The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude
Abstract
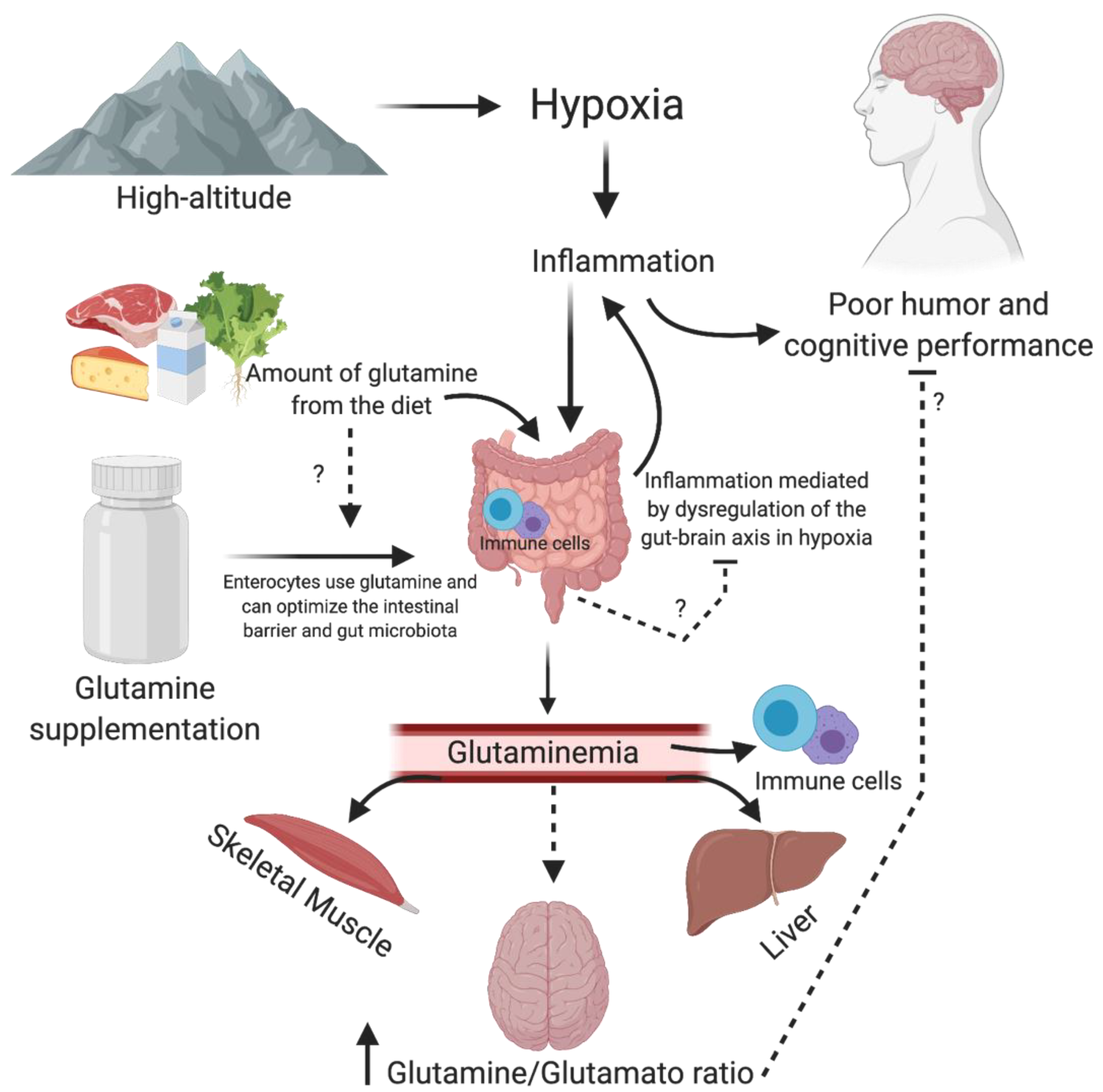
:1. Introduction
Study Type and Search Strategy
2. Hypoxia
3. Hypoxia and Mood
4. Hypoxia and Cognition
5. Inflammation, Cognition and Mood
6. Glutamine, Inflammation, Mood and Cognition
7. Conclusions and Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lafuente, J.V.; Bermudez, G.; Camargo-Arce, L.; Bulnes, S. Blood-brain barrier changes in high altitude. Cns. Neurol. Disord. Drug Targets 2016, 15, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Virues-Ortega, J.; Garrido, E.; Javierre, C.; Kloezeman, K.C. Human behaviour and development under high-altitude conditions. Dev. Sci. 2006, 9, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Tschop, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschop, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Coeffier, M.; Miralles-Barrachina, O.; Le Pessot, F.; Lalaude, O.; Daveau, M.; Lavoinne, A.; Lerebours, E.; Dechelotte, P. Influence of glutamine on cytokine production by human gut in vitro. Cytokine 2001, 13, 148–154. [Google Scholar] [CrossRef]
- Wernerman, J. Glutamine supplementation. Ann. Intensive Care 2011, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Castell, L.M.; Newsholme, E.A. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997, 13, 738–742. [Google Scholar] [CrossRef]
- Bailey, D.M.; Castell, L.M.; Newsholme, E.A.; Davies, B. Continuous and intermittent exposure to the hypoxia of altitude: Implications for glutamine metabolism and exercise performance. Br. J. Sports Med. 2000, 34, 210–212. [Google Scholar] [CrossRef] [Green Version]
- Caris, A.V.; Da Silva, E.T.; Dos Santos, S.A.; Tufik, S.; Dos Santos, R.V.T. Effects of carbohydrate and glutamine supplementation on oral mucosa immunity after strenuous exercise at high altitude: A double-blind randomized trial. Nutrients 2017, 9, 692. [Google Scholar] [CrossRef]
- Caris, A.V.; Lira, F.S.; de Mello, M.T.; Oyama, L.M.; dos Santos, R.V. Carbohydrate and glutamine supplementation modulates the Th1/Th2 balance after exercise performed at a simulated altitude of 4500 m. Nutrition 2014, 30, 1331–1336. [Google Scholar] [CrossRef]
- Honda, Y.; Tani, H.; Masuda, A.; Kobayashi, T.; Nishino, T.; Kimura, H.; Masuyama, S.; Kuriyama, T. Effect of prior O2 breathing on ventilatory response to sustained isocapnic hypoxia in adult humans. J. Appl. Physiol. 1996, 81, 1627–1632. [Google Scholar] [CrossRef]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P.; et al. The role of glutamine in the complex interaction between gut microbiota and health: A narrative review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, J.; Sidoryk-Wegrzynowicz, M.; Zielinska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Nava, R.C.; Zuhl, M.N.; Moriarty, T.A.; Amorim, F.T.; Bourbeau, K.C.; Welch, A.M.; McCormick, J.J.; King, K.E.; Mermier, C.M. The effect of acute glutamine supplementation on markers of inflammation and fatigue during consecutive days of simulated wildland firefighting. J. Occup. Environ. Med. 2019, 61, e33–e42. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, B.J.; Immink, M.A.; Colzato, L.S. Influences of glutamine administration on response selection and sequence learning: A randomized-controlled trial. Sci. Rep. 2017, 7, 2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, D.C.; da Silva, J.C.; Matos, F.O.; Okano, A.H.; Bazotte, R.B.; Avelar, A. The effect of a short period of supplementation with glutamine dipeptide on the cognitive responses after a resistance training session of women with HIV/AIDS: A randomized double-blind placebo-controlled crossover study. Biomed Res. Int. 2018, 2018, 2525670. [Google Scholar] [CrossRef] [Green Version]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Hypolito, T.; Godois, A.D.M.; Pereira, J.R.R.; Garcia, A.B.O.; Lara, R.S.B.; Rogero, M.M.; Tirapegui, J. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients 2018, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Mishra, K.P.; Ganju, L. Influence of high altitude exposure on the immune system: A review. Immunol. Invest. 2010, 39, 219–234. [Google Scholar] [CrossRef]
- Walter, D.H.; Fichtlscherer, S.; Sellwig, M.; Auch-Schwelk, W.; Schachinger, V.; Zeiher, A.M. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J. Am. Coll. Cardiol. 2001, 37, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Richard, N.A.; Sahota, I.S.; Widmer, N.; Ferguson, S.; Sheel, A.W.; Koehle, M.S. Acute mountain sickness, chemosensitivity, and cardiorespiratory responses in humans exposed to hypobaric and normobaric hypoxia. J. Appl. Physiol. 2014, 116, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Fowler, B.; Prlic, H. A comparison of visual and auditory reaction time and P300 latency thresholds to acute hypoxia. Aviat. Space Environ. Med. 1995, 66, 645–650. [Google Scholar]
- Gao, Y.X.; Li, P.; Jiang, C.H.; Liu, C.; Chen, Y.; Chen, L.; Ruan, H.Z.; Gao, Y.Q. Psychological and cognitive impairment of long-term migrators to high altitudes and the relationship to physiological and biochemical changes. Eur. J. Neurol. 2015, 22, 1363–1369. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, X.Y.; Fu, C.; Shen, X.F.; Wu, Y.H.; Wang, T. Effects of acute mild and moderate hypoxia on human mood state. Space Med. Med. Eng. 2000, 13, 1–5. [Google Scholar]
- Bolmont, B.; Thullier, F.; Abraini, J.H. Relationships between mood states and performances in reaction time, psychomotor ability, and mental efficiency during a 31-day gradual decompression in a hypobaric chamber from sea level to 8848 m equivalent altitude. Physiol. Behav. 2000, 71, 469–476. [Google Scholar] [CrossRef]
- Shibata, T.; Yamagata, H.; Uchida, S.; Otsuki, K.; Hobara, T.; Higuchi, F.; Abe, N.; Watanabe, Y. The alteration of hypoxia inducible factor-1 (HIF-1) and its target genes in mood disorder patients. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 43, 222–229. [Google Scholar] [CrossRef]
- Nicolas, M.; Thullier-Lestienne, F.; Bouquet, C.; Gardette, B.; Gortan, C.; Richalet, J.P.; Abraini, J.H. A study of mood changes and personality during a 31-day period of chronic hypoxia in a hypobaric chamber (Everest-Comex 97). Psychol. Rep. 2000, 86, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, W.A.; Ensign, W.Y.; Mills, P.J. Negative mood endures after completion of high-altitude military training. Ann. Behav. Med. 2005, 29, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodzko-Zajko, W.J.; Moore, K.A. Physical fitness and cognitive functioning in aging. Exerc. Sport Sci. Rev. 1994, 22, 195–220. [Google Scholar] [CrossRef]
- De Aquino Lemos, V.; Antunes, H.K.; dos Santos, R.V.; Lira, F.S.; Tufik, S.; de Mello, M.T. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012, 49, 1298–1306. [Google Scholar] [CrossRef]
