Pro-Inflammatory Diet Is Associated with Adiposity during Childhood and with Adipokines and Inflammatory Markers at 11 Years in Mexican Children
Abstract
1. Introduction
2. Materials and Methods
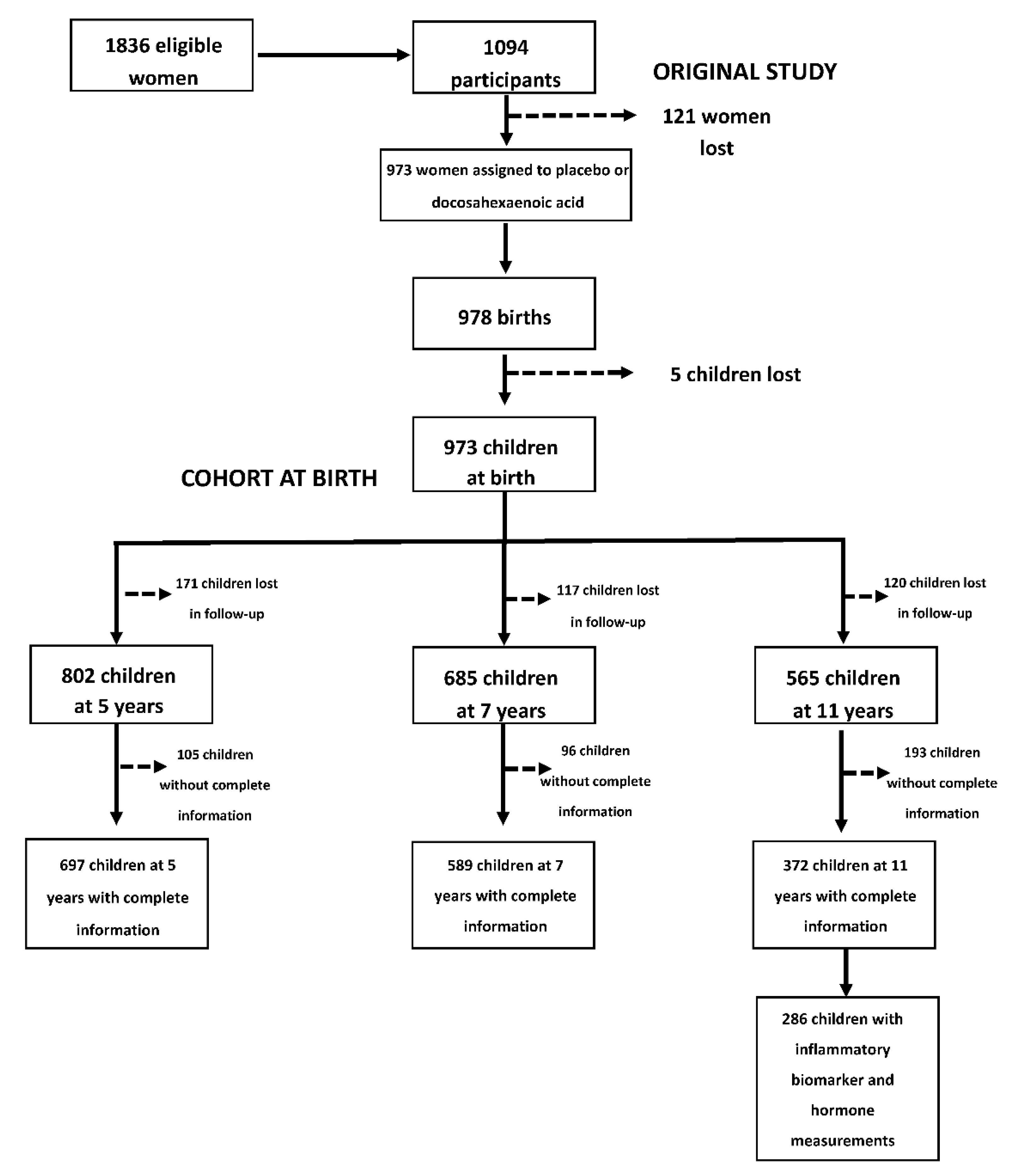
2.1. Study Design and Participants
2.2. Study Variables
2.2.1. Exposure Variable
2.2.2. Outcome Variables
2.3. Inflammatory Biomarkers and Adipokines at 11 Years
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Energy, Fiber and Nutrients from Diet According to Dietary Inflammatory Index Tertiles
3.2. C-DII Trajectories (5–11 Years) by Sex
3.3. C-DII and Adiposity from 5 to 11 Years
3.4. C-DII (11y), Inflammatory Biomarkers and Adipokines at 11 Years
3.5. C-DII Trajectories (5–11 Years), Inflammatory Biomarkers and Adipokines at 11 Years
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernández Cordero, S.; Ramírez Silva, I.; Perichart, O.; Mazariegos, M.; Reyes, H.; Ariza, A.C. Overweight and obesity in children: Determinants from a life course perspective. In Current State of Public Policy and Recommendations to Prevent and Control Obesity in Mexico; Rivera Dommarco, J.Á., Colchero, M., Fuentes, M., González de Cosío Martínez, T., Aguilar Salinas, C., Hernández Licona, G., Barquera, S., Eds.; National Institute of Public Health: Cuernav, Mexico, 2018; pp. 89–106. [Google Scholar]
- Lassek, W.D.; Gaulin, S.J. Menarche is related to fat distribution. Am. J. Phys. Anthr. 2007, 133, 1147–1151. [Google Scholar] [CrossRef]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose tissue development and expansion from the womb to adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef]
- Bandini, L.G.; Must, A.; Naumova, E.N.; Anderson, S.; Caprio, S.; I Spadano-Gasbarro, J.; Dietz, W.H.; Spadano-Gasbarro, J. Change in leptin, body composition and other hormones around menarche—A visual representation. Acta Paediatr. 2008, 97, 1454–1459. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S75–S78. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.; Jönsson, L.; Latulippe, M.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S31–S34. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Stoppa-Vaucher, S.; Dirlewanger, M.A.; Meier, C.A.; De Moerloose, P.; Reber, G.; Roux-Lombard, P.; Combescure, C.; Saudan, S.; Schwitzgebel, V. Inflammatory and prothrombotic states in obese children of european descent. Obesity 2012, 20, 1662–1668. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Shivappa, N.; E Steck, S.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Khan, S.; Wirth, M.D.; Ortaglia, A.; Alvarado, C.R.; Shivappa, N.; Hurley, T.; Hébert, J.R. Design, development and construct validation of the children’s dietary inflammatory index. Nutrients 2018, 10, 993. [Google Scholar] [CrossRef]
- Ramirez-Silva, I.; A Rivera, J.; Trejo-Valdivia, B.; Stein, A.D.; Martorell, R.; Romieu, I.; Barraza-Villarreal, A.; Avila-Jiménez, L.; Ramakrishnan, U. Relative weight gain through age 4 years is associated with increased adiposity, and higher blood pressure and insulinemia at 4–5 years of age in mexican children. J. Nutr. 2018, 148, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C.A.; Moshfegh, A.J.; Baer, D.J.; Kretsch, M.J. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J. Nutr. 2006, 136, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- López-Olmedo, N.; Carriquiry, A.L.; Rodríguez-Ramírez, S.; Ramírez-Silva, I.; Espinosa, J.; Hernandez, L.; Campirano, F.; Martínez-Tapia, B.; Rivera, J.A. Usual intake of added sugars and saturated fats is high while dietary fiber is low in the Mexican population. J. Nutr. 2016, 146, S1856–S1865. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Silva, I.; Rodríguez-Ramírez, S.; Barragán-Vázquez, S.; Castellanos-Gutiérrez, A.; Reyes-García, A.; Martínez-Piña, A.; Pedroza-Tobías, A. Prevalence of inadequate intake of vitamins and minerals in the Mexican population correcting by nutrient retention factors, Ensanut 2016. Salud Pública México 2020, 62, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Silva, I.; Barragán-Vázquez, S.; Mongue-Urrea, A.; Mejía-Rodríguez, F.; Rodríguez-Ramírez, S.C.; Rivera-Dommarco, J.A.; Flores Aldana, M.; Villalpando, S.; Orjuela, M.; Ancira, M.; et al. Base Base de Alimentos de México 2012 (BAM): Compilación de la Composición de los Alimentos Frecuentemente Consumidos en el país. Version 18.1.2. 2019. Available online: http://kin.insp.mx/aplicaciones/Redpidieta (accessed on 22 May 2019).
