Dietary Chloride Deficiency Syndrome: Pathophysiology, History, and Systematic Literature Review
Abstract
1. Introduction
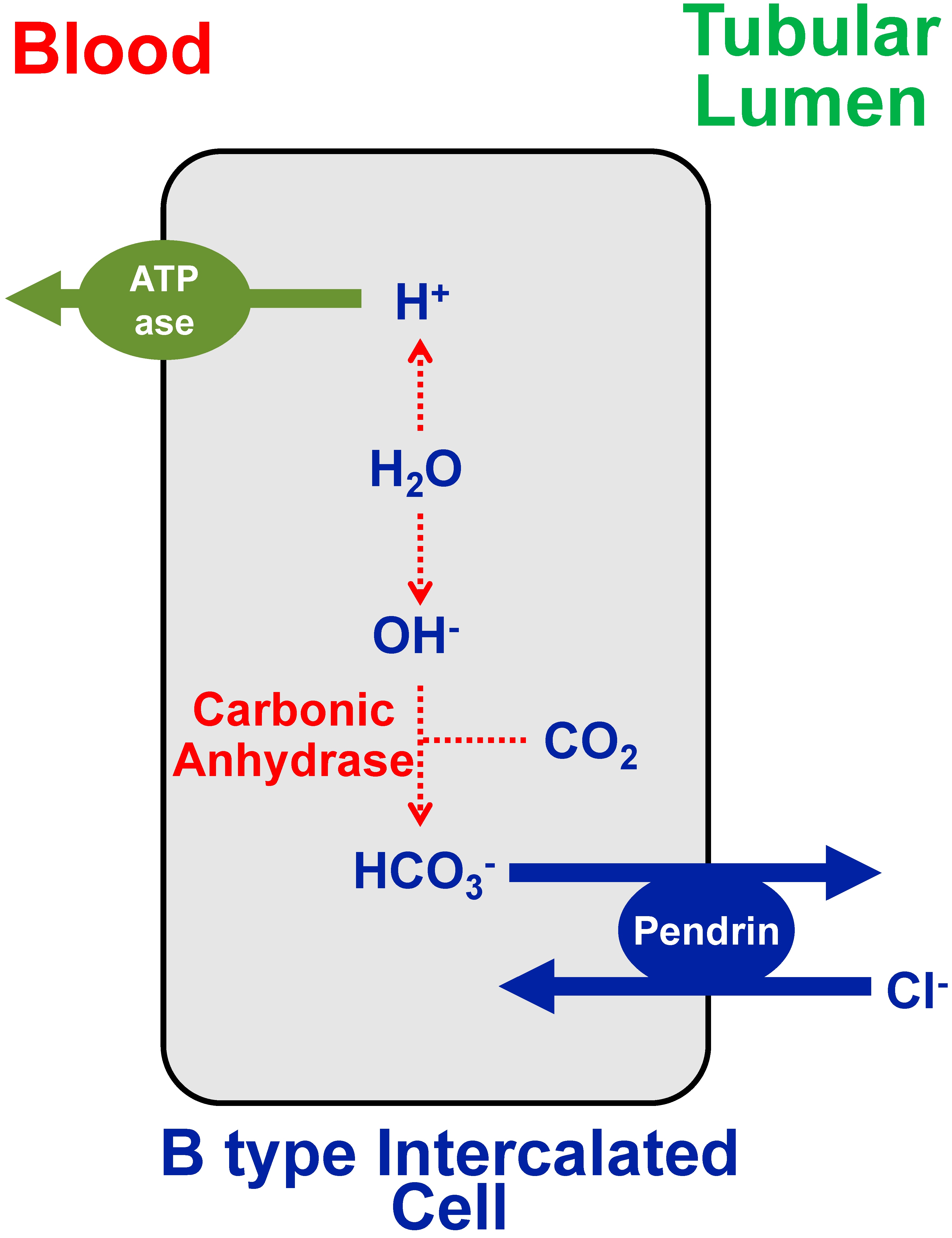
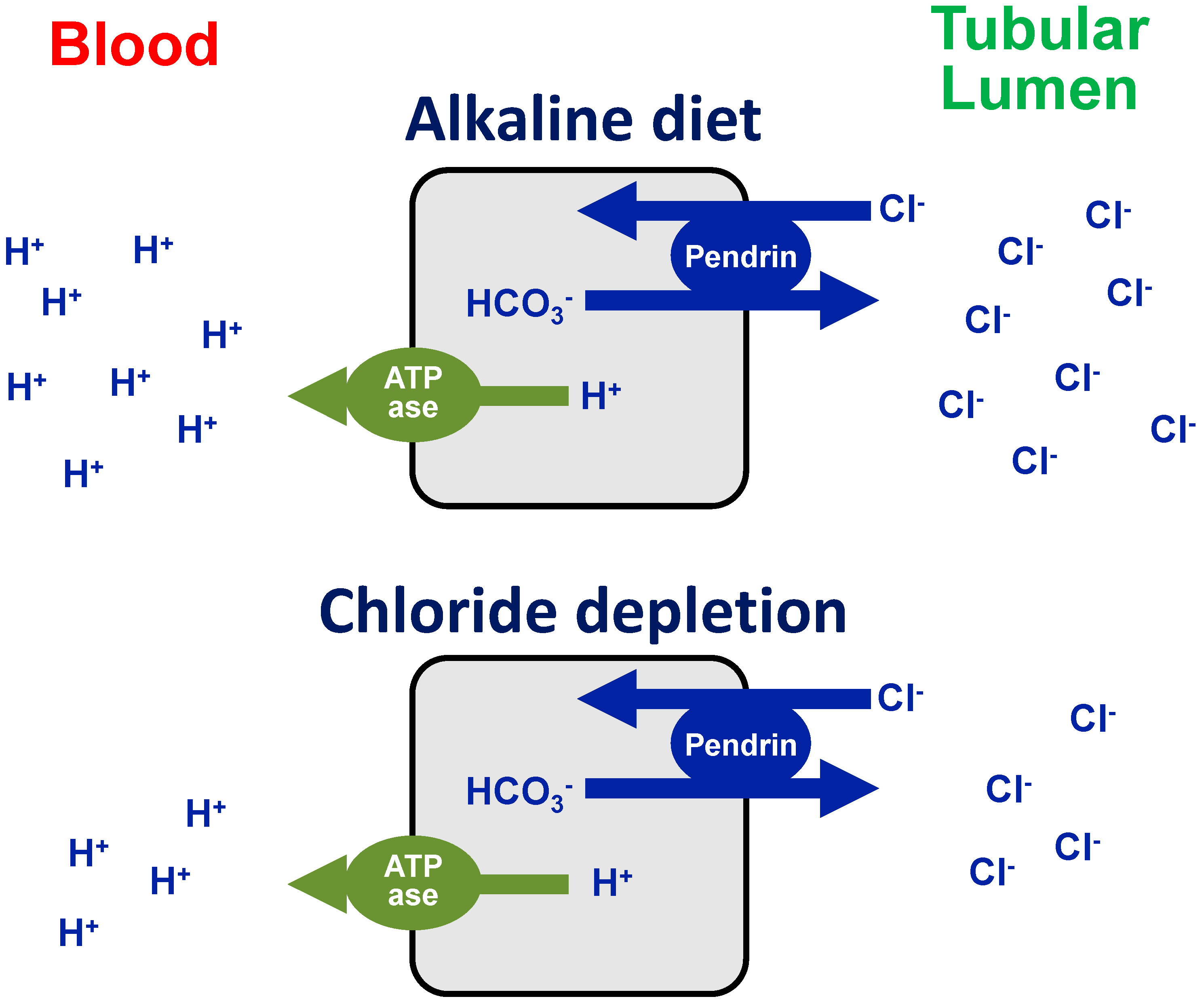
2. Chloride Deficiency Metabolic Alkalosis
3. Epidemic and Sporadic Dietary Chloride Depletion Alkalosis: A Systematic Review
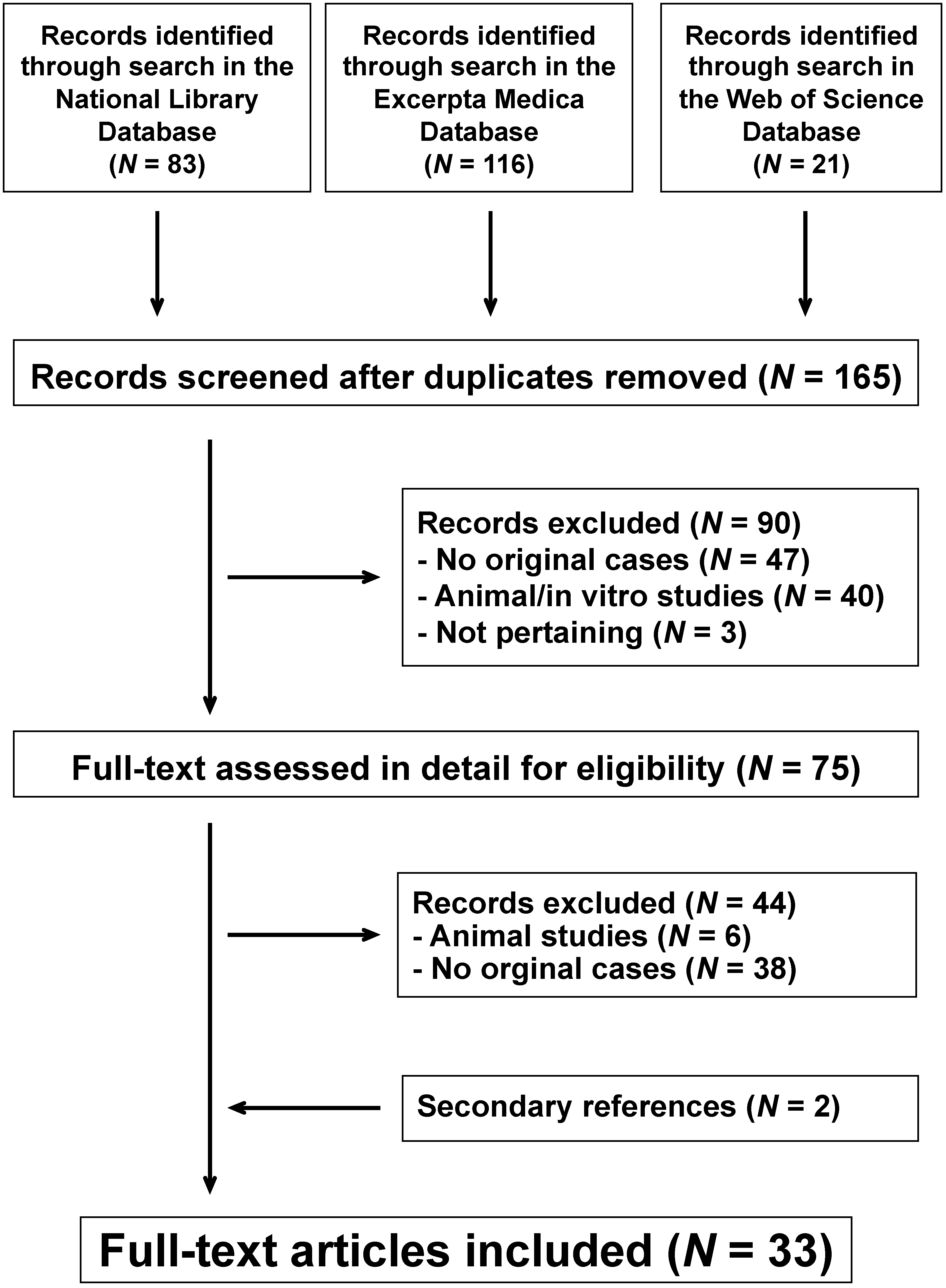
3.1. Literature Search Strategy
3.2. Data Extraction
3.3. Search Results
3.4. Epidemic Dietary Chloride Depletion Alkalosis in Infants Fed a Low-Chloride Formula Milk
3.4.1. Historical Background
3.4.2. Outbreaks in the United States of America (1979) and Spain (1981)
3.5. Dietary Chloride Depletion Alkalosis after the Initial (1979–1981) Outbreaks
4. Possible Long-Term Sequelae after Exposure to a Low-Chloride Formula Milk
4.1. Cognitive Deficits
4.2. Increased Liking for Salty Foods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy, S., 3rd. The chloride depletion syndrome. Adv. Pediatr. 1984, 31, 235–257. [Google Scholar] [PubMed]
- Soifer, J.T.; Kim, H.T. Approach to Metabolic Alkalosis. Emerg. Med. Clin. N. Am. 2014, 32, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Mersin, S.S.; Ramelli, G.P.; Laux-End, R.; Bianchetti, M.G. Urinary chloride excretion distinguishes between renal and extrarenal metabolic alkalosis. Eur. J. Nucl. Med. Mol. Imaging 1995, 154, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Luke, R.G.; Galla, J.H. It Is Chloride Depletion Alkalosis, Not Contraction Alkalosis. J. Am. Soc. Nephrol. 2012, 23, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Al-Bataineh, M.M.; Pastor-Soler, N.M. Collecting Duct Intercalated Cell Function and Regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Infant metabolic alkalosis and soy-based formula—United States. 1979. MMWR. Morb. Mortal. Wkly. Rep. 1996, 45, 985–988. [Google Scholar]
