ± 2.16 | 12.91 ± 0.05 | 12.25 ± 0.60 | −13.9 |
| C group | 20.01 ± 1.18 a,* | 17.01 ± 0.45 b | 16.50 ± 0.27 * | 21.67 ± 0.53 a,c,d,* | 52.2 |
| n-6 PUFA | Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | |
|---|---|---|---|---|---|---|---|
| Linoleic acid (18:2n-6) | S group | 525.33 ± 32.43 | 1534.81 ± 153.60 a | 1411.13 ± 67.48 a | 964.554 ± 11.09 a,b,c | 1330.23 ± 43.46 a | 153.2 |
| C group | 1731.06 ± 25.28 a | 827.076 ± 33.06 a,b,* | 677.56 ± 9.56 a,b,c,* | 830.28 ± 30.48 a.b,d,* | 58.0 | ||
| γ-linolenic acid (18:3n-6) | S group | 14.26 ± 0.82 | 30.98 ± 1.64 a | 32.65 ± 1.95 a | 24.18 ± 0.58 a,c | 33.77 ± 1.93 a,d | 136.8 |
| C group | 50.34 ± 2.50 a,b,* | 21.85 ± 0.85 a,b,* | 17.18 ± 0.44 b,* | 21.93 ± 1.89 a,b,* | 53.7 | ||
| 11,14-eicosadienoic acid (20:2n-6) | S group | 1.26 ± 0.10 | 3.81 ± 0.07 a | 1.68 ± 0.23 b | 6.62 ± 0.26 a,b,c | 5.36 ± 0.20 a,b,c,d | 325.3 |
| C group | 8.37 ± 0.22 a,* | 10.50 ± 0.19 a,b,* | 16.37 ± 0.33 a,b,c,* | 13.69 ± 0.42 a,b,c,d,* | 986.5 | ||
| Arachidonic acid (20:4n-6) | S group | 259.06 ± 19.06 | 435.25 ± 4.67 a | 454.75 ± 4.23 a | 460.06 ± 10.96 a | 479.56 ± 18.85 a | 85.1 |
| C group | 416.30 ± 0.47 a,* | 395.02 ± 12.34 a,* | 349.73 ± 5.49 a,b,* | 316.38 ± 3.57 a,b,c,* | 22.1 |
| n-3 PUFA | Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | |
|---|---|---|---|---|---|---|---|
| α-linolenic acid (18:3n-3) | S group | 16.37 ± 0.96 | 57.46 ± 4.27 a | 55.85 ± 3.97 a | 34.53 ± 0.66 a,b,c | 42.33 ± 1.83 a,b,c | 158.5 |
| C group | 82.63 ± 1.21 a,* | 47.12 ± 2.13 a,b | 42.73 ± 0.99 a,b,* | 74.23 ± 6.04 a,c,d,* | 353.4 | ||
| Dihomo-α-linolenic acid (20:3n-3) | S group | 15.23 ± 1.07 | 26.60 ± 0.34 a | 30.45 ± 0.33 a | 36.16 ± 0.75 a,b | 44.19 ± 3.48 a,b,c,d | 190.1 |
| C group | 28.09 ± 1.10 a | 36.35 ± 1.43 a,b,* | 47.69 ± 0.67 a,b,c,* | 49.09 ± 1.44 a,b,c | 222.3 | ||
| Eicosapentaenoic acid (20:5n-3) | S group | 3.97 ± 0.34 | 3.87 ± 0.61 a | 10.39 ± 1.15 a | 8.97 ± 0.21 a | 11.71 ± 0.56 a,b | 194.9 |
| C group | 17.00 ± 0.49 a,* | 27.35 ± 0.97 a,b,* | 36.70 ± 0.99 a,b,c,* | 48.11 ± 1.01 a,b,c,d,* | 1111.8 | ||
| Docosahexaenoic acid (22:6n-3) | S group | 188.79 ± 12.06 | 228.63 ± 3.43 a | 283.76 ± 7.76 a,b | 273.89 ± 6.12 a,b | 259.47 ± 5.15 a | 37.4 |
| C group | 297.89 ± 5.55 a,* | 314.45 ± 12.18 a | 295.23 ± 2.63 a,* | 313.31 ± 3.03 a,* | 65.9 |
| Fatty Acids | Day 0 | Day 7 | Day 14 | Day 28 | Day 56 | ∆% | |
|---|---|---|---|---|---|---|---|
| SFA | S group | 690.66 ± 49.46 | 1694.58 ± 110.10 a | 1767.14 ± 61.13 a | 1691.58 ± 22.29 a | 2041.59 ± 56.74 a,b,d | 195.5 |
| C group | 1998.79 ± 8.37 a | 1824.68 ± 82.56 a | 2259.49 ± 13.78 a,b,c,* | 2104.20 ± 51 a,c | 204.6 | ||
| MUFA | S group | 305.02 ± 19.07 | 1555.54 ± 167.30 a | 1551.91 ± 87.71 a | 1625.98 ± 25.26 a | 2408.39 ± 64.61 a,b,c,d | 689.5 |
