The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy
Abstract
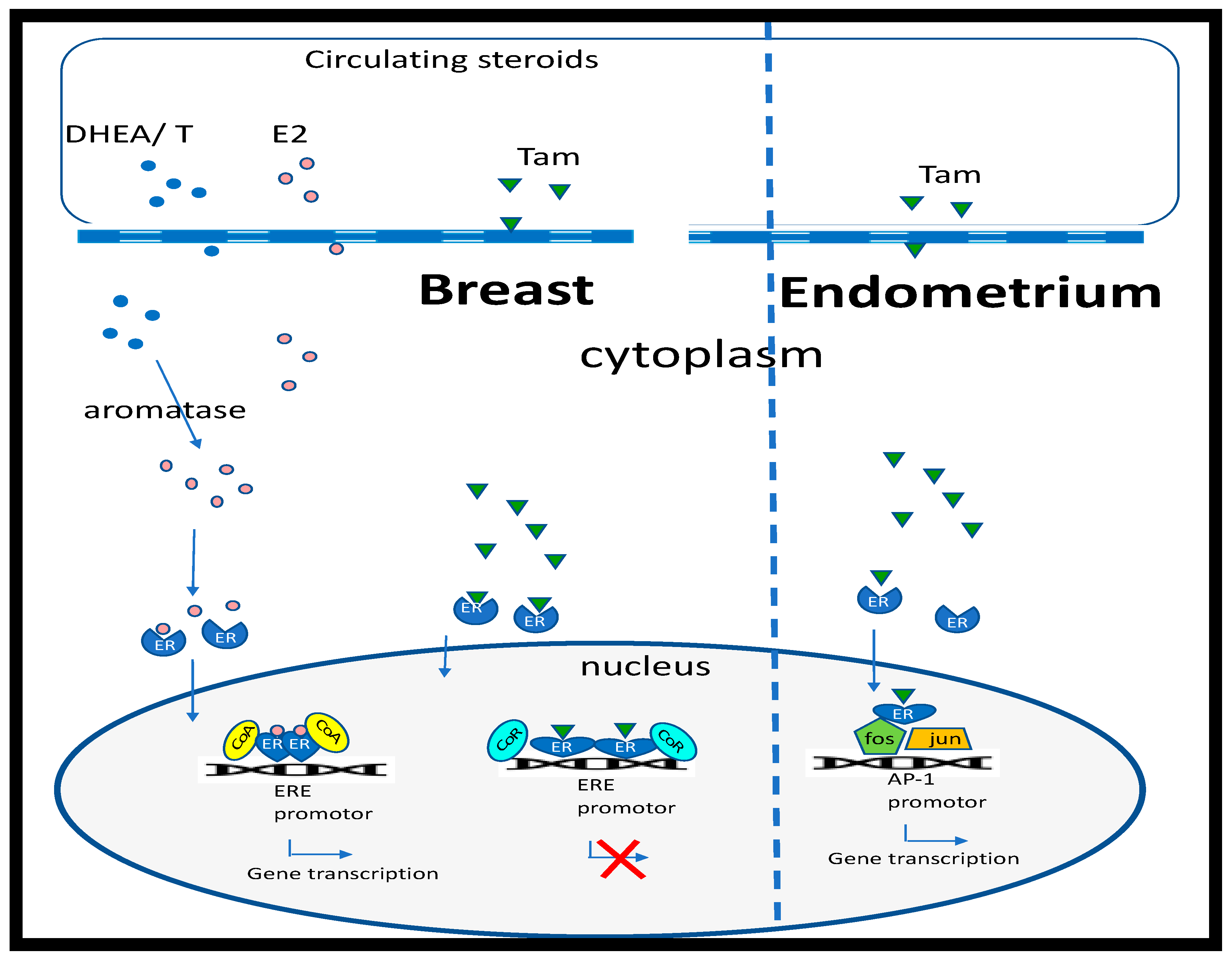
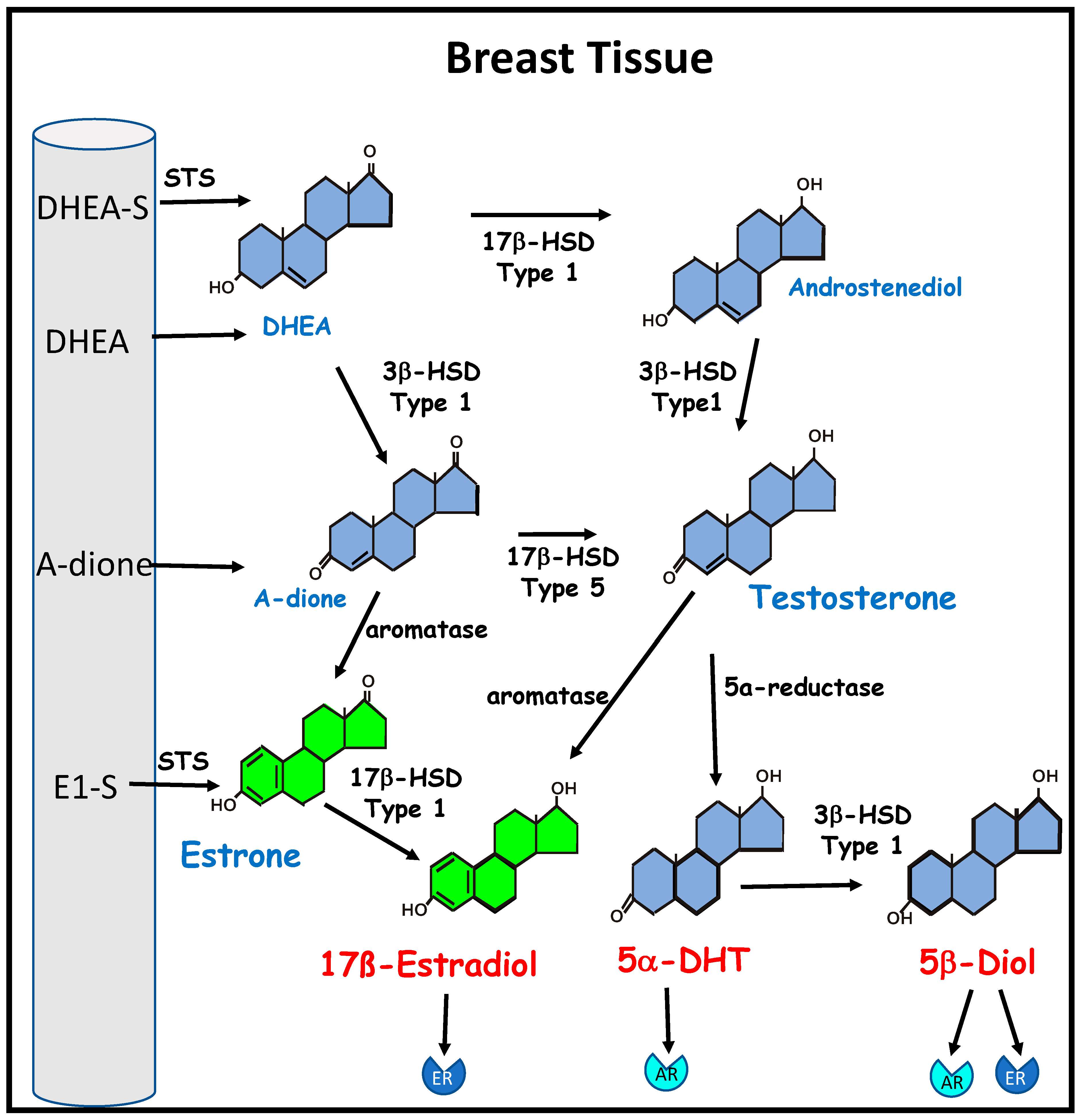
1. Estrogens and Breast Cancer
2. Breast Cancer Treatment
3. Estrogen and Hair Growth
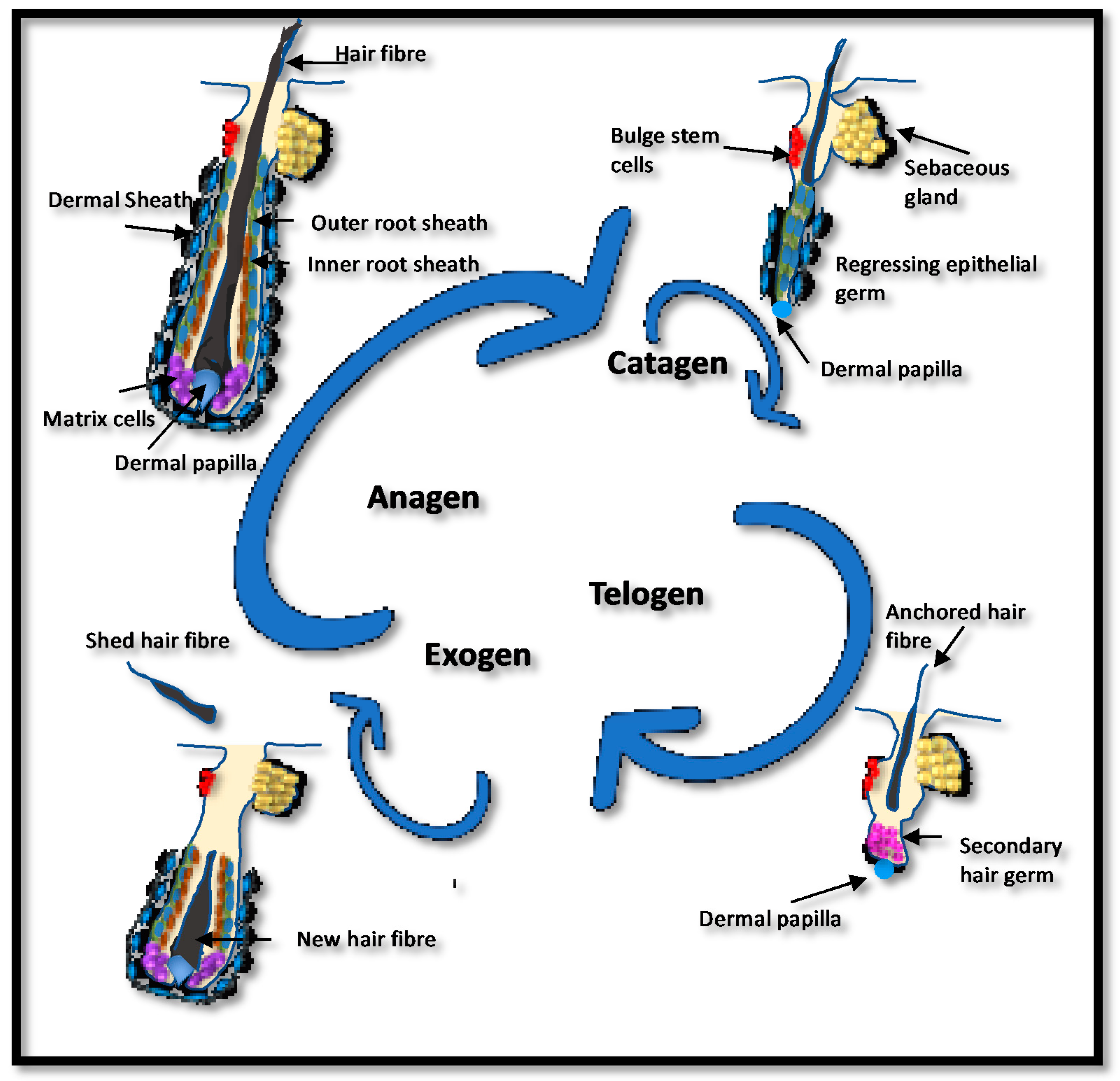
4. Endocrine Therapy-Induced Hair Loss (ETIHL)
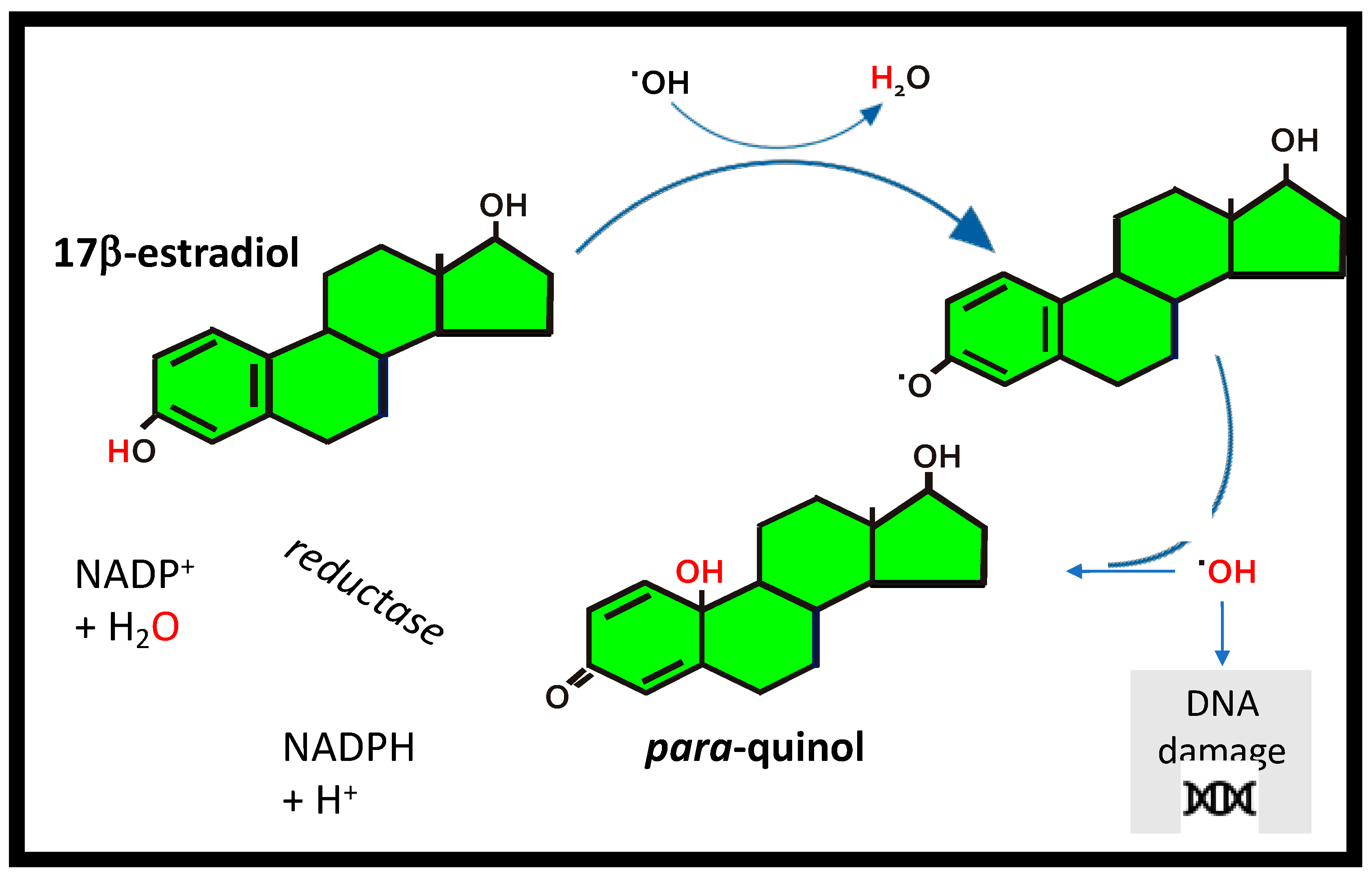
5. Estrogens as Antioxidants
6. Natural Ingredients and Their Effect on Breast Cancer and Hair Growth
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Dall, G.V.; Britt, K.L. Estrogen Effects on the Mammary Gland in Early and Late Life and Breast Cancer Risk. Front. Oncol. 2017, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001, 344, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Wang, J.P.; Li, Y.; Fan, P.; Liu, G.; Zhang, N.; Conaway, M.; Wang, H.; Korach, K.S.; Bocchinfuso, W.; et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int. J. Cancer 2010, 127, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Su, K.; Zeng, J. Clinicopathological classification and traditional prognostic indicators of breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8500–8505. [Google Scholar]
- Fortner, R.T.; Sisti, J.; Chai, B.; Collins, L.C.; Rosner, B.; Hankinson, S.E.; Tamimi, R.M.; Eliassen, A.H. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: Results from the Nurses’ Health Studies. Breast Cancer Res. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ERβ. Identification and characterisation of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef]
- Cui., J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Nguyen, P.; Kushner, P.J. Differential SERM effects on corepressor binding dictate ERalpha activity in vivo. J. Biol. Chem. 2003, 278, 6912–6920. [Google Scholar] [CrossRef] [PubMed]
- Paech, K.; Webb, P.; Kuiper, G.G.; Nilsson, S.; Gustafsson, J.; Kushner, P.J.; Scanlan, T.S. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997, 277, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Evers, N.M.; van den Berg, J.H.J.; Wang, S.; Melchers, D.; Houtman, R.; de Haan, L.H.J.; Ederveen, A.G.H.; Groten, J.P.; Rietjens, I.M.C.M. Cell proliferation and modulation of interaction of estrogen receptors with coregulators induced by ERα and ERβ agonists. J. Steroid Biochem. Mol. Biol. 2014, 143, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Minireview: Extranuclear steroid receptors: Roles in modulation of cell functions. Mol. Endocrinol. 2011, 25, 377–384. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Sainson, R.C.; Kim, J.K.; Hughes, C.C.; Levin, E.R. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007, 282, 22278–22288. [Google Scholar] [CrossRef]
- Levin, E.R. G-protein-coupled receptor 30: Estrogen receptor or collaborator? Endocrinology 2009, 150, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Fan, P.; Zhang, Z.; Bao, Y.; Song, R.X.; Yue, W. Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids 2009, 74, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Song, P.; Li, Y.; Dong, Y.; Liang, Y.; Qu, H.; Qi, D.; Lu, Y.; Jin, X.; Guo, Y.; Jia, Y.; et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef]
- Hartman, J.; Lindberg, K.; Morani, A.; Inzunza, J.; Strom, A.; Gustafsson, J.A. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006, 66, 11207–11213. [Google Scholar] [CrossRef]
- Song, W.; Tang, L.; Xu, Y.; Sun, Q.; Yang, F.; Guan, X. ERbeta1 inhibits metastasis of androgen receptor-positive triple-negative breast cancer by suppressing ZEB1. J. Exp. Clin. Cancer Res. 2017, 36, 75. [Google Scholar] [CrossRef]
- Ruddy, S.C.; Lau, R.; Cabrita, M.A.; McGregor, C.; McKay, B.C.; Murphy, L.C.; Wright, J.S.; Durst, T.; Pratt, M.A.C. Preferential estrogen receptor beta ligands reduce Bcl-2 expression in hormone-resistant breast cancer cells to increase autophagy. Mol. Cancer Ther. 2014, 13, 1882–1893. [Google Scholar] [CrossRef]
- Salhab, M.; Reed, M.; Al Sarakbi, W.; Jiang, W.G.; Mokbel, K. The role of aromatase and 17β hydroxysteroid dehydrogenase type 1 mRNA expression in predicting the clinical outcome of human breast cancer. Breast Cancer Res. Treat. 2006, 99, 155–162. [Google Scholar] [CrossRef]
- Santen, R.J.; Martel, J.; Hoagland, M.; Naftolin, F.; Roa, L.; Harada, N.; Hafer, L.; Zaino, R.; Pauley, R.; Santner, S. Demonstration of aromatase activity and its regulation in breast tumor and benign breast fibroblasts. Breast Cancer Res. Treat. 1998, 49 (Suppl. 9), S93–S99. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Bogush, T.A.; Polezhaev, B.B.; Mamichev, I.A.; Bogush, E.A.; Polotsky, B.E.; Tjulandin, S.A.; Ryabov, A.B. Tamoxifen Never Ceases to Amaze: New Findings on Non-Estrogen Receptor Molecular Targets and Mediated Effects. Cancer Investig. 2018, 36, 211–220. [Google Scholar] [CrossRef]
- Sainsbury, R. The development of endocrine therapy for women with breast cancer. Cancer Treat. Rev. 2013, 39, 507–517. [Google Scholar] [CrossRef]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57, 9S–16S. [Google Scholar] [CrossRef]
- Bergin, A.R.T.; Loi, S. Triple-negative breast cancer: Recent treatment advances. F1000 Res. 2019, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Droog, M.; Beelen, K.; Linn, S.; Zwart, W. Tamoxifen resistance: From bench to bedside. Eur. J. Pharmacol. 2013, 717, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hachisuga, T.; Miyakawa, T.; Tsujioka, H.; Horiuchi, S.; Emoto, M.; Kawarabayashi, T. K-ras mutation in tamoxifen-related endometrial polyps. Cancer 2003, 98, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. The Past, Present, and Future of Selective Estrogen Receptor Modulation. Ann. N. Y. Acad. Sci. 2001, 949, 72–79. [Google Scholar] [CrossRef]
- Karatas, F.; Sahin, S.; Sever, A.R.; Altundag, K. Management of hair loss associated with endocrine therapy in patients with breast cancer: An overview. Springerplus 2016, 5, 585. [Google Scholar] [CrossRef]
- Randall, V.A.; Hibberts, N.A.; Thornton, M.J.; Hamada, K.; Merrick, A.E.; Kato, S.; Jenner, T.J.; De Oliveira, I.; Messenger, A.G. The hair follicle: A paradoxical androgen target organ. Horm Res. 2000, 54, 243–250. [Google Scholar] [CrossRef]
- Randall, V.A.; Ebling, F.J. Seasonal changes in human hair growth. Br. J. Dermatol. 1991, 124, 146–151. [Google Scholar] [CrossRef]