- Roy, S., 3rd; Arant, B.S., Jr. Alkalosis from chloride-deficient Neo-Mull-Soy. N. Engl. J. Med. 1979, 301, 615. [Google Scholar]
- Roy, S., 3rd; Arant, B.S., Jr. Hypokalemic metabolic alkalosis in normotensive infants with elevated plasma renin activity and hyperaldosteronism: Role of dietary chloride deficiency. Pediatrics 1981, 67, 423–429. [Google Scholar]
- Greenberg, F.; Cordero, J.; Erickson, J.; Roy, S. Withdrawal of two soy-based infant formulæ. Lancet 1979, 314, 462. [Google Scholar] [CrossRef]
- Garin, E.H.; Geary, D.; Richard, G.A. Soybean formula (Neo-Mull-Soy) metabolic alkalosis in infancy. J. Pediatr. 1979, 95, 985–987. [Google Scholar] [CrossRef]
- Grossman, H.; Duggan, E.; McCamman, S.; Welchert, E.; Hellerstein, S. The dietary chloride deficiency syndrome. Pediatrics 1980, 66, 366–374. [Google Scholar]
- Kallen, R.J.; Aronson, D.J. Metabolic alkalosis in identical twins receiving a low-chloride formula (pseudo-Bartter’ssyndrome). Can. Med. Assoc. J. 1980, 123, 527–530. [Google Scholar] [PubMed]
- Linshaw, M.A.; Harrison, H.L.; Gruskin, A.B.; Prebis, J.; Harris, J.; Stein, R.; Jayaram, M.; Preston, D.; DiLiberti, J.; Baluarte, H.J.; et al. Hypochloremic alkalosis in infants associated with soy protein formula. J. Pediatr. 1980, 96, 635–640. [Google Scholar] [CrossRef]
- Reznik, V.M.; Griswold, W.R.; Mendoza, S.A.; McNeal, R.M. Neo-Mull-Soy metabolic alkalosis: A model of Bartter’s syndrome? Pediatrics 1980, 66, 784–786. [Google Scholar] [PubMed]