- De Aquino-Lemos, V.; Santos, R.V.; Antunes, H.K.; Lira, F.S.; Luz Bittar, I.G.; Caris, A.V.; Tufik, S.; de Mello, M.T. Acute physical exercise under hypoxia improves sleep, mood and reaction time. Physiol. Behav. 2016, 154, 90–99. [Google Scholar] [CrossRef]
- Kryskow, M.A.; Beidleman, B.A.; Fulco, C.S.; Muza, S.R. Performance during simple and complex military psychomotor tasks at various altitudes. Aviat. Space Environ. Med. 2013, 84, 1147–1152. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Han, L.; Wang, T.; Wei, Y. Effects of acute moderate hypoxia on human performance of arithmetic. Hang Tian Yi Xue Yu Yi Xue Gong Cheng Space Med. Med. Eng. 1998, 11, 391–395. [Google Scholar]
- Shukitt-Hale, B.; Banderet, L.E.; Lieberman, H.R. Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. Int. J. Aviat. Psychol. 1998, 8, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Bjursten, H.; Ederoth, P.; Sigurdsson, E.; Gottfredsson, M.; Syk, I.; Einarsson, O.; Gudbjartsson, T. S100B profiles and cognitive function at high altitude. High Alt. Med. Biol. 2010, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Brown, E.; Taylor, C.T. Hypoxia-sensitive pathways in intestinal inflammation. J. Physiol. 2018, 596, 2985–2989. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Indias, I.; Torres, M.; Montserrat, J.M.; Sanchez-Alcoholado, L.; Cardona, F.; Tinahones, F.J.; Gozal, D.; Poroyko, V.A.; Navajas, D.; Queipo-Ortuno, M.I.; et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 2015, 45, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jiao, L.; Liu, R.; Zhang, Y.; Ji, Q.; Zhang, H.; Gao, X.; Ma, Y.; Shi, H.N. The effect of exposure to high altitude and low oxygen on intestinal microbial communities in mice. PLoS ONE 2018, 13, e0203701. [Google Scholar] [CrossRef]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Small, S.A.; Schobel, S.A.; Buxton, R.B.; Witter, M.P.; Barnes, C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011, 12, 585–601. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Humphreys, A.G.; Lehman, J.C.; Diamond, D.M.; Rose, G.M.; Meaney, M.J. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Deckersbach, T.; Dougherty, D.D.; Rauch, S.L. Functional imaging of mood and anxiety disorders. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2006, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W.; Mold, C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Hu, S.L.; Xiong, W.; Dai, Z.Q.; Zhao, H.L.; Feng, H. Cognitive changes during prolonged stay at high altitude and its correlation with c-reactive protein. PLoS ONE 2016, 11, e0146290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, A.; Alexander, J.J. Complement and blood-brain barrier integrity. Mol. Immunol. 2014, 61, 149–152. [Google Scholar] [CrossRef]
- Johnsen, E.; Fathian, F.; Kroken, R.A.; Steen, V.M.; Jorgensen, H.A.; Gjestad, R.; Loberg, E.M. The serum level of C-reactive protein (CRP) is associated with cognitive performance in acute phase psychosis. BMC Psychiatry 2016, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Lotrich, F.E.; Butters, M.A.; Aizenstein, H.; Marron, M.M.; Reynolds, C.F., 3rd; Gildengers, A.G. The relationship between interleukin-1 receptor antagonist and cognitive function in older adults with bipolar disorder. Int. J. Geriatr. Psychiatry 2014, 29, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Fritzenwanger, M.; Jung, C.; Goebel, B.; Lauten, A.; Figulla, H.R. Impact of short-term systemic hypoxia on phagocytosis, cytokine production, and transcription factor activation in peripheral blood cells. Mediat. Inflamm. 2011, 2011, 429501. [Google Scholar] [CrossRef]