- Lynfield, Y.L. Effect of pregnancy on the human hair cycle. J. Investig. Dermatol. 1960, 35, 323–327. [Google Scholar] [CrossRef]
- Labrie, F. Intracrinology and menopause: The science describing the cell-specific intracellular formation of estrogens and androgens from DHEA and their strictly local action and inactivation in peripheral tissues. Menopause 2019, 26, 220–224. [Google Scholar] [CrossRef]
- Chen, W.; Yang, C.-C.; Todorova, A.; Al Khuzaei, S.; Chiu, H.-C.; Worret, W.I.; Ring, J. Hair loss in elderly women. Eur. J. Dermatol. 2010, 20, 145–151. [Google Scholar] [CrossRef]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br. J. Dermatol. 2009, 161, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Association analysis of oestrogen receptor beta gene (ESR2) polymorphisms with female pattern hair loss. Br. J. Dermatol. 2012, 166, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.J.; Taylor, A.H.; Mulligan, K.; Al-Azzawi, F.; Lyon, C.; O’Driscoll, J.; Messenger, A.G. The distribution of estrogen receptor beta (ERbeta) is distinct to that of ERalpha and the androgen receptor in human skin and the pilosebaceous unit. J. Investig. Dermatol. Symp. Proc. 2003, 8, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.; Taylor, A.H.; Meskiri, A.; Sharpe, D.T.; Thornton, M.J. Differing responses of human follicular and nonfollicular scalp cells in an in vitro wound healing assay: Effects of estrogen on vascular endothelial growth factor secretion. Wound Repair Regen. 2008, 16, 243–253. [Google Scholar] [CrossRef]
- Labrie, F. Adrenal androgens and intracrinology. Semin. Reprod. Med. 2004, 22, 299–309. [Google Scholar] [CrossRef]
- Pomari, E.; Dalla Valle, L.; Pertile, P.; Colombo, L.; Thornton, M.J. Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts. FASEB J. 2015, 29, 508–524. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, V.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Price, V.H. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J. Investig. Dermatol. 1997, 109, 296–300. [Google Scholar] [CrossRef]
- Sánchez, P.; Serrano-Falcón, C.; Torres, J.M.; Serranoortega, S.; Ortega, E. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch. Dermatol. Res. 2018, 310, 77–83. [Google Scholar] [CrossRef]
- Rebora, A.; Guarrera, M. Kenogen. A new phase of the hair cycle? Dermatology 2002, 205, 108–110. [Google Scholar] [CrossRef]
- Gallicchio, L.; Calhoun, C.; Helzlsouer, K.J. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast Cancer Res. Treat. 2013, 142, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Saggar, V.; Wu, S.; Dickler, M.N.; Lacouture, M.E. Alopecia with endocrine therapies in patients with cancer. Oncologist 2013, 18, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Iorio, A.; Scali, E.; Fortuna, M.; Mari, E.; Maxia, C.; Gerardi, M.; Framarino, M.; Carlesimo, M. Aromatase inhibitors induce ‘male pattern hair loss’ in women? Ann. Oncol. 2013, 24, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Gateley, C.; Bundred, N. Alopecia and breast disease. Br. Med. J. 1997, 314, 481. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Shapiro, J.; Chan, D.; Fornier, M.; Modi, S.; Gajria, D.; Dusza, S.; Goldfarb, S.; Lacouture, M.E. Endocrine Therapy-Induced Alopecia in Patients with Breast Cancer. JAMA Dermatol. 2018, 154, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, S.K.; Kim, H.U.; Ihm, C.W. Pattern Alopecia during Hormonal Anticancer Therapy in Patients with Breast Cancer. Ann. Dermatol. 2014, 26, 743–746. [Google Scholar] [CrossRef]
- Richardson, T.E.; Yang, S.H.; Wen, Y.; Simpkins, J.W. Estrogen protection in Friedreich’s ataxia skin fibroblasts. Endocrinology 2011, 152, 2742–2749. [Google Scholar] [CrossRef][Green Version]
- Prokai, L.; Prokai-Tatrai, K.; Perjesi, P.; Zharikova, A.D.; Perez, E.J.; Liu, R.; Simpkins, J.W. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Natl. Acad. Sci. USA 2003, 100, 11741–11746. [Google Scholar] [CrossRef]
- Richardson, T.E.; Yu, A.E.; Wen, Y.; Yang, S.H.; Simpkins, J.W. Estrogen prevents oxidative damage to the mitochondria in Friedreich’s ataxia skin fibroblasts. PLoS ONE 2012, 7, e34600. [Google Scholar] [CrossRef]
- Behl, C.; Holsboer, F. The female sex hormone oestrogen as a neuroprotectant. Trends Pharmacol. Sci. 1999, 20, 441–444. [Google Scholar] [CrossRef]
- Baker, M.E.; Lathe, R. The promiscuous estrogen receptor: Evolution of physiological estrogens and response to phytochemicals and endocrine disruptors. J. Steroid Biochem. Mol. Biol. 2018, 184, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Stuart, J.A. Resveratrol interacts with estrogen receptor-β to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase. Free Radic. Biol. Med. 2011, 50, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Adnan, M.; Rahman, M.M. Natural Sources of Tocotrienols: A Note on Absorption. J. In Silico In Vitr. Pharmacol. 2017, 3, 20. [Google Scholar] [CrossRef]
- Zhang, J.; Riby, J.E.; Conde, L.; Grizzle, W.E.; Cui, X.; Skibola, C.F. A Fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines. BMC Complement. Altern. Med. 2016, 16, 151. [Google Scholar] [CrossRef]
- Hosking, A.M.; Juhasz, M.; Mesinkovska, N.A. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Ski. Appendage Disord. 2019, 5, 72–89. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process. Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2016, 46, 185–196. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Bai, L.; Pan, M.H. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Asgarpanah, J.; Roohi, E. Phytochemistry and pharmacological properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6, 3689–3693. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Lee, J.; Huang, Y.; Singh, S.V. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol. Carcinog. 2011, 50, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, C.; Cheniti, A.; Connétable, S.; Piccardi, N.; Vincenzi, C.; Tosti, A. Effect of a nutritional supplement on hair loss in women. J. Cosmet. Dermatol. 2015, 14, 76–82. [Google Scholar]