- Wolfsdorf, J.I. Failure to thrive and metabolic alkalosis. Adverse effects of a chloride-deficient formula in two infants. JAMA 1980, 243, 1068–1070. [Google Scholar] [CrossRef]
- Arnold, W.C.; Warren, R.H. Clinical incidence and causes of metabolic alkalosis in children. J. Ark. Med. Soc. 1983, 80, 186–188. [Google Scholar]
- Roy, S., 3rd. Perspectives on adverse effects of milks and infant formulas used in infant feeding. J. Am. Diet. Assoc. 1983, 82, 373–377. [Google Scholar]
- Rodriguez-Soriano, J.; Vallo, A.; Castillo, G.; Oliveros, R.; Cea, J.M.; Balzategui, M.J. Biochemical features of dietary chloride deficiency syndrome: A comparative study of 30 cases. J. Pediatr. 1983, 103, 209–214. [Google Scholar] [CrossRef]
- Asnes, R.S.; Wisotsky, D.H.; Migel, P.F.; Seigle, R.L.; Levy, J. The dietary chloride deficiency syndrome occurring in a breast-fed infant. J. Pediatr. 1982, 100, 923–924. [Google Scholar] [CrossRef]
- Hill, I.D.; Bowie, M.D. Chloride deficiency syndrome due to chloride-deficient breast milk. Arch. Dis. Child. 1983, 58, 224–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wack, R.P.; Roscelli, J.D. Chloride deficiency syndrome in older exclusively breast-fed infants. Arch. Pediatr. Adolesc. Med. 1994, 148, 438–441. [Google Scholar] [CrossRef]
- De Vizia, B.; Mansi, A.; Giangregorio, A.; Troncone, R. Metabolic alkalosis due to the use of an oligoantigenic diet in infancy. Acta Paediatr. 1995, 84, 103–105. [Google Scholar] [CrossRef]
- D’Eufemia, P.; Lucarelli, S.; Giardini, O.; Cardi, E. Metabolic alkalosis. Acta Paediatr. 1996, 85, 1518. [Google Scholar] [CrossRef]