- Taylor, C.T. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J. Physiol. 2008, 586, 4055–4059. [Google Scholar] [CrossRef]
- Franco, D.L.; Mainez, J.; Vega, S.; Sancho, P.; Murillo, M.M.; de Frutos, C.A.; del Castillo, G.; Lopez-Blau, C.; Fabregat, I.; Nieto, M.A. Snail1 suppresses TGF-beta-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J. Cell Sci. 2010, 123, 3467–3477. [Google Scholar] [CrossRef] [Green Version]
- Demasi, M.; Cleland, L.G.; Cook-Johnson, R.J.; Caughey, G.E.; James, M.J. Effects of hypoxia on monocyte inflammatory mediator production: Dissociation between changes in cyclooxygenase-2 expression and eicosanoid synthesis. J. Biol. Chem. 2017, 292, 15993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaus, J.; Vandeleur, C.L.; von Kanel, R.; Lasserre, A.M.; Strippoli, M.P.; Gholam-Rezaee, M.; Castelao, E.; Marques-Vidal, P.; Bovet, P.; Merikangas, K.; et al. Associations between mood, anxiety or substance use disorders and inflammatory markers after adjustment for multiple covariates in a population-based study. J. Psychiatr. Res. 2014, 58, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, S.M.; Scully, P.; Scott, L.V.; Dinan, T.G. Cytokine profiles in bipolar affective disorder: Focus on acutely ill patients. J. Affect. Disord. 2006, 90, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Dominguez, A.; Hernandez, M.E.; Berlanga, C.; Gutierrez-Mora, D.; Moreno, J.; Heinze, G.; Pavon, L. Immune variations in bipolar disorder: Phasic differences. Bipolar Disord. 2007, 9, 596–602. [Google Scholar] [CrossRef]
- Lawless, N.P.; Dillard, T.A.; Torrington, K.G.; Davis, H.Q.; Kamimori, G. Improvement in hypoxemia at 4600 meters of simulated altitude with carbohydrate ingestion. Aviat. Spaceand Environ. Med. 1999, 70, 874–878. [Google Scholar]
- Bailey, D.M.; Davies, B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt. Med. Biol. 2001, 2, 21–29. [Google Scholar] [CrossRef]
- Oliver, S.J.; Golja, P.; Macdonald, J.H. Carbohydrate supplementation and exercise performance at high altitude: A randomized controlled trial. High Alt. Med. Biol. 2012, 13, 22–31. [Google Scholar] [CrossRef]
- Charlot, K.; Pichon, A.; Richalet, J.P.; Chapelot, D. Effects of a high-carbohydrate versus high-protein meal on acute responses to hypoxia at rest and exercise. Eur. J. Appl. Physiol. 2013, 113, 691–702. [Google Scholar] [CrossRef]
- Caris, A.V.; Da Silva, E.T.; Dos Santos, S.A.; Lira, F.S.; Oyama, L.M.; Tufik, S.; Dos Santos, R.V. Carbohydrate supplementation influences serum cytokines after exercise under hypoxic conditions. Nutrients 2016, 8, 706. [Google Scholar] [CrossRef]
- Santos, S.A.; Silva, E.T.; Caris, A.V.; Lira, F.S.; Tufik, S.; Dos Santos, R.V. Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J. Hum. Nutr. Diet. 2016, 29, 516–522. [Google Scholar] [CrossRef]