- Rushton, D.H. Nutritional factors and hair loss. Clin. Exp. Dermatol. 2002, 27, 396–404. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Santamaria, R.; Irace, C.; Piccolo, M.; Maisto, M.; Novellino, E. Annurca Apple Nutraceutical Formulation Enhances Keratin Expression in a Human Model of Skin and Promotes Hair Growth and Tropism in a Randomized Clinical Trial. J. Med. Food. 2018, 21, 90–103. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef]
- Pozo-Guisado, E.; Merino, J.M.; Mulero-Navarro, S.; Lorenzo-Benayas, M.J.; Centeno, F.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int. J. Cancer 2005, 115, 74–84. [Google Scholar] [CrossRef]
- Ronghe, A.; Chatterjee, A.; Bhat, N.K.; Padhye, S.; Bhat, H.K. Tamoxifen synergizes with 4-(E)-{(4-hydroxyphenylimino)-methylbenzene, 1,2-diol} and 4-(E)-{(p-tolylimino)-methylbenzene-1,2-diol}, novel azaresveratrol analogs, in inhibiting the proliferation of breast cancer cells. Oncotarget 2016, 7, 51747–51762. [Google Scholar] [CrossRef] [PubMed]
- Juchaux, F.; Sellathurai, T.; Perrault, V.; Boirre, F.; Delannoy, P.; Bakkar, K.; Albaud, J.; Gueniche, A.; Cheniti, A.; Dal Belo, S.; et al. A combination of pyridine-2, 4-dicarboxylic acid diethyl ester and resveratrol stabilizes hypoxia-inducible factor 1-alpha and improves hair density in female volunteers. Int. J. Cosmet. Sci. 2020, 42, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Meganathan, P.; Veerasenan, S.D.; Selvaduray, K.R. Tocotrienols and breast cancer: The evidence to date. Genes Nutr. 2012, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Nesaretnam, K.; Leoni, G.; Ambra, R.; Canali, R.; Bolli, A.; Marino, M.; Virgili, F.A. Novel mechanism of natural vitamin E tocotrienol activity: Involvement of ERbeta signal transduction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E427–E437. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Guantario, B.; Leoni, G.; Nesaretnam, K.; Ronci, M.B.; Canali, R.; Virgili, F. Tocotrienols induce endoplasmic reticulum stress and apoptosis in cervical cancer cells. Genes Nutr. 2016, 11, 32. [Google Scholar] [CrossRef]
- Guthrie, N.; Gapor, A.; Chambers, A.F.; Carroll, K.K. Inhibition of Proliferation of Estrogen Receptor-Negative MDA-MB-435 and -Positive MCF-7 Human Breast Cancer Cells by Palm Oil Tocotrienols and Tamoxifen, Alone and in Combination. J. Nutr. 1997, 127, 544S–548S. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Selvaduray, K.R.; Abdul Razak, G.; Veerasenan, S.D.; Gomez, P.A. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: A pilot clinical trial. Breast Cancer Res. 2010, 12, R81. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Elangovan, S.; Wu, J.M. Differential Suppression of Proliferation in MCF-7 and MDA-MB-231 Breast Cancer Cells Exposed to Alpha-, Gamma- And Delta-Tocotrienols Is Accompanied by Altered Expression of Oxidative Stress Modulatory Enzymes. Anticancer Res. 2010, 30, 4169–4176. [Google Scholar]
- Ahmed, N.S.; Ghatak, S.; El Masry, M.S.; Gnyawali, S.C.; Roy, S.; Amer, M.; Everts, H.; Sen, C.K.; Khanna, S. Epidermal E-Cadherin Dependent β-Catenin Pathway is Phytochemical Inducible and Accelerates Anagen Hair Cycling. Mol. Ther. 2017, 25, 2502–2512. [Google Scholar] [CrossRef]
- Beoy, L.A.; Woei, W.J.; Hay, Y.K. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop. Life Sci Res. 2010, 21, 91–99. [Google Scholar]
- Hostanska, K.; Suter, A.; Melzer, J.; Saller, R. Evaluation of cell death caused by an ethanolic extract of Serenoae repentis fructus (Prostasan) on human carcinoma cell lines. Anticancer Res. 2007, 27, 873–881. [Google Scholar] [PubMed]
- Di Silverio, F.; D’Eramo, G.; Lubrano, C.; Flammia, G.P.; Sciarra, A.; Palma, E.; Caponera, M.; Sciarra, F. Evidence that Serenoa repens extract displays an antiestrogenic activity in prostatic tissue of benign prostatic hypertrophy patients. Eur. Urol. 1992, 21, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Strauch, G.; Perles, P.; Vergult, G.; Gabriel, M.; Gibelin, B.; Cummings, S.; Malbecq, W.; Malice, M.P. Comparison of finasteride (Proscar) and Serenoa repens (Permixon) in the inhibition of 5-alpha reductase in healthy male volunteers. Eur. Urol. 1994, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J. Altern. Complement. Med. 2002, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Mari, E.; Scarno, M.; Garelli, V.; Maxia, C.; Scali, E.; Iorio, A.; Carlesimo, M. Comparitive effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: A two-year study. Int. J. Immunopathol. Pharmacol. 2012, 25, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Wessagowit, V.; Tangjaturonrusamee, C.; Kootiratrakarn, T.; Bunnag, T.; Pimonrat, T.; Muangdang, N.; Pichai, P. Treatment of male androgenetic alopecia with topical products containing Serenoa repens extract. Australas. J. Dermatol. 2016, 57, 76–82. [Google Scholar] [CrossRef]
- Večeřa, R.; Orolin, J.; Škottová, N.; Kazdová, L.; Oliyarnik, O.; Ulrichová, J.; Šimánek, V. The influence of maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat. Plant Foods Hum. Nutr. 2007, 62, 59–63. [Google Scholar] [CrossRef]
- Fano, D.; Vásquez-Velásquez, C.; Gonzales-Castañeda, C.; Guajardo-Correa, E.; Orihuela, P.A.; Gonzales, G.F. N-Butanol and Aqueous Fractions of Red Maca Methanolic Extract Exerts Opposite Effects on Androgen and Oestrogens Receptors (Alpha and Beta) in Rats with Testosterone-Induced Benign Prostatic Hyperplasia. Evid. Based Complement. Altern. Med. 2017, 2017, 9124240. [Google Scholar] [CrossRef]
- Meissner, H.O.; Mscisz, A.; Reich-Bilinska, H.; Mrozikiewicz, P.; Bobkiewicz-Kozlowska, T.; Kedzia, B.; Lowicka, A.; Barchia, I. Hormone-Balancing Effect of Pre-Gelatinized Organic Maca (Lepidium peruvianum Chacon): (III) Clinical responses of early-postmenopausal women to Maca in double blind, randomized, Placebo-controlled, crossover configuration, outpatient study. Int. J. Biomed. Sci. 2006, 2, 375–394. [Google Scholar]
- Atwa, M.A.; Youssef, N.; Bayoumy, N.M. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-α) in patients with alopecia areata: Association with clinical type and severity. Int. J. Dermatol. 2016, 55, 666–672. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gasco, M.; Lozada-Requena, I. Role of maca (Lepidium meyenii) consumption on serum interleukin-6 levels and health status in populations living in the Peruvian Central Andes over 4000 m of altitude. Plant Foods Hum. Nutr. 2013, 68, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. J. Med. Life 2016, 9, 79–83. [Google Scholar] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2016, 7, 339–346. [Google Scholar] [CrossRef]