- Mantadakis, E.; Bitsori, M.; Fitrolaki, D.; Michaeloudi, E.; Briassoulis, G.; Spanaki, A.-M. Life-threatening hypokalemia in an infant (Case Presentation). Acta Paediatr. 2009, 98, 1234–1235. [Google Scholar] [CrossRef]
- Medina, O.M.; González, J.L.; Nieto, V.G.; Ramírez, S.R.; Pérez, C.M. Alcalosis metabólica de origen dietético en un lactante. An. Pediatría 2009, 70, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Osuagwu, M.N.; Gordon, M.; Akobeng, A.K. Severe hypokalemia due to an inappropriate formula milk. Clin. Pediatr. 2010, 51, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Fourreau, D.; Peretti, N.; Hengy, B.; Gillet, Y.; Courtil-Teyssedre, S.; Hess, L.; Loras-Duclaux, I.; Caron, N.; Didier, C.; Cour-Andlauer, F.; et al. Complications carentielles suite à l’utilisation de « laits » végétaux, chez des nourrissons de deux mois et demi à 14 mois (quatre cas). Presse Médicale 2013, 42, e37–e43. [Google Scholar] [CrossRef]
- Park, E.-S.; Seo, J.-H.; Lim, J.-Y.; Park, C.-H.; Woo, H.-O.; Youn, H.-S. Hypoelectrolytemia due to inadequate diet. Pediatr. Nephrol. 2005, 21, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, J.; Aramaki, S.; Yokochi, K. Dietary chloride deficiency due to new liquid nutritional products. Pediatr. Int. 2009, 51, 197–200. [Google Scholar] [CrossRef]
- Kamel, K.S.; Ethier, J.; Levin, A.; Halperin, M.L. Hypokalemia in the “beautiful people”. Am. J. Med. 1990, 88, 534–536. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Bovée, D.M.; Geerse, D.A.; Visser, W.J. Diet-exercise-induced hypokalemic metabolic alkalosis. Am. J. Med. 2020. [Google Scholar] [CrossRef]
- Hellerstein, S.; Duggan, E.; Grossman, H.M.; McCamman, S.; Sharma, P.; Welchert, E. Metabolic alkalosis and Neo-Mull-Soy. J. Pediatr. 1979, 95, 1083–1084. [Google Scholar] [CrossRef]