| C group | 2017.12 ± 12.99 a,* | 2179.19 ± 99.01 a,* | 4124.39 ± 5.66 a,b,c,* | 3576.26 ± 103.00 a,b,c,d,* | 1072.4 | ||
| PUFA | S group | 1024.32 ± 66.86 | 2325.86 ± 168.70 a | 2280.69 ± 75.50 a | 1809.00 ± 18.63 a,b,c | 2206.65 ± 62.31 a | 115.4 |
| C group | 2631.73 ± 27.35 a | 1679.74 ± 63.16 a,b,* | 1483.22 ± 17.60 a,b,* | 1667.06 ± 36.96 a,b,* | 62.7 | ||
| n-6 | S group | 799.33 ± 52.42 | 2004.87 ± 160.00 a | 1900.23 ± 71.01 a | 1455.42 ± 13.77 a,b,c | 1848.93 ± 54.55 a | 131.3 |
| C group | 2206.09 ± 26.50 a | 1254.44 ± 46.44 a,b,* | 1060.85 ± 13.96 a,b,c,* | 1182.30 ± 31.24 a,b,* | 47.9 | ||
| n-3 | S group | 224.38 ± 14.44 | 320.99 ± 8.66 a | 380.46 ± 8.84 a | 353.57 ± 6.57 a | 357.72 ± 9.11 a | 59.4 |
| C group | 426.23 ± 3.31 a,* | 425.30 ± 16.72 a | 422.37 ± 4.15 a,* | 484.75 ± 6.79 a,b,c,d,* | 116.0 | ||
| SUM | S group | 2020.12 ± 135.40 | 5408.39 ± 272.70 a | 5599.73 ± 38.21 a | 5126.56 ± 51.41 a | 6656.63 ± 180.60 a,b,d | 229.5 |
| C group | 6647.63 ± 38.21 a,* | 5683.60 ± 244.70 a,b | 7867.11 ± 28.08 a,b,c,* | 7522.60 ± 132.00 a,b,c,* | 272.3 | ||
| n-6/n-3 | S group | 3.56 ± 0.00 | 6.22 ± 0.33 a | 4.99 ± 0.16 a,b | 4.11 ± 0.06 b,c | 5.16 ± 0.08 a,b,d | 44.9 |
| C group | 5.18 ± 0.06 a,* | 2.95 ± 0.00 a,b,* | 2.51 ± 0.01 a,b,c,* | 2.43 ± 0.04 a,b,c,* | −31.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, M.M.; Godoy, G.; Fernandes, I.d.L.; Manin, L.P.; Zappielo, C.; Masi, L.N.; Oliveira, V.A.B.d.; Visentainer, J.V.; Curi, R.; Bazotte, R.B. The Dietary Replacement of Soybean Oil by Canola Oil Does Not Prevent Liver Fatty Acid Accumulation and Liver Inflammation in Mice. Nutrients 2020, 12, 3667. https://doi.org/10.3390/nu12123667
Antunes MM, Godoy G, Fernandes IdL, Manin LP, Zappielo C, Masi LN, Oliveira VABd, Visentainer JV, Curi R, Bazotte RB. The Dietary Replacement of Soybean Oil by Canola Oil Does Not Prevent Liver Fatty Acid Accumulation and Liver Inflammation in Mice. Nutrients. 2020; 12(12):3667. https://doi.org/10.3390/nu12123667
Chicago/Turabian StyleAntunes, Marina Masetto, Guilherme Godoy, Ingrid de Lima Fernandes, Luciana Pelissari Manin, Caroline Zappielo, Laureane Nunes Masi, Vivian Araújo Barbosa de Oliveira, Jesuí Vergílio Visentainer, Rui Curi, and Roberto Barbosa Bazotte. 2020. "The Dietary Replacement of Soybean Oil by Canola Oil Does Not Prevent Liver Fatty Acid Accumulation and Liver Inflammation in Mice" Nutrients 12, no. 12: 3667. https://doi.org/10.3390/nu12123667
APA StyleAntunes, M. M., Godoy, G., Fernandes, I. d. L., Manin, L. P., Zappielo, C., Masi, L. N., Oliveira, V. A. B. d., Visentainer, J. V., Curi, R., & Bazotte, R. B. (2020). The Dietary Replacement of Soybean Oil by Canola Oil Does Not Prevent Liver Fatty Acid Accumulation and Liver Inflammation in Mice. Nutrients, 12(12), 3667. https://doi.org/10.3390/nu12123667