- Nejati-Koshki, K.; Akbarzadeh, A.; Pourhassan-Moghaddam, M. Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell Int. 2014, 14, 66. [Google Scholar] [CrossRef]
- Hallman, K.; Aleck, K.; Dwyer, B.; Lloyd, V.; Quigley, M.; Sitto, N.; Siebert, A.E.; Dinda, S. The effects of turmeric (curcumin) on tumor suppressor protein (p53) and estrogen receptor (ERα) in breast cancer cells. Breast Cancer 2017, 9, 153–161. [Google Scholar]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef]
- Singh, M.; Singh, N. Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol. Cell Biochem. 2011, 347, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.Y.; Zhang, C.J. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran. J. Reprod. Med. 2013, 11, 415–422. [Google Scholar]
- Jung, S.; Otberg, N.; Thiede, G.; Richter, H.; Sterry, W.; Panzner, S.; Lademann, J. Innovative liposomes as a transfollicular drug delivery system: Penetration into porcine hair follicles. J. Investig. Dermatol. 2006, 126, 1728–1732. [Google Scholar] [CrossRef]
- Konrádsdóttir, F.; Ogmundsdóttir, H.; Sigurdsson, V.; Loftsson, T. Drug targeting to the hair follicles: A cyclodextrin-based drug delivery. AAPS PharmSciTech 2009, 10, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.; Taira, N.; Maruta, H.; Tawata, S. Artepillin C and Other Herbal PAK1-blockers: Effects on Hair Cell Proliferation and Related PAK1-dependent Biological Function in Cell Culture. Phytother. Res. 2016, 30, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Lee, J.; Jung, E.; Kim, S.C.; Kang, J.I.; Lee, J.; Kim, Y.W.; Sung, Y.K.; Kang, H.K.; Park, D. A cell-based system for screening hair growth-promoting agents. Arch. Dermatol. Res. 2009, 301, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Pumthong, G.; Asawanonda, P.; Varothai, S.; Jariyasethavong, V.; Triwongwaranat, D.; Suthipinittharm, P.; Ingkaninan, K.; Leelapornpisit, P.; Waranuch, N. Curcuma aeruginosa, a novel botanically derived 5α-reductase inhibitor in the treatment of male-pattern baldness: A multicenter, randomized, double-blind, placebo-controlled study. J. Dermatol. Treat. 2012, 23, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Vanden Berghe, T.; Vanden Berghe, W. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Saggam, A.; Tillu, G.; Dixit, S.; Chavan-Gautam, P.; Borse, S.; Joshi, K.; Patwardhan, B. Withania somnifera (L.) Dunal: A potential therapeutic adjuvant in cancer. J. Ethnopharmacol. 2020, 255, 112759. [Google Scholar] [CrossRef]
- Khazal, K.F.; Samuel, T.; Hill, D.L.; Grubbs, C.J. Effect of an extract of Withania somnifera root on estrogen receptor-positive mammary carcinomas. Anticancer Res. 2013, 33, 1519–1523. [Google Scholar]
- Zhang, X.; Mukerji, R.; Samadi, A.K.; Cohen, M.S. Down-regulation of estrogen receptor-alpha and rearranged during transfection tyrosine kinase is associated with withaferin a-induced apoptosis in MCF-7 breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 84. [Google Scholar] [CrossRef]
- Sehrawat, A.; Samanta, S.K.; Hahm, E.R.; St Croix, C.; Watkins, S.; Singh, S.V. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion 2019, 47, 282–293. [Google Scholar] [CrossRef]
- Ghosh, K.; De, S.; Mukherjee, S.; Das, S.; Ghosh, A.N.; Sengupta, S.B. Withaferin A induced impaired autophagy and unfolded protein response in human breast cancer cell-lines MCF-7 and MDA-MB-231. Toxicol. In Vitro 2017, 44, 330–338. [Google Scholar] [CrossRef]
- Szarc vel Szic, K.; Op de Beeck, K.; Ratman, D.; Wouters, A.; Beck, I.M.; Declerck, K.; Heyninck, K.; Fransen, E.; Bracke, M.; De Bosscher, K.; et al. Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS ONE 2014, 9, e87850. [Google Scholar] [CrossRef] [PubMed]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on ashwagandha: A rasayana (rejuvenator) of ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Olff, M.; Güzelcan, Y.; de Vries, G.J.; Assies, J.; Gersons, B.P. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology 2006, 31, 1220–1230. [Google Scholar] [CrossRef]
- Vincent, M.; Yogiraj, K. A Descriptive Study of Alopecia Patterns and their Relation to Thyroid Dysfunction. Int. J. Trichol. 2013, 5, 57–60. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef]
- van Beek, N.; Bodó, E.; Kromminga, A.; Gáspár, E.; Meyer, K.; Zmijewski, M.A.; Slominski, A.; Wenzel, B.E.; Paus, R. Thyroid hormones directly alter human hair follicle functions: Anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J. Clin. Endocrinol. Metab. 2008, 93, 4381–4388. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Sellami, R.; Masmoudi, J.; Ouali, U.; Mnif, L.; Amouri, M.; Turki, H.; Jaoua, A. The relationship between alopecia areata and alexithymia, anxiety and depression: A case-control study. Indian J. Dermatol. 2014, 59, 421. [Google Scholar] [CrossRef]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair loss: Common causes and treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Jiang, X.; Qu, Q.; Li, M.; Miao, S.; Li, X.; Cai, W. Horsetail mixture on rheumatoid arthritis and its regulation on TNF-alpha and IL-10. Pak. J. Pharm. Sci. 2014, 27, 2019–2023. [Google Scholar] [PubMed]
- Park, E.Y.; Jeon, H. Antioxidant and Anti-inflammatory Activities of Equisetum hyemale. Nat. Prod. Sci. 2008, 14, 239–243. [Google Scholar]
- Cetojević-Simin, D.D.; Canadanović-Brunet, J.M.; Bogdanović, G.M.; Djilas, S.M.; Cetković, G.S.; Tumbas, V.T.; Stojiljković, B.T. Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense L.) extracts. J. Med. Food 2010, 13, 452–459. [Google Scholar]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-Reductase, IL-6 Secretion, and Oxidation Process of Equisetum debile Roxb. ex Vaucher Extract as Functional Food and Nutraceuticals Ingredients. Nutrients 2017, 10, 9. [Google Scholar] [CrossRef]
- Atalay, P.B.; Kuku, G.; Tuna, B.G. Effects of carbendazim and astaxanthin co-treatment on the proliferation of MCF-7 breast cancer cells. In Vitr. Cell. Dev. Biol. Anim. 2019, 55, 113–119. [Google Scholar] [CrossRef]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef]