- Chutorian, A.M.; LaScala, C.P.; Ores, C.N.; Nass, R. Cerebral dysfunction following infantile dietary chloride deficiency. Pediatr. Neurol. 1985, 1, 335–341. [Google Scholar] [CrossRef]
- Willoughby, A.; A Moss, H.; Hubbard, V.S.; Bercu, B.B.; I Graubard, B.; Vietze, P.M.; Chang, C.C.; Berendes, H.W. Developmental outcome in children exposed to chloride-deficient formula. Pediatrics 1987, 79, 851–857. [Google Scholar]
- Willoughby, A.; I Graubard, B.; Hocker, A.; Storr, C.; Vietze, P.; Thackaberry, J.M.; A Gerry, M.; McCarthy, M.; Gist, N.F.; Magenheim, M. Population-based study of the developmental outcome of children exposed to chloride-deficient infant formula. Pediatrics 1990, 85, 485–490. [Google Scholar] [PubMed]
- Malloy, M.H.; Graubard, B.; Moss, H.; McCarthy, M.; Gwyn, S.; Vietze, P.; Willoughby, A.; Rhoads, G.G.; Berendes, H. Hypochloremic metabolic alkalosis from ingestion of a chloride-deficient infant formula: Outcome 9 and 10 years later. Pediatrics 1991, 87, 811–822. [Google Scholar]
- Malloy, M.H.; Willoughby, A.; Graubard, B.; Lynch, J.; McCarthy, M.; Moss, H.; Vietze, P.; Rhoads, G.; Berendes, H. Exposure to a chloride-deficient formula during infancy: Outcome at ages 9 and 10 years. Pediatrics 1990, 86, 601–610. [Google Scholar]
- Kaleita, T.A.; Kinsbourne, M.; Menkes, J.H. A neurobehavioral syndrome after failure to thrive on chloride-deficient formula. Dev. Med. Child Neurol. 2008, 33, 626–635. [Google Scholar] [CrossRef]
- Stein, L.J.; Cowart, B.J.; Epstein, A.N.; Pilot, L.J.; Laskin, C.R.; Beauchamp, G.K. Increased liking for salty foods in adolescents exposed during infancy to a chloride-deficient feeding formula. Appetite 1996, 27, 65–77. [Google Scholar] [CrossRef]