- Ramírez Silva, I.; Barragán-Vázquez, S.; Rodríguez Ramírez, S.; Rivera Dommarco, J.A.; Mejía-Rodríguez, F.; Barquera Cervera, S.; Tolentino Mayo, L.; Flores Aldana, M.; Villalpando Hernández, S.; Ancira Moreno, M.; et al. Base de Alimentos de México 2016 (BAM): Compilación de la Composición de los Alimentos Frecuentemente Consumidos en el país. Version 18.1.1. 2019. Available online: http://kin.insp.mx/aplicaciones/Redpidieta (accessed on 22 May 2019).
- WHO. Growth Reference 5–19 Years [Internet]. World Health Organization. 2007. Available online: http://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 22 May 2019).
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Habicht, J.-P. Estandarización de métodos epidemiológicos cuantitativos sobre el terreno. Sanit Panam. 1974, LXXV, 375–384. [Google Scholar]
- Higgins, P.B.; Fields, D.A.; Hunter, G.R.; Gower, B.A. Effect of scalp and facial hair on air displacement plethysmography estimates of percentage of body fat. Obesity 2001, 9, 326–330. [Google Scholar] [CrossRef]
- Fields, A.D.; Higgins, P.B.; Hunter, G.R. Assessment of body composition by air-displacement plethysmography: Influence of body temperature and moisture. Dyn. Med. 2004, 3, 3. [Google Scholar] [CrossRef]
- Siri, E.W. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar]
- Olsson, U. Confidence intervals for the mean of a log-normal distribution. J. Stat. Educ. 2005, 13. [Google Scholar] [CrossRef]
- Todendi, P.F.; Possuelo, L.G.; Klinger, E.I.; Reuter, C.P.; Burgos, M.S.; Moura, D.J.; Fiegenbaum, M.; Valim, A.R. Low-grade inflammation markers in children and adolescents: Influence of anthropometric characteristics and CRP and IL6 polymorphisms. Cytokine 2016, 88, 177–183. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Mente, A.; De Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Bawaked, R.A.; Schröder, H.; Ribas-Barba, L.; Izquierdo-Pulido, M.; Aranceta, J.; Fíto, M.; Serra-Majem, L. Association of diet quality with dietary inflammatory potential in youth. Food Nutr. Res. 2017, 61, 1328961. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; González-Jiménez, E.; Rueda-Medina, B.; Tovar-Gálvez, M.I.; Ramírez-Vélez, R.; Correa-Bautista, J.E.; Schmidt-RioValle, J. Dietary inflammatory index and cardiovascular risk factors in Spanish children and adolescents. Res. Nurs. Health 2018, 41, 448–458. [Google Scholar] [CrossRef]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; Da Costa, T.H.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114. [Google Scholar] [CrossRef]
- Rivera, J.A.; De Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in Latin America: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Cuevas-Nasú, L.; Gaona-Pineda, B.E.; Gómez-Acosta, L.M.; del Carmen Morales-Ruán, M.; Hernández-Ávila, M.; Rivera-Dommarco, J.Á. Overweight and obesity in children and adolescents, 2016. Halfway national health and nutrition survey update. Salud Pública México 2018, 60, 244–253. [Google Scholar] [CrossRef]
- Levy, T.S.; Romero-Martínez, M.; Nasu, L.C.; Méndez-Gómez-Humarán, I.; Avila-Arcos, M.A.; Rivera, J.A. The Mexican national health and nutrition survey as a basis for public policy planning: Overweight and obesity. Nutrients 2019, 11, 1727. [Google Scholar] [CrossRef]
- Aslani, Z.; Qorbani, M.; Hébert, J.R.; Shivappa, N.; Motlagh, M.E.; Asayesh, H.; Mahdavi-Gorabi, A.; Kelishadi, R. Association of dietary inflammatory index with anthropometric indices in children and adolescents: The weight disorder survey of the childhood and adolescence surveillance and prevention of adult non-communicable disease (CASPIAN)-IV study. Br. J. Nutr. 2019, 121, 340–350. [Google Scholar] [CrossRef]
- Ramallal, R.; Toledo, E.; Martínez, J.A.; Shivappa, N.; Hébert, J.R.; Martínez-González, M.A.; Ruiz-Canela, M. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: The SUN cohort. Obesity 2017, 25, 997–1005. [Google Scholar] [CrossRef]
- Navarro, P.; Shivappa, N.; Hébert, J.R.; Mehegan, J.; Murrin, C.M.; Kelleher, C.C.; Phillips, C.M. Predictors of the dietary inflammatory index in children and associations with childhood weight status: A longitudinal analysis in the lifeways cross-generation cohort study. Clin. Nutr. 2020, 39, 2169–2179. [Google Scholar] [CrossRef]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Associations of prenatal and early life dietary inflammatory potential with childhood adiposity and cardiometabolic risk in project viva. Pediatr. Obes. 2017, 13, 292–300. [Google Scholar] [CrossRef]
- Rendo-Urteaga, T.; De Moraes, A.C.F.; Torres-Leal, F.L.; Manios, Y.; Gottand, F.; Sjöström, M.; Kafatos, A.; Widhalm, K.; De Henauw, S.; Molnár, D.; et al. Leptin and adiposity as mediators on the association between early puberty and several biomarkers in European adolescents: The HELENA Study. J. Pediatr. Endocrinol. Metab. 2018, 31, 1221–1229. [Google Scholar]
- Nieuwenhuis, D.; Pujol-Gualdo, N.; Arnoldussen, I.A.C.; Kiliaan, A.J. Adipokines: A gear shift in puberty. Obes. Rev. 2020, 21, e13005. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity [Internet]. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef]
- Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Beltrán, A.A.; Ibáñez, C.A.; Vega, C.C.; Lomas-Soria, C.; Castro-Rodríguez, D.C.; Elías-López, A.L.; Nathanielsz, P.W.; et al. Maternal obesity accelerates rat offspring metabolic ageing in a sex-dependent manner. J. Physiol. 2019, 597, 5549–5563. [Google Scholar] [CrossRef]
- Zambrano, E.; Ibáñez, C.; Martínez-Samayoa, P.M.; Lomas-Soria, C.; Durand-Carbajal, M.; Rodríguez-González, G.L. Maternal obesity: Lifelong metabolic outcomes for offspring from poor developmental trajectories during the perinatal period. Arch. Med. Res. 2016, 47, 1–12. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zárate, M.L.; Villalobos-Flores, L.E.; García-González, C.; Morales-Hernández, R.; Nuñez-Hernández, J.A.; Hernández-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernández-Guerrero, C.; et al. Gut microbiota and endothelial dysfunction markers in obese Mexican children and adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Nishide, R.; Ando, M.; Funabashi, H.; Yoda, Y.; Nakano, M.; Shima, M. Association of serum hs-CRP and lipids with obesity in school children in a 12-month follow-up study in Japan. Environ. Health Prev. Med. 2014, 20, 116–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aburawi, E.H.; Al Hamad, S.; Yasin, J.; Almekhaini, L.A.; Souid, A.K. Dyslipidemia, subclinical inflammation, hepatic cholestasis and endothelial dysfunction in schoolchildren with excess fat: A study from the United Arab Emirates. PLoS ONE 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Murdolo, G.; Nowotny, B.; Celi, F.; Donati, M.; Bini, V.; Papi, F.; Gornitzka, G.; Castellani, S.; Roden, M.; Falorni, A.; et al. Inflammatory adipokines, high molecular weight adiponectin, and insulin resistance: A population-based survey in prepubertal schoolchildren. PLoS ONE 2011, 6, e17264. [Google Scholar] [CrossRef]
- Butte, N.F.; Comuzzie, A.G.; Cai, G.; Cole, S.A.; Mehta, N.R.; Bacino, C.A. Genetic and environmental factors influencing fasting serum adiponectin in hispanic children. J. Clin. Endocrinol. Metab. 2005, 90, 4170–4176. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Cheng, H.; Zhao, X.; Hou, D.; Yan, Y.; Cianflone, K.; Li, M.; Mi, J. Leptin and leptin-to-adiponectin ratio predict adiposity gain in nonobese children over a six-year period. Child. Obes. 2017, 13, 213–221. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hebert, J.R. A population-based dietary inflammatory index predicts levels of c-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef]
- Ruscica, M.; Corsini, A.; Ferri, N.; Banach, M.; Sirtori, C.R. Clinical approach to the inflammatory etiology of cardiovascular diseases. Pharmacol. Res. 2020, 159, 104916. [Google Scholar] [CrossRef] [PubMed]
- Luglio, H.F.; Van Baak, M.A.; Mariman, E.C.M.; Sulistyoningrum, D.C.; Huriyati, E.; Lee, Y.Y.; Muda, W.A.M.W. Dietary inflammatory index score and its association with body weight, blood pressure, lipid profile, and leptin in indonesian adults. Nutrients 2019, 11, 148. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agallium, I.; Hinglem, M.; Houm, L.; Hurley, T.G.; Jiao, L.; et al. Validation of the dietary inflammatory index in the women’s health initiative. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Coheley, L.M.; Shivappa, N.; Hebert, J.R.; Lewis, R.D. Dietary inflammatory index® and cortical bone outcomes in healthy adolescent children. Osteoporos. Int. 2019, 30, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Almeida-De-Souza, J.; Santos, R.; Barros, R.; Abreu, S.; Moreira, C.; Lopes, L.; Mota, J.; Moreira, P. Dietary inflammatory index and inflammatory biomarkers in adolescents from LabMed physical activity study. Eur. J. Clin. Nutr. 2017, 72, 710–719. [Google Scholar] [CrossRef]
- Canto-Osorio, F.; Denova-Gutierrez, E.; Sánchez-Romero, L.M.; Salmerón, J.; Barrientos-Gutierrez, T. Dietary inflammatory index and metabolic syndrome in Mexican adult population. Am. J. Clin. Nutr. 2020, 112, 373–380. [Google Scholar] [CrossRef]
| Children’s Dietary Inflammatory Index | BMI Z Score | Log-Abdominal Circumference | Log-Skinfold Sum |
|---|---|---|---|
| C-DII boys | |||
| β (95% CI) | 0.016 (−0.019–0.050) | 0.000 (−0.003–0.003) | 0.004 (−0.008–0.016) |
| % of change | - | 0.01 (−0.33–0.35) | 0.42 (−0.79–1.65) |
| p value | 0.37 | 0.96 | 0.49 |
| C-DII girls | |||
| β (95% CI) | 0.048 (0.011–0.085) | 0.004 (0.00–0.008) | 0.017 (0.004–0.030) |
| % of change | - | 0.41 (0.05–0.78) | 1.76 (0.46–3.08) |
| p value | 0.01 | 0.03 | 0.01 |
| p for C-DII-sex interaction | 0.21 | 0.11 | 0.14 |
| Body Mass Index (BMI) * | Fat Mass (kg) ** | |||||||
|---|---|---|---|---|---|---|---|---|
| Inflammatory Biomarkers | Normal Weight (NW) n = 161 P50 (p25–p75) | Overweight (OW) n = 72 P50 (p25–p75) | p Value * NW vs. OW | Obese (Ob) n = 53 P50 (p25–p75) | p Value * NW vs. OB | β | 95% CI | p Value |
| hsCRP (mg/L) | 0.48 (0.23–1.08) | 1.17 (0.50–2.42) | <0.001 | 3.70 (1.26–4.84) | <0.001 | 0.10 | 0.08–0.13 | <0.001 |
| IL-6 (pg/mL) | 0.50 (0.22–0.87) | 0.60 (0.27–0.97) | 0.31 | 0.50 (0.19–0.91) | 1.00 | 0.00 | −0.01–0.01 | 0.86 |
| Adiponectin (ng/mL) | 14.55 (11.91–17.43) | 14.97 (10.77–18.57) | 1.00 | 15.01 (13.06–19.28) | 0.21 | 0.06 | −0.03–0.15 | 0.17 |
| Leptin (ng/mL) | 5.39 (2.67–9.78) | 6.23 (2.49–12.11) | 1.00 | 9.20 (3.78–12.89) | 0.02 | 0.19 | 0.05–0.32 | 0.01 |
| Adiponectin/leptin ratio | 2.62 (1.28–5.66) | 2.56 (1.25–4.85) | 1.00 | 2.02 (1.08–3.97) | 0.13 | −0.04 | −0.10–0.01 | 0.14 |
| Inflammatory Biomarkers and Hormones * | OR | 95% CI | p Value |
|---|---|---|---|
| hsCRP | |||
| <3 mg/L | 1.00 (Ref.) | ||
| ≥3mg/L | 1.29 | 0.99, 1.69 | 0.06 |
| Leptin | |||
| <1.88 ng/mL | 1.00 (Ref.) | ||
| 1.88–<5.68 ng/mL | 1.30 | 1.01–1.67 | 0.04 |
| 5.68–<13.00 ng/mL | 1.38 | 1.0–1.78 | 0.01 |
| ≥13.00 ng/mL | 1.34 | 1.02–1.76 | 0.03 |
| Adiponectin | |||
| ≥19.63 ng/mL | 1.00 (Ref.) | ||
| <19.63–13.76 ng/mL | 1.01 | 0.79–1.27 | 0.96 |
| <13.76 ng/mL | 1.01 | 0.80–1.28 | 0.92 |
| Adiponectin/leptin ratio | |||
| ≥23.43 | 1.00 (Ref.) | ||
| <23.43–2.45 | 1.52 | 0.97–2.39 | 0.07 |
| <2.45 | 1.75 | 1.11–2.76 | 0.02 |
| IL-6 | |||
| <0.55 pg/mL | 1.00 (Ref.) | ||
| 0.55–<6.23 pg/mL | 0.99 | 0.83–1.18 | 0.90 |
| ≥6.23 pg/mL | 0.86 | 0.53–1.41 | 0.55 |
| Inflammatory Biomarkers and Hormones | C-DII Trajectories in Boys, n = 150 | C-DII Trajectories in Girls, n = 133 | |||
|---|---|---|---|---|---|
| Trajectory 1 | Trajectory 2 | Trajectory 3 | Trajectory 1 | Trajectory 2 | |
| Log-hsCRP | Ref. | Ref. | |||
| β (95%CI) | 0.01 (−0.44–0.46) | 0.05 (−0.32–0.41) | 0.05 (−0.41–0.51) | ||
| % change | 1.05 (−35.34–57.93) | 4.71 (−27.09–50.38) | 5.23 (−33.44–66.53) | ||
| p value | 0.96 | 0.80 | 0.82 | ||
| Log-IL-6 | Ref. | Ref. | |||
| β (95%CI) | −0.028 (−0.53–0.47) | −0.008 (−0.42–0.40) | −0.006 (−0.46–0.45) | ||
| % change | −2.76 (−41.15–60.64) | −0.82 (−34.10–49.18) | −0.62 (−37.21–57.27) | ||
| p value | 0.91 | 0.97 | 0.98 | ||
| Log-Leptin | Ref. | Ref. | |||
| β (95%CI) | 0.29 (−0.17–0.76) | 0.56 (0.19–0.94) | −0.15 (−0.55–0.24) | ||
| % change | 33.98 (−15.71–112.97) | 75.24 (20.49–155.05) | −14.22 (−42.46–27.88) | ||
| p value | 0.21 | <0.01 | 0.45 | ||
| Log-Adiponectin | Ref. | Ref. | |||
| β (95%CI) | −0.03 (−0.21–0.15) | 0.08 (−0.06–0.23) | 0.08 (−0.09–0.25) | ||
| % change | −2.92 (−18.94–16.27) | 8.88 (−5.90–25.98) | 8.53 (−8.58–28.86) | ||
| p value | 0.75 | 0.25 | 0.35 | ||
| Log-Adiponectin/leptin ratio | Ref. | Ref. | |||
| β (95%CI) | −0.32 (−0.82–0.18) | −0.48 (−0.88–−0.07) | 0.23 (−0.18–0.65) | ||
| % change | −27.54 (−56.03–19.41) | −37.89 (−58.54–−6.96) | 26.52 (−16.36–91.39) | ||
| p value | 0.20 | 0.02 | 0.26 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán-Vázquez, S.; Ariza, A.C.; Ramírez Silva, I.; Pedraza, L.S.; Rivera Dommarco, J.A.; Ortiz-Panozo, E.; Zambrano, E.; Reyes Castro, L.A.; Shivappa, N.; Hébert, J.R.; et al. Pro-Inflammatory Diet Is Associated with Adiposity during Childhood and with Adipokines and Inflammatory Markers at 11 Years in Mexican Children. Nutrients 2020, 12, 3658. https://doi.org/10.3390/nu12123658
Barragán-Vázquez S, Ariza AC, Ramírez Silva I, Pedraza LS, Rivera Dommarco JA, Ortiz-Panozo E, Zambrano E, Reyes Castro LA, Shivappa N, Hébert JR, et al. Pro-Inflammatory Diet Is Associated with Adiposity during Childhood and with Adipokines and Inflammatory Markers at 11 Years in Mexican Children. Nutrients. 2020; 12(12):3658. https://doi.org/10.3390/nu12123658
Chicago/Turabian StyleBarragán-Vázquez, Sofia, Ana Carolina Ariza, Ivonne Ramírez Silva, Lilia Susana Pedraza, Juan A. Rivera Dommarco, Eduardo Ortiz-Panozo, Elena Zambrano, Luis A. Reyes Castro, Nitin Shivappa, James R. Hébert, and et al. 2020. "Pro-Inflammatory Diet Is Associated with Adiposity during Childhood and with Adipokines and Inflammatory Markers at 11 Years in Mexican Children" Nutrients 12, no. 12: 3658. https://doi.org/10.3390/nu12123658
APA StyleBarragán-Vázquez, S., Ariza, A. C., Ramírez Silva, I., Pedraza, L. S., Rivera Dommarco, J. A., Ortiz-Panozo, E., Zambrano, E., Reyes Castro, L. A., Shivappa, N., Hébert, J. R., Martorell, R., Stein, A. D., Barraza-Villarreal, A., Romieu, I., Avila-Jiménez, L., & Ramakrishnan, U. (2020). Pro-Inflammatory Diet Is Associated with Adiposity during Childhood and with Adipokines and Inflammatory Markers at 11 Years in Mexican Children. Nutrients, 12(12), 3658. https://doi.org/10.3390/nu12123658