- Naji, T.; Niazi, S.; Hamedani, P.S.K. The Cytotoxic Effects of Astaxanthin on Breast Cancer Cells. In Proceedings of the International Conference on BioMedical Sciences (ICBMS19), Istanbul, Turkey, 27–28 September 2019. Conference Book. [Google Scholar]
- Karimian, A.; Hajizadeh Moghaddam, A.; Mir Mohammadrezaei, F. Effect of Astaxanthin on cell viability in T-47D and MDA-MB-231 Breast Cancer Cell Lines. Multidiscip. Cancer Investig. 2017, 2017, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Moussavou, G.; Kwak, D.H.; Obiang-Obonou, B.W.; Maranguy, C.A.; Dinzouna-Boutamba, S.D.; Lee, D.H.; Pissibanganga, O.G.; Ko, K.; Seo, J.I.; Choo, Y.K. Anticancer effects of different seaweeds on human colon and breast cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural characterization and anticancer activity (MCF7 and MDA-MB-231) of polysaccharides fractionated from brown seaweed Sargassum Wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Fucoidans Stimulate Immune Reaction and Suppress Cancer Growth. Anticancer Res. 2017, 37, 6041–6046. [Google Scholar]
- Malyarenko, O.S.; Zdobnova, E.V.; Silchenko, A.S.; Kusaykin, M.I.; Ermakova, S.P. Radiosensitizing effect of the fucoidan from brown alga Fucus evanescens and its derivative in human cancer cells. Carbohydr. Polym. 2019, 205, 465–471. [Google Scholar] [CrossRef]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Meher, J.G.; Singh, P.; Raval, K.; Kumar, A.; et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol. 2019, 122, 1100–1114. [Google Scholar] [CrossRef]
- Abudabbus, A.; Badmus, J.A.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of Fucoidan and Chemotherapeutic Agent Combinations on Malignant and Non-malignant Breast Cell Lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients with Breast Cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef]
- Kang, J.I.; Kim, M.K.; Lee, J.H.; Jeon, Y.J.; Hwang, E.K.; Koh, Y.S.; Hyun, J.W.; Kwon, S.Y.; Yoo, E.S.; Kang, H.K. Undariopsis peterseniana Promotes Hair Growth by the Activation of Wnt/β-Catenin and ERK Pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef]
- Shin, H.; Cho, A.R.; Kim, D.Y.; Munkhbayer, S.; Choi, S.J.; Jang, S.; Kim, S.H.; Shin, H.C.; Kwon, O. Enhancement of Human Hair Growth Using Ecklonia cava Polyphenols. Ann. Dermatol. 2016, 28, 15–21. [Google Scholar] [CrossRef]
- Kang, J.I.; Kim, S.C.; Kim, M.K.; Boo, H.J.; Jeon, Y.J.; Koh, Y.S.; Yoo, E.S.; Kang, S.M.; Kang, H.K. Effect of Dieckol, a component of Ecklonia cava, on the promotion of hair growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, K.; Nakamura, T. Induction of hepatocyte growth factor by fucoidan and fucoidan-derived oligosaccharides. J. Pharm. Pharmacol. 2008, 60, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Jindo, T.; Tsuboi, R.; Takamori, K.; Ogawa, H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J. Investig. Dermatol. 1998, 110, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Kim, T.S.; Kwon, H.J.; Lee, S.P.; Kang, M.H.; Kim, B.J.; Kim, M.N. Efficacy of Cistanche Tubulosa and Laminaria Japonica Extracts (MK-R7) Supplement in Preventing Patterned Hair Loss and Promoting Scalp Health. Clin. Nutr. Res. 2015, 4, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, D.H. Comparison of Saccharina japonica-Undaria pinnatifida Mixture and Minoxidil on Hair Growth Promoting Effect in Mice. Arch. Plast. Surg. 2016, 43, 498–505. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- Piccolo, M.; Ferraro, M.G.; Maione, F.; Maisto, M.; Stornaiuolo, M.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Induction of Hair Keratins Expression by an Annurca Apple-Based Nutraceutical Formulation in Human Follicular Cells. Nutrients 2019, 11, 3041. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, H.; Ye, Y.; Liu, L.; Li, S.; Zhang, J.; Luo, R. Safflower polysaccharide inhibits the proliferation and metastasis of MCF-7 breast cancer cell. Mol. Med. Rep. 2015, 11, 4611–4616. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef]
- Cai, J.; Wen, R.; Li, W.; Wang, X.; Tian, H.; Yi, S.; Zhang, L.; Li, X.; Jiang, C.; Li, H. Oil body bound oleosin-rhFGF9 fusion protein expressed in safflower (Carthamus tinctorius L.) stimulates hair growth and wound healing in mice. BMC Biotechnol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and-negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, H.; Ahmadi Ashtiani, H.; Aghaei, M.; Ehsani, A.; Barikbin, B. Combination of herbal extracts and platelet-rich plasma induced dermal papilla cell proliferation: Involvement of ERK and Akt pathways. J. Cosmet. Dermatol. 2013, 12, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Heinzelmann, T.; Schultz, K.D.; Furkert, J.; Fechner, K.; Czarnetzki, B.M. Hair growth induction by substance P. Lab. Investig. 1994, 71, 134–140. [Google Scholar] [PubMed]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng. Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Song, K.H.; Woo, J.K.; Park, M.H.; Rhee, M.H.; Choi, C.; Oh, S.H. Ginsenoside Rp1 from Panax ginseng Exhibits Anti-cancer Activity by Down-regulation of the IGF-1R/Akt Pathway in Breast Cancer Cells. Plant Foods Hum. Nutr. 2011, 66, 298–305. [Google Scholar] [CrossRef]
- Keum, D.I.; Pi, L.Q.; Hwang, S.T.; Lee, W.S. Protective effect of Korean Red Ginseng against chemotherapeutic drug-induced premature catagen development assessed with human hair follicle organ culture model. J. Ginseng. Res. 2016, 40, 169–175. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.N.; Hong, Y.D.; Park, B.C.; Na, Y. Panax ginseng extract antagonizes the effect of DKK-1-induced catagen-like changes of hair follicles. Int. J. Mol. Med. 2017, 40, 1194–1200. [Google Scholar] [CrossRef]
| NUTRACEUTICAL | PROPERTIES | BREAST CANCER | HAIR GROWTH | POTENTIAL ADJUVANT |
|---|---|---|---|---|