- Lavagno, C.; Camozzi, P.; Renzi, S.; Lava, S.A.G.; Simonetti, G.D.; Bianchetti, M.G.; Milani, G.P. Breastfeeding-associated hypernatremia: A systematic review of the literature. J. Hum. Lact. 2016, 32, 67–74. [Google Scholar] [CrossRef]
- Berend, K.; Van Hulsteijn, L.H.; Gans, R.O.B. Chloride: The queen of electrolytes? Eur. J. Intern. Med. 2012, 23, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Deschênes, G. Sodium—Not harmful? Pediatr. Nephrol. 2020, 35, 1771–1776. [Google Scholar] [CrossRef]
| Conditions presenting with normal blood pressure | |
| - Chloride deficiency | |
| Renal chloride losses | Thiazide or loop diuretics* |
| Congenital or acquired chloride (and sodium) losing tubular disorders (e.g.: Bartter or Gitelman syndromes) | |
| Excessive sweating | Physical labor |
| Hot and humid conditions | |
| High sweat salt concentration (e.g.: cystic fibrosis) | |
| Gastrointestinal losses | Vomiting*, nasogastric suction |
| Congenital chloride diarrhea, Zollinger-Ellison syndrome, villous adenoma, high-volume ileostomy losses | |
| Transient neonatal chloride deficiency secondary maternal chloride deficiency | Maternal eating disorder |
| Mother taking thiazide or loop diuretics | |
| Mother affected by a chloride (and sodium) losing disorder | |
| Poor dietary chloride intake | |
| - Alkali intake (e.g.: alkaline diet, baking soda) or administration, milk alkali syndrome, severe potassium depletion | |
| Conditions presenting with arterial hypertension | |
| Liddle syndrome, apparent mineralocorticoid excess syndrome | |
| Primary hyperaldosteronism | |
| Excess licorice ingestion | |
| Finding | