Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. (6S)-5-Methyl-THF-Na Pharmacokinetic Study
2.1.1. Subjects and Settings
2.1.2. Study Design
2.1.3. Study Objectives and Sample Size Estimation
2.1.4. Blood Analyses and Analytical Methods
2.1.5. Statistical Analyses
2.2. AUCs for (6S)-5-Methyl-THF-Ca Versus Folic Acid and Methods of Indirect Comparisons with the AUCs for (6S)-5-Methyl-THF-Na
Methods of Indirect Comparisons between Na and Ca Salts of 5-Methyl-THF
3. Results
3.1. (6S)-5-Methyl-THF-Na Pharmacokinetic Study
3.1.1. Baseline Characteristics and Folate Markers during the Screening Visit
3.1.2. Changes of Plasma Concentrations of (6S)-5-Methyl-THF and Total Folate
3.1.3. Pharmacokinetic Markers after (6S)-5-Methyl-THF-Na and Folic Acid Intake
3.1.4. Subgroup Analyses of AUCs of Plasma (6S)-5-Methyl-THF after Intake of (6S)-5-Methyl-THF-Na and Folic Acid
3.1.5. Safety
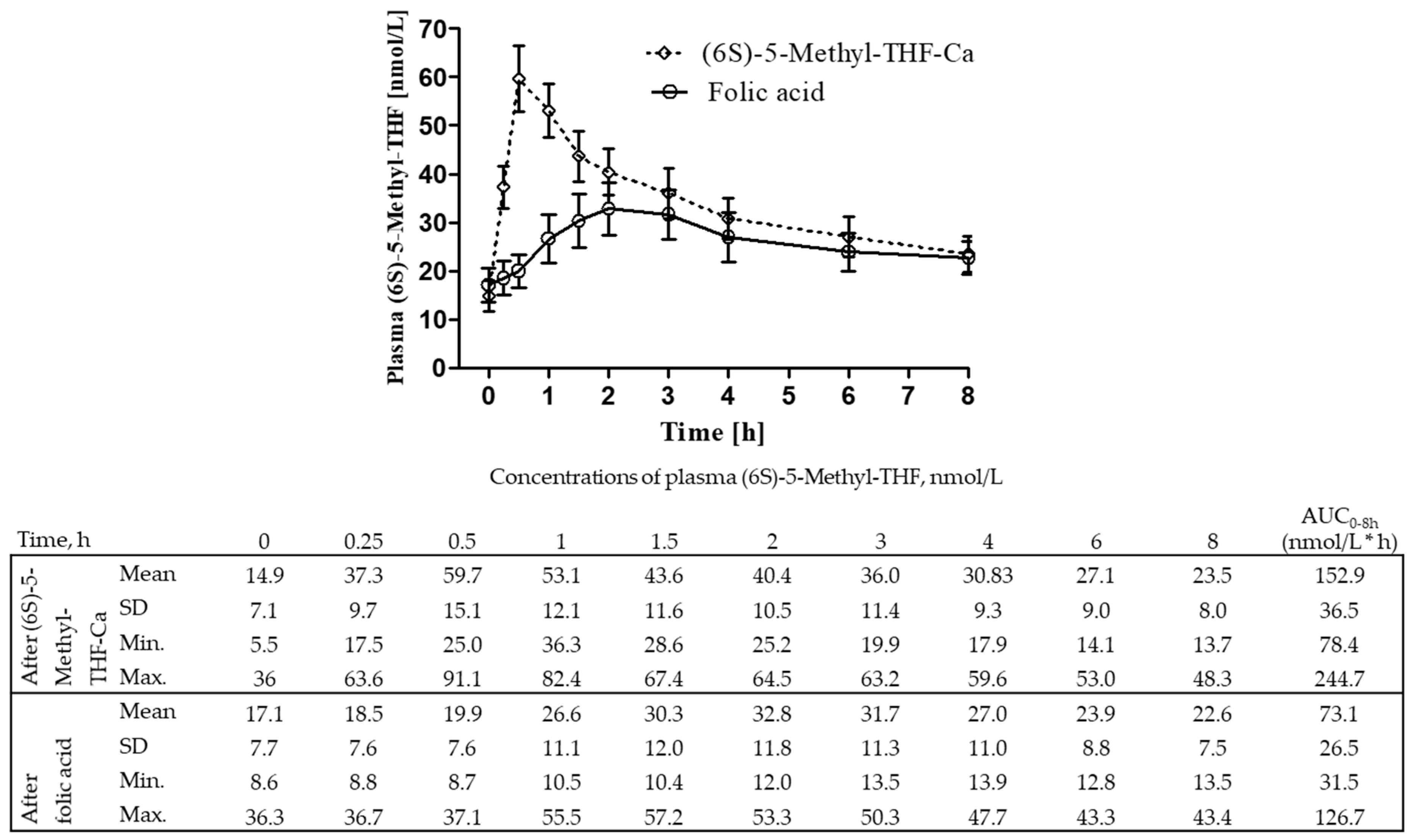
3.2. The AUCs of Plasma (6S)-5-Methyl-THF after (6S)-5-Methyl-THF-Ca Versus Folic Acid
3.3. Indirect Comparison of the AUCs between the (6S)-5-Methyl-THF Salts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bernstein, L.H.; Gutstein, S.; Weiner, S.V. Gamma glutamyl carboxypeptidase (conjugase), the folic acid-releasing enzyme of intestinal mucosa. Am. J. Clin. Nutr. 1970, 23, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J. Nutr. 2010, 140, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- van Gool, J.D.; Hirche, H.; Lax, H.; De, S.L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Pena-Rosas, J.P.; Fernandez-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, CD007950. [Google Scholar] [CrossRef]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef]
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ Among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011–2016. J. Nutr. 2020, 150, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Obeid, R.; Kasoha, M.; Kirsch, S.H.; Munz, W.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am. J. Clin. Nutr. 2010, 92, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-Methyltetrahydrofolate and Folic Acid Levels in Maternal and Cord Blood Serum: Longitudinal Evaluation of Japanese Pregnant Women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Hornstra, J.; Rocha, M.; Jansen, G.; Assaraf, Y.; Lasry, I.; Blom, H.; Smulders, Y.M. Folic Acid Impairs the Uptake of 5-Methyltetrahydrofolate in Human Umbilical Vascular Endothelial Cells. J. Cardiovasc. Pharmacol. 2017, 70, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, E.C.; Hong, X.; Selhub, J.; Paul, L.; Wood, R.A.; Matsui, E.C.; Keet, C.A.; Wang, X. Association Between Folate Metabolites and the Development of Food Allergy in Children. J. Allergy Clin. Immunol. Pract. 2020, 8, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Prinz-Langenohl, R.; Bramswig, S.; Tobolski, O.; Smulders, Y.M.; Smith, D.E.; Finglas, P.M.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: A randomized placebo-controlled study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venn, B.J.; Green, T.J.; Moser, R.; McKenzie, J.E.; Skeaff, C.M.; Mann, J. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]-5-methyltetrahydrofolate and folic acid. J. Nutr. 2002, 132, 3353–3355. [Google Scholar] [CrossRef] [Green Version]
- Pentieva, K.; McNulty, H.; Reichert, R.; Ward, M.; Strain, J.J.; McKillop, D.J.; McPartlin, J.M.; Connolly, E.; Molloy, A.; Kramer, K.; et al. The short-term bioavailabilities of [6S]-5-methyltetrahydrofolate and folic acid are equivalent in men. J. Nutr. 2004, 134, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Lamers, Y.; Prinz-Langenohl, R.; Bramswig, S.; Pietrzik, K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am. J. Clin. Nutr. 2006, 84, 156–161. [Google Scholar] [CrossRef]
- Henderson, A.M.; Aleliunas, R.E.; Loh, S.P.; Khor, G.L.; Harvey-Leeson, S.; Glier, M.B.; Kitts, D.D.; Green, T.J.; Devlin, A.M. l-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J. Nutr. 2018, 148, 885–890. [Google Scholar] [CrossRef] [Green Version]
- Lamers, Y.; Prinz-Langenohl, R.; Moser, R.; Pietrzik, K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004, 79, 473–478. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Knapp, J.P.; Herrmann, W.; Obeid, R. Quantification of key folate forms in serum using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 68–75. [Google Scholar] [CrossRef]
- Kok, R.M.; Smith, D.E.; Dainty, J.R.; Van Den Akker, J.T.; Finglas, P.M.; Smulders, Y.M.; Jakobs, C.; de, M.K. 5-Methyltetrahydrofolic acid and folic acid measured in plasma with liquid chromatography tandem mass spectrometry: Applications to folate absorption and metabolism. Anal. Biochem. 2004, 326, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Patanwala, I.; King, M.J.; Barrett, D.A.; Rose, J.; Jackson, R.; Hudson, M.; Philo, M.; Dainty, J.R.; Wright, A.J.; Finglas, P.M.; et al. Folic acid handling by the human gut: Implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014, 100, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, A.M.; Crider, K.S.; Rogers, L.M.; Cannon, M.J.; Berry, R.J. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 421–423. [Google Scholar] [PubMed]
- Crider, K.S.; Devine, O.; Hao, L.; Dowling, N.F.; Li, S.; Molloy, A.M.; Li, Z.; Zhu, J.; Berry, R.J. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014, 349, g4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, L.A.; Sherwood, K.L.; Pawlosky, R.; Ito, S.; O’Connor, D.L. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am. J. Clin. Nutr. 2006, 83, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troesch, B.; Demmelmair, J.; Gimpfl, M.; Hecht, C.; Lakovic, G.; Roehle, R.; Sipka, L.; Trisic, B.; Vusurovic, M.; Schoop, R.; et al. Suitability and safety of L-5-methyltetrahydrofolate as a folate source in infant formula: A randomized-controlled trial. PLoS ONE 2019, 14, e0216790. [Google Scholar] [CrossRef] [Green Version]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.E.; Mikael, L.G.; Leung, K.Y.; Levesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, M.V.; Randazzo, O.; La, F.M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.G.; Stenroos, E.S.; Spychala, J.R.; Chatkupt, S.; Ming, S.X.; Buyske, S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): A risk factor for spina bifida acting in mothers during pregnancy? Am. J. Med. Genet. A 2004, 124A, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, R.D.; Choumenkovitch, S.F.; Troen, A.P.; Jacques, P.F.; D’Agostino, R.; Selhub, J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J. Nutr. 2008, 138, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Philip, D.; Buch, A.; Moorthy, D.; Scott, T.M.; Parnell, L.D.; Lai, C.Q.; Ordovas, J.M.; Selhub, J.; Rosenberg, I.H.; Tucker, K.L.; et al. Dihydrofolate reductase 19-bp deletion polymorphism modifies the association of folate status with memory in a cross-sectional multi-ethnic study of adults. Am. J. Clin. Nutr. 2015, 102, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Pietrzik, K.; Lamers, Y.; Bramswig, S.; Prinz-Langenohl, R. Calculation of red blood cell folate steady state conditions and elimination kinetics after daily supplementation with various folate forms and doses in women of childbearing age. Am. J. Clin. Nutr. 2007, 86, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Yang, M.; Li, G.M.; Qiu, Y.; Zheng, J.; Du, X.; Wang, J.L.; Liu, R.W. The MTHFR C677T polymorphism contributes to an increased risk for vascular dementia: A meta-analysis. J. Neurol. Sci. 2010, 294, 74–80. [Google Scholar] [CrossRef]
- Gilbody, S.; Lewis, S.; Lightfoot, T. Methylenetetrahydrofolate Reductase (MTHFR) Genetic Polymorphisms and Psychiatric Disorders: A HuGE Review. Am. J. Epidemiol. 2006, 165, 1–13. [Google Scholar] [CrossRef]
- Cronin, S.; Furie, K.L.; Kelly, P.J. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: Evidence from a cumulative meta-analysis. Stroke 2005, 36, 1581–1587. [Google Scholar] [CrossRef] [Green Version]

| All | Men | Women | |
|---|---|---|---|
| N | 24 | 12 | 12 |
| Age, years | 29.7 (7.5) | 32.1 (9.2) | 27.3 (4.6) |
| BMI, kg/m2 | 23.1 (2.8) | 23.6 (2.6) | 22.6 (3.0) |
| Systolic blood pressure, mmHg | 121 (16) | 130 (10) | 112 (15) |
| Diastolic blood pressure, mmHg | 74 (10) | 77 (10) | 71 (9) |
| Heart rate, bpm | 70 (9) | 71 (7) | 69 (10) |
| Hemoglobin, g/dl | 14.3 (1.3) | 15.2 (0.8) | 13.4 (1.0) |
| tHcy, µmol/L | 7.6 (1.8) | 7.8 (1.4) | 7.4 (2.1) |
| Vitamin B12, pmol/L | 271 (80) | 291 (86) | 251 (70) |
| Plasma folate, nmol/L | 15.9 (6.0) | 14.9 (3.7) | 16.9 (7.6) |
| RBC-folate, nmol/L | 664 (174) | 669 (183) | 658 (173) |
| Time, h | 0 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Test compound: (6S)-5-Methyl-THF-Na, 436 µg | |||||||||
| (6S)-5-Methyl-THF | 13.6 (6.4) | 19.2 (10.5) | 42.8 (17.9) | 46.8 (14.2) | 34.5 (10.5) | 39.6 (9.3) | 26.6 (9.0) | 25.5 (9.3) | 22.3 (8.4) |
| Change from t0 | / | 5.6 (6.2) | 29.2 (14.1) | 33.2 (10.2) | 20.8 (5.7) | 16.0 (4.5) | 13.0 (4.2) | 11.9 (4.3) | 8.7 (3.8) |
| Total folate | 16.4 (6.7) | 19.6 (7.7) | 36.3 (12.3) | 37.3 (9.7) | 30.8 (7.1) | 27.6 (6.4) | 25.7 (6.1) | 25.8 (7.7) | 23.6 (7.3) |
| Change from t0 a | / | 3.1 (4.2) | 19.9 (9.4) | 20.8 (7.3) | 14.4 (3.8) | 11.1 (3.2) | 9.3 (3.5) | 9.4 (2.3) | 7.8 (2.7) |
| Reference compound: folic acid, 400 µg | |||||||||
| (6S)-5-Methyl-THF | 13.5 (6.0) | 13.4 (6.3) | 16.1 (7.6) | 21.5 (9.1) | 23.7 (8.8) | 21.5 (7.8) | 20.1 (7.0) | 20.9 (8.9) | 19.7 (8.9) |
| Change from t0 | / | −0.1 (2.3) | 2.6 (3.2) | 8.0 (4.0) | 10.1 (3.9) | 8.0 (3.0) | 6.6 (2.3) | 7.3 (4.9) | 6.2 (4.5) |
| Total folate | 15.5 (5.6) | 16.1 (7.5) | 25.5 (8.8) | 35.8 (10.5) | 26.7 (7.3) | 23.6 (7.1) | 22.8 (7.1) | 23.0 (6.9) | 22.3 (8.1) |
| Change from t0 a | / | 0.6 (2.7) | 10.0 (6.1) | 20.3 (8.1) | 11.2 (3.2) | 8.1 (2.8) | 7.3 (2.6) | 7.5 (3.6) | 6.8 (3.8) |
| Folate Compound | |||
|---|---|---|---|
| (6S)-5-Methyl-THF-Na | Folic Acid | p | |
| Plasma (6S)-5-Methyl-THF | |||
| AUC0–8 h, nmol/L*h, mean (SD) | 126.0 (33.6) | 56.0 (25.3) | <0.0001 |
| Cmax, nmol/L, mean (SD) and (range) | 36.8 (10.8) (14.4, 53.0) | 11.1 (4.1) (5.3, 24.7) | <0.0001 |
| Tmax, minutes, median (25th–75th Percentiles) (range) | 60 (30–60) (30, 240) | 120 (120–165) (60, 480) | 0.0002 a |
| Plasma total folate | |||
| AUC0–8 h, nmol/L*h, mean (SD) | 89.2 (19.1) | 71.1 (17.8) | 0.0007 |
| AUC0–8 h, nmol/L*h, mean (SD) | 91.6 (18.0) | 69.9 (17.3) | <0.0001 b |
| Cmax, nmol/L, mean (SD) and (range) | 23.9 (7.3) (12.1, 42.2) | 20.7 (7.7) (7.5, 33.0) | 0.0564 |
| Cmax, nmol/L, mean (SD) and (range) | 24.9 (6.7) (16.0, 42.2) | 20.3 (7.8) (7.5, 33.0) | 0.0074 b |
| Tmax, minutes, median (25th–75th Percentiles) (range) | 30 (30–60) (30, 360) | 60 (60–60) (30, 180) | 0.0534 a |
| Tmax, minutes, median (25th–75th Percentile) (range) | 30 (30–60) (30, 60) | 60 (60–60) (30, 180) | 0.0026 a,b |
| AUC0–8 h of (6S)-5-Methyl-THF [nmol/L*h] | p for AUCs between the Interventions a | p for Interaction Product × Subgroup b | ||
|---|---|---|---|---|
| (6S)-5-Methyl-THF-Na | Folic acid | |||
| Men | 111.9 (28.9) | 51.6 (18.5) | <0.0001 | |
| Women | 140.1 (33.0) | 60.5 (30.9) | <0.0001 | 0.4938 |
| Post-hoc subgroup analyses according to biomarker concentrations at screening c | ||||
| Plasma folate low | 125.7 (28.9) | 54.7 (21.9) | <0.0001 | |
| Plasma folate high | 126.3 (39.1) | 57.4 (29.2) | <0.0001 | 0.8040 |
| RBC-folate low | 121.0 (34.4) | 54.5 (21.9) | 0.0002 | |
| RBC-folate high | 130.9 (33.6) | 57.5 (29.2) | <0.0001 | 0.6833 |
| Vitamin B12 low | 142.4 (30.0) | 57.2 (31.2) | <0.0001 | |
| Vitamin B12 high | 109.6 (29.6) | 54.9 (19.1) | <0.0001 | 0.0503 |
| tHcy low | 127.6 (40.2) | 58.1 (29.5) | <0.0001 | |
| tHcy high | 124.3 (27.2) | 54.0 (21.4) | <0.0001 | 0.7090 |
| Hemoglobin low | 129.6 (42.2) | 57.7 (30.8) | <0.0001 | |
| Hemoglobin high | 122.3 (23.6) | 54.4 (19.6) | <0.0001 | 0.9101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeid, R.; Schön, C.; Pietrzik, K.; Menzel, D.; Wilhelm, M.; Smulders, Y.; Knapp, J.-P.; Böhni, R. Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients 2020, 12, 3623. https://doi.org/10.3390/nu12123623
Obeid R, Schön C, Pietrzik K, Menzel D, Wilhelm M, Smulders Y, Knapp J-P, Böhni R. Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients. 2020; 12(12):3623. https://doi.org/10.3390/nu12123623
Chicago/Turabian StyleObeid, Rima, Christiane Schön, Klaus Pietrzik, Daniel Menzel, Manfred Wilhelm, Yvo Smulders, Jean-Pierre Knapp, and Ruth Böhni. 2020. "Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts" Nutrients 12, no. 12: 3623. https://doi.org/10.3390/nu12123623