| Resveratrol | Naturally occurring polyphenolic stilbene found in, blueberries, raspberries, mulberries, grapes and red wine. Can signal via ERα and ERβ. An effective antioxidant with strong anti-inflammatory properties. | Inhibits estrogen-induced breast carcinogenesis via induction of NRF2-mediated protective pathways [79]. Induction of apoptosis in ER +ve MCF-7 cells is via inhibition of the ERα-dependent PI3K pathway [80]. Resveratrol analogues combined with tamoxifen have a synergistic effect on inhibiting proliferation of ER +ve and ER-ve breast cancer cells [81]. | Using the sensitive probe DCFH-DA, it was shown to significantly reduce oxygen peroxide-induced oxidative stress generated in hair follicles and hair matrix cells [82]. Furthermore, a clinical study of 79 women suffering from hair loss treated with a topical combination of pyridine-2, 4-dicarboxylic acid diethyl ester and resveratrol reported significantly increased hair density after 1.5 months [82]. | Anti-carcinogenic. Synergizes with tamoxifen. Anti-inflammatory. Antioxidant. Potential to improve hair density in women and reduce oxidative stress. |
| Tocotrienols | Hydrophobic phenolic antioxidants with structural features allow binding to ERα and ERβ. Found in plant seeds, e.g., rice bran, oil palm and annatto; belong to the vitamin E family [64]. | Exhibit high affinity for ERβ and promote nuclear translocation, modulating cell morphology, caspase-3 activation, DNA fragmentation and apoptosis [83,84]. Binding to ERβ induces apoptosis in breast cancer cells [84,85]. Can synergize with tamoxifen to inhibit proliferation [86] with a clinical trial suggesting breast cancer survival may be extended by combining tocotrienol with tamoxifen therapy [87]. Proliferation of ER-negative breast cancer cell line can also be inhibited by α-, γ- and δ-tocotrienols [86,88]. | Induce murine hair follicle development and stimulate anagen hair cycling by suppressing epidermal E-cadherin followed by a 4-fold induction of β-catenin and its nuclear translocation [89]. In an 8-month treatment of 38 patients with hair loss, increased hair counts in 34.5% [90]; thought to be due to inhibition of lipid peroxidation and reduction in oxidative stress in the hair follicle. | ERβ agonist. Anti-carcinogenic. Synergizes with tamoxifen. Antioxidant. Potential to improve hair cycling and reduce oxidative stress. |
| Saw palmetto (Serenoa repens) | American dwarf tree berries. Competitive, 5α reductase (1 and 2) inhibitor. Multiple sites of action—different pharmacodynamic profile to finasteride [66]. Increases 3α-hydroxysteroid-dehydrogenase activity, converting 5α-DHT to a weaker metabolite, androstanediol [66]. | Induces dose-dependent inhibition of ER + ve/−ve breast cancer cell line proliferation. Inhibition 2.5x greater (p < 0.01) in MCF-7 cells (ER +ve) than MDA MB231 cells (ER −ve) [91]. The anti-proliferative effect was triggered by the induction of apoptosis. Has anti-estrogenic activity in prostate tissue of men with BPH [92]. Furthermore, decreases 5α-DHT and estradiol plasma levels in men [93]. | Most studies have been conducted on men with androgenetic alopecia. Daily treatment with 200 mg in 26 men with androgenetic alopecia saw improvement in 60% compared to 11% with placebo [94]. A daily oral supplement of 320 mg compared to 1 mg finasteride in 100 men saw more growth on frontal and vertex scalp in 68% of the finasteride cohort, while 38% of the saw palmetto group had increased hair growth on the vertex [95]. A topical application saw increased terminal hair counts after 12 and 24 weeks [96]. | 5α-reductase inhibitor. Anti-carcinogenic. Potential to improve terminal hair counts, particularly on vertex. |
| Maca (Lepidium meyenii) | Plant roots (Andes) contain high levels of flavonolignans and glucosinolates. Protective against inflammation, increases antioxidants and has hormonal balancing properties. Supports the antioxidant system by activating SOD and GSH [97]. | Has anti-proliferative activity against cancer, including breast cancer [63,64]. In rats with BPH), oral supplementation of a butanol fraction of red maca reduces prostate size by restoring ERβ expression without changing ARs and ERα expression [98]. In postmenopausal women, after 2 months, Maca stimulated estradiol and suppressed cortisol [99]. It modulates estrogen levels by targeting only ERβ and/or re-establishing hormonal homeostasis through the hypothalamus–pituitary–ovarian axis [99]. | IL-6 has been implicated in forms of hair loss such as AA [100]. Blood analysis of individuals consuming Maca has shown a reduced level of IL-6 [101], suggesting potential application for treatment of AA. Maca has the ability to support the antioxidant defense system by activating SOD and GSH [97], which are powerful antioxidants that lower levels of oxidative stress in forms of hair loss such as AGA [102]. | Anti-carcinogenic. Anti-inflammatory. Antioxidant. Potential to reduce oxidative stress in the hair follicle and improve hair growth. |
| Curcumin | Curcumin is the main metabolite in turmeric (Curcuma longa) root, with anti-inflammatory and antioxidant properties [72]. It down-regulates inducible nitric oxide and cyclooxygenase-2; inhibits nuclear factor-kB signaling, decreasing pro-inflammatory cytokines, e.g., TNF-α and IL-1, which are implicated in hair loss [103]. It is recognized as a strong anti-cancer agent, including breast cancer, in traditional medicine [104]. | Inhibits proliferation of T-47D breast cancer cells in a dose-dependent manner by downregulating ERα [105,106]. It has antioxidant and anti-inflammatory action and can induce cell cycle arrest in breast cancer cells [107]. Studies on other estrogen-sensitive cancer cell lines, have demonstrated a capacity to counteract the proliferative effect of estradiol [108] as well as the synthesis of estradiol [109]. | Encapsulating curcumin in liposomes enhances penetration in porcine hair follicles by 70% [110]. Similarly, a cyclodextrin complex enhances curcumin follicle penetration [111]. In vitro it blocked expression of genes that inhibit hair growth, e.g., PAK1 and TGF-β1 [112,113]. Topical application (5%) or in combination with 5% minoxidil was assessed in 87 men with androgenetic alopecia for 6 months, demonstrating that while curcumin alone did not stimulate hair growth, a combination with minoxidil showed a significant improvement, suggesting they may act in a synergistic manner [114]. | Anti-carcinogenic. Anti-inflammatory. Antioxidant. Synergizes with minoxidil with the potential to improve hair growth. |
| Ashwagandha (Withania somnifera) | Derived from the root and leaf of the plant, which is particularly rich in withanolides, which are the main active ingredients and have anti-inflammatory and adaptogenic properties [74]. Ashwagandha and its actives have been proposed as anti-cancer agents that are able to modulate apoptotic, proliferative and metastatic markers in cancer [115,116]. | In breast cancer, it demonstrates chemo-preventive activity in female rats following administration of the mammary carcinogen methylnitrosourea by significantly reducing the rate of cell proliferation in mammary tumors [117]. In MCF-7 cells, withaferin, the main active ingredient, suppressed ER-α protein by 90%, while expression of ER-β protein increased by 20–30% [75]. Withaferin-mediated down-regulation of ER-α protein expression correlated with a decrease in its nuclear level, suppression of its mRNA level, and inhibition of estrogen-dependent activation of ERE2e1b-luciferase reporter gene [75]. In another study, it was shown that ashwagandha inhibited proliferation and induced apoptosis in the MCF-7 breast cancer cell line by down-regulating ERα protein levels via proteasome-dependent ERα degradation [118]. Furthermore, withaferin alters the mitochondria dynamic resulting in apoptosis in breast cancer cells [119] and impairs cancer autophagy resulting in proteotoxicity and death [120]. Since withaferin has shown activity in triple negative breast cancer cells MDA-MB-231, by blocking their invasiveness and associated markers, the active has been suggested for further (pre)clinical development to defeat aggressive metastatic breast cancer [121]. | Ashwagandha extract has anti-stress and anti-anxiety properties [122]. Being a powerful adaptogen, the extract has the ability to keep cortisol levels at a healthy homeostatic level while improving one’s resistance to stress [123,124]. Chronic stress activates the HPA axis by increasing cortisol levels, which, in turn, inhibits the HPT axis and reduces serum T3 and T4 levels [125]. Endocrine disorders, such as hypothyroidism, can cause hair loss [126]. Treatment with ashwagandha lowers serum cortisol levels by downregulation of the HPA axis, which, in turn, upregulates the HPT axis to normalize the thyroid hormone level [127]. Thyroid hormones, such as T3, are integral to hair growth and wellness [128]. High-concentration, full-spectrum ashwagandha root extract can improve resistance towards stress and anxiety [129]. Anxiety can contribute to hair loss as evidenced by a study finding a high prevalence of anxiety symptoms in patients with alopecia areata [130]. Stress has been associated with hair loss, such as in the case of telogen effluvium, characterized by a non-scarring, non-inflammatory alopecia of relatively sudden onset caused by physiologic or emotional stress [131]. Use of ashwagandha would then reduce the impact of stress on hair loss. | Anti-inflammatory. Anti-carcinogenic. Suppresses ERα. Increases ERβ. Reduces cortisol. Normalizes thyroid hormone levels. No direct hair growth studies, but effect on circulating cortisol and T3 will impact hair growth. |
| Horsetail (Equisetum arvense) | Used in traditional medicine and has strong anti-inflammatory and antioxidant properties [73]. Down-regulates TNF-α and upregulates anti-inflammatory IL-10 in rheumatoid arthritis patients [132]. Suppresses free radicals and inflammatory mediators in IFN- γ and LPS-stimulated murine macrophages [133]. | Extracts demonstrate antioxidative effects in two lipid peroxidation systems and anti-proliferative activity in human tumor cell lines [134]. | Extracts inhibit 5α-reductase and decrease IL-6 secretion in LPS-stimulated macrophages and are not toxic against human follicle dermal papilla cells [135]. | Anticarcinogenic. Anti-inflammatory. Antioxidant. 5α-reductase inhibitor. |
| Astaxanthin | A carotenoid produced by Haematococcus pluvialis (fresh-water algae). This blood-red pigment accumulates when algae are subjected to stress [67]. A powerful antioxidant, superior to other carotenoids. It preserves membrane integrity by insertion in the bilayer, protecting the redox state and mitochondrial function. It decreases ROS and increases production of antioxidants, e.g., catalase, GSH and SOD [68,69]. | In ER +ve MCF-7 cells, it causes a significant accumulation of cells in the G2/M phase [136]. It significantly reduces proliferation and migration of MCF-7 cells [137] and, after 24 hours, leads to a significant decrease in viability [138]. It also significantly reduces T47D viability in a dose-dependent manner [139]. Its ability to reduce proliferation and viability and to increase apoptosis of cancer cells is associated with an increased expression of PPARγ [140]. Another mechanism by which it inhibits breast cancer cells by reducing proliferation is via inactivation of the PI3K/AKT pathway [140]. | Dysfunction of mitochondrial respiration delays hair regeneration [141]. Astaxanthin can protect mitochondria from oxidative damage by helping the scavenging of ROS from follicle cells [68]. | Powerful antioxidant. Anti-carcinogenic. Has potential to improve hair growth by protecting from oxidative stress. |
| Kelp (brown seaweed) | Brown seaweeds rich in polysaccharides (e.g., alginic acid and fucoidan), vitamin B12, iron, iodine, phlorotannins and fucoxanthin, with biological properties [142]. In rats, it has a dual action as an antagonistic of ERα and as an aromatase inhibitor, suggesting a protective role in the initiation and progression of estrogen-dependent cancers [65]. | Treatment of MCF-7 and MDA-MB-231 breast cancer cells with Sargassum muticum methanol extract significantly inhibits proliferation and angiogenesis and increases apoptosis in a time- and dose-dependent manner [65,143]. Polysaccharides from Sargassum wightii induce apoptosis of MCF7 and MDA-MB-231 cells by increasing ROS, mitochondrial membrane cleavage and nuclei damage [144]. In animal models of breast cancer, fucoidan, one of the main polysaccharides in seaweed, stimulated an immune response [145]. As an adjuvant it increased sensitization to radiation and chemotherapy in breast cancer cells and animal models [146,147,148]. The co-administration of fucoidan on pharmacokinetics of letrozole and tamoxifen in patients with breast cancer showed it was well tolerated and did not alter plasma concentration of the drugs [149]. | An extract from brown seaweed Undariopsis peterseniana, rich in fucoxanthinone, promoted hair growth in rat hair follicles ex vivo and stimulated rat dermal papilla cell proliferation by activating the Wnt/β-Catenin pathway [150]. The brown alga Ecklonia cava, rich in phlorotannins, stimulated human follicle dermal papilla cell proliferation and hair fiber elongation in human hair follicles ex vivo and hair growth in mice [151,152]. Fucoidan, a prominent polysaccharide in brown seaweed, stimulated production of HGF, which stimulates the hair cycle [153,154]. A patent on fucoidan as a hair-restoring agent was filed in 2000 (EP1234568B1). A double-blinded, placebo-controlled clinical trial showed seaweed extracts in supplements helped to prevent patterned hair loss and promoted scalp health in men and women [155]. A study on mice showed a mixture of seaweed extracts was as effective for hair growth promotion as minoxodil [156]. | Anti-carcinogenic. Does not interfere with plasma levels of tamoxifen or aromatase inhibitors. Contains supplements important for hair growth, e.g., B12 and iron [77]. Direct effects on dermal papilla cells and hair growth. |
| Malus pumila Miller cv. Annurca (apple fruits) | Polyphenols with a high content of oligomeric procyanidins, specifically procyanidin B2. | Apple polyphenolic compounds had a significant antiproliferative action on MCF-7 cells. An amount of 500 μM of Annurca flesh polyphenols extract (AFPE) induced a cell cycle arrest at G2/M. AFPE was also capable of inducing morphological changes as evidenced by nuclear condensation [157]. | Significantly stimulates the synthesis of cytokeratins in the human keratinocyte HaCaT cell line [78] and primary cultures of human hair keratinocytes [158] in vitro and protects cultured human dermal papilla from oxidative stress [158]. Oral supplementation (800 mg) significantly increased hair growth, density and keratin content in a cohort of 250 patients (116 men and 134 women; 30–83 years of age) randomly divided into two subgroups (each one of 125 subjects, 58 men and 67 women) after 2 months [78]. | Strong antioxidant. Anti-carcinogenic. Stimulates keratin synthesis. Stimulates hair growth and density. |
| Carthamus Tinctorius L. (Safflower) | Active constituents include flavonoids, phenylethanoid glycosides, coumarins, fatty acids and steroids. Its oil has high nutritional value, consisting of 70% polyunsaturated fatty acid (i.e., linoleic acid) and 10% monounsaturated oleic acid. | Significantly increases apoptotic rate of the MCF-7 cells in a dose-dependent manner by down-regulating expression of Bcl-2 and upregulating Bcl-2-associated X protein in a time-dependent manner. Additionally, it significantly reduced expression of MMP-9 increased expression of TIMP-1 [159]. | Suppresses the expression of TGF-β1 and significantly increases length of hair follicles in culture by stimulating the expression of VEGF and KGF [160]. Promotes hair growth, at least in part, by upregulating expression of β-catenin [161]. | Antioxidant. Anti-inflammatory. Anti-carcinogenic. Stimulates hair growth. |
| Capsicum annuum | Chile pepper with high phenolic content and antioxidant activity. | Capsaicin is potent inhibiter of ER +ve (MCF-7, T47D, BT-474) and ER-ve (SKBR-3, MDA-MB231) breast cancer cell lines, associated with G0/G1 cell-cycle arrest, increased levels of apoptosis and reduced protein expression of human epidermal growth factor receptor (EGFR), HER2, activated extracellular-regulated kinase (ERK) and cyclin D1. Further blocked breast cancer cell migration in vitro and decreased tumors by 50%, growing orthotopically in immunodeficient mice [162]. | Significantly increases IGF-I production in har follicles, promoting hair growth [163]. Capsaicin as an isolated active, when injected intradermally into the back skin of C57BL/6 mice with all follicles in the telogen phase of hair cycle, induced significant hair growth (anagen), which was associated with substantial mast cell degranulation [164]. | |
| Panax ginseng | The three key ingredients are saponins (ginsenoside), polysaccharides and phenolic compounds. Ginsenosides are categorized into two groups based on their chemical structure, i.e., oleanane type (five-ring structure) and dammarane type (four-ring structure) [165]. | Anticancer properties include induction of apoptosis, blocking angiogenesis, and inhibiting proliferation in cancer cell lines including MCF-7. [166]. Inhibits breast cancer cell proliferation and both anchorage-dependent and -independent breast cancer cell colony formation. In addition, it decreased the stability of the IGF-1R protein in breast cancer cells. suggesting that IGF-1R is an important target for treatment and prevention of breast cancer [167]. | In clinical studies, red ginseng combined with topical minoxidil increases its effectiveness at promoting hair growth in human clinical studies. Moreover, it promotes the proliferation of human dermal follicle papilla cells and keratinocytes and enhances hair anagen in the mouse [165]. In chemotherapy-induced alopecia, it can protect against premature catagen [168], and in vitro, it has been shown to stimulate proliferation and inhibit apoptosis in hair follicle outer root sheath keratinocytes [169]. | Anti-carcinogenic. Antioxidant. Anti-inflammatory. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Acqua, G.; Richards, A.; Thornton, M.J. The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy. Nutrients 2020, 12, 3537. https://doi.org/10.3390/nu12113537
Dell’Acqua G, Richards A, Thornton MJ. The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy. Nutrients. 2020; 12(11):3537. https://doi.org/10.3390/nu12113537
Chicago/Turabian StyleDell’Acqua, Giorgio, Aleksander Richards, and M. Julie Thornton. 2020. "The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy" Nutrients 12, no. 11: 3537. https://doi.org/10.3390/nu12113537
APA StyleDell’Acqua, G., Richards, A., & Thornton, M. J. (2020). The Potential Role of Nutraceuticals as an Adjuvant in Breast Cancer Patients to Prevent Hair Loss Induced by Endocrine Therapy. Nutrients, 12(11), 3537. https://doi.org/10.3390/nu12113537