- Lefferts, W.K.; Hughes, W.E.; White, C.N.; Brutsaert, T.D.; Heffernan, K.S. Effect of acute nitrate supplementation on neurovascular coupling and cognitive performance in hypoxia. Appl. Physiol. Nutr. Metab. 2016, 41, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Masschelein, E.; van Thienen, R.; Wang, X.; Van Schepdael, A.; Thomis, M.; Hespel, P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 2012, 113, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, D.P.; Treichler, D.P.; Ganger, C.T.t.; Schneider, A.C.; Ueda, K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J. Appl. Physiol. 2015, 118, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Vanhatalo, A.; Fulford, J.; Bailey, S.J.; Blackwell, J.R.; Winyard, P.G.; Jones, A.M. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J. Physiol. 2011, 589, 5517–5528. [Google Scholar] [CrossRef] [Green Version]
- Hennis, P.J.; Mitchell, K.; Gilbert-Kawai, E.; Bountziouka, V.; Wade, A.; Feelisch, M.; Grocott, M.P.; Martin, D.S. Effects of dietary nitrate supplementation on symptoms of acute mountain sickness and basic physiological responses in a group of male adolescents during ascent to Mount Everest Base Camp. Nitric Oxide 2016, 60, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Parry-Billings, M.; Budgett, R.; Koutedakis, Y.; Blomstrand, E.; Brooks, S.; Williams, C.; Calder, P.C.; Pilling, S.; Baigrie, R.; Newsholme, E.A. Plasma amino acid concentrations in the overtraining syndrome: Possible effects on the immune system. Med. Sci. Sports Exerc. 1992, 24, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Blannin, A.K.; Bishop, N.C.; Robson, P.J.; Gleeson, M. Effect of oral glutamine supplementation on human neutrophil lipopolysaccharide-stimulated degranulation following prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Krzywkowski, K.; Petersen, E.W.; Ostrowski, K.; Link-Amster, H.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. J. Appl. Physiol. 2001, 91, 832–838. [Google Scholar] [CrossRef]
- Krieger, J.W.; Crowe, M.; Blank, S.E. Chronic glutamine supplementation increases nasal but not salivary IgA during 9 days of interval training. J. Appl. Physiol. 2004, 97, 585–591. [Google Scholar] [CrossRef]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045S–2049S. [Google Scholar] [CrossRef]
- Sasaki, E.; Umeda, T.; Takahashi, I.; Arata, K.; Yamamoto, Y.; Tanabe, M.; Oyamada, K.; Hashizume, E.; Nakaji, S. Effect of glutamine supplementation on neutrophil function in male judoists. Luminescence 2013, 28, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Legault, Z.; Bagnall, N.; Kimmerly, D.S. The influence of oral l-glutamine supplementation on muscle strength recovery and soreness following unilateral knee extension eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Kjaer, M. Glucoregulation during exercise: The role of the neuroendocrine system. Sports Med. 2005, 35, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Newsholme, E.A. Intracellular distribution of some enzymes of the glutamine utilisation pathway in rat lymphocytes. Biochem. Biophys. Res. Commun. 1986, 138, 318–322. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Evans, J.; Calder, P.C.; Newsholme, E.A. Does glutamine contribute to immunosuppression after major burns? Lancet 1990, 336, 523–525. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhao, L.; Chen, M.; Bai, G.; Hu, Y.; Hu, W.; Yan, Z.; Gao, H. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim. Biophys. Acta 2017, 1863, 266–273. [Google Scholar] [CrossRef]
- Thielen, J.W.; Hong, D.; Rohani Rankouhi, S.; Wiltfang, J.; Fernandez, G.; Norris, D.G.; Tendolkar, I. The increase in medial prefrontal glutamate/glutamine concentration during memory encoding is associated with better memory performance and stronger functional connectivity in the human medial prefrontal-thalamus-hippocampus network. Hum. Brain Mapp. 2018, 39, 2381–2390. [Google Scholar] [CrossRef] [Green Version]
- De Kieviet, J.F.; Oosterlaan, J.; van Zwol, A.; Boehm, G.; Lafeber, H.N.; van Elburg, R.M. Effects of neonatal enteral glutamine supplementation on cognitive, motor and behavioural outcomes in very preterm and/or very low birth weight children at school age. Br. J. Nutr. 2012, 108, 2215–2220. [Google Scholar] [CrossRef]
- Arwert, L.I.; Deijen, J.B.; Drent, M.L. Effects of an oral mixture containing glycine, glutamine and niacin on memory, GH and IGF-I secretion in middle-aged and elderly subjects. Nutr. Neurosci. 2003, 6, 269–275. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Kahana, M.; Wolfson, R.; Ren, H.; Musch, M.M.; Chang, E.B. Glutamine reduces cytokine release, organ damage, and mortality in a rat model of endotoxemia. Shock 2001, 16, 398–402. [Google Scholar] [CrossRef]
- De Souza, A.Z.; Zambom, A.Z.; Abboud, K.Y.; Reis, S.K.; Tannihao, F.; Guadagnini, D.; Saad, M.J.; Prada, P.O. Oral supplementation with L-glutamine alters gut microbiota of obese and overweight adults: A pilot study. Nutrition 2015, 31, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felger, J.C. Role of inflammation in depression and treatment implications. Handb. Exp. Pharm. 2018. [Google Scholar] [CrossRef]
- Kiernan, E.A.; Smith, S.M.; Mitchell, G.S.; Watters, J.J. Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia. J. Physiol. 2016, 594, 1563–1577. [Google Scholar] [CrossRef]
- Karl, J.P.; Berryman, C.E.; Young, A.J.; Radcliffe, P.N.; Branck, T.A.; Pantoja-Feliciano, I.G.; Rood, J.C.; Pasiakos, S.M. Associations between the gut microbiota and host responses to high altitude. Am. J. Physiol. Gastrointest Liver Physiol. 2018. [Google Scholar] [CrossRef]
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 2014, 817, 357–371. [Google Scholar] [CrossRef]
- Novotny, M.; Klimova, B.; Valis, M. Microbiome and cognitive impairment: Can any diets influence learning processes in a positive way? Front. Aging Neurosci. 2019, 11, 170. [Google Scholar] [CrossRef]
- Huang, T.T.; Lai, J.B.; Du, Y.L.; Xu, Y.; Ruan, L.M.; Hu, S.H. Current understanding of gut microbiota in mood disorders: An update of human studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- L’Huillier, C.; Jarbeau, M.; Achamrah, N.; Belmonte, L.; Amamou, A.; Nobis, S.; Goichon, A.; Salameh, E.; Bahlouli, W.; do Rego, J.L.; et al. Glutamine, but not branched-chain amino acids, restores intestinal barrier function during activity-based anorexia. Nutrients 2019, 11, 1348. [Google Scholar] [CrossRef] [Green Version]
- Akisu, M.; Baka, M.; Huseyinov, A.; Kultursay, N. The role of dietary supplementation with L-glutamine in inflammatory mediator release and intestinal injury in hypoxia/reoxygenation-induced experimental necrotizing enterocolitis. Ann. Nutr. Metab. 2003, 47, 262–266. [Google Scholar] [CrossRef]
- Han, T.; Li, X.; Cai, D.; Zhong, Y.; Chen, L.; Geng, S.; Yin, S. Effect of glutamine on apoptosis of intestinal epithelial cells of severe acute pancreatitis rats receiving nutritional support in different ways. Int. J. Clin. Exp. Pathol. 2013, 6, 503–509. [Google Scholar] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin Pr. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Amores-Sanchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroon, E.; Miller, A.H. Inflammation effects on brain glutamate in depression: Mechanistic considerations and treatment implications. Curr. Top Behav. Neurosci. 2017, 31, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Bye, R.; Scheltinga, M.; Ziegler, T.R.; Jacobs, D.O.; Wilmore, D.W. Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. Jpn. J. Parenter Enter. Nutr. 1993, 17, 422–427. [Google Scholar] [CrossRef]
- Hasler, G.; Buchmann, A.; Haynes, M.; Muller, S.T.; Ghisleni, C.; Brechbuhl, S.; Tuura, R. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl. Psychiatry 2019, 9, 170. [Google Scholar] [CrossRef]
- O’Donnell-Luria, A.H.; Lin, A.P.; Merugumala, S.K.; Rohr, F.; Waisbren, S.E.; Lynch, R.; Tchekmedyian, V.; Goldberg, A.D.; Bellinger, A.; McFaline-Figueroa, J.R.; et al. Brain MRS glutamine as a biomarker to guide therapy of hyperammonemic coma. Mol. Genet. Metab. 2017, 121, 9–15. [Google Scholar] [CrossRef]
- Kusumoto, I. Industrial production of L-glutamine. J. Nutr. 2001, 131, 2552S–2555S. [Google Scholar] [CrossRef] [Green Version]
- Holecek, M. Side effects of long-term glutamine supplementation. Jpn. J. Parenter Enter. Nutr. 2013, 37, 607–616. [Google Scholar] [CrossRef]
- Oppong, K.N.; Al-Mardini, H.; Thick, M.; Record, C.O. Oral glutamine challenge in cirrhotics pre- and post-liver transplantation: A psychometric and analyzed EEG study. Hepatology 1997, 26, 870–876. [Google Scholar] [CrossRef]
- Lenders, C.M.; Liu, S.; Wilmore, D.W.; Sampson, L.; Dougherty, L.W.; Spiegelman, D.; Willett, W.C. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur. J. Clin. Nutr. 2009, 63, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
| MeSH, Entry Terms and Keywords Combinations Used to Search the Articles. | |
|---|---|
| #1 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND Mood disorders |
| #2 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND Cognition disorders |
| #3 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND inflammation OR neurogenic inflammation |
| #4 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND Glutamine OR L-Glutamine OR L Glutamine |
| #5 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND Cognition OR Cognition disorders AND Affect OR Mood disorders AND inflammation OR neurogenic inflammation |
| #6 | Hypoxia OR High Altitude OR Altitude Sickness OR Mountain Sickness OR Simulated Altitude AND Cognition OR Cognition disorders AND Affect OR Mood disorders AND inflammation OR neurogenic inflammation AND OR L-Glutamine OR L Glutamine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos Quaresma, M.; Souza, W.; Lemos, V.; Caris, A.; Thomatieli-Santos, R. The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude. Nutrients 2020, 12, 3627. https://doi.org/10.3390/nu12123627
Dos Santos Quaresma M, Souza W, Lemos V, Caris A, Thomatieli-Santos R. The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude. Nutrients. 2020; 12(12):3627. https://doi.org/10.3390/nu12123627
Chicago/Turabian StyleDos Santos Quaresma, MVL, WYG Souza, VA Lemos, AV Caris, and RV Thomatieli-Santos. 2020. "The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude" Nutrients 12, no. 12: 3627. https://doi.org/10.3390/nu12123627
APA StyleDos Santos Quaresma, M., Souza, W., Lemos, V., Caris, A., & Thomatieli-Santos, R. (2020). The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude. Nutrients, 12(12), 3627. https://doi.org/10.3390/nu12123627






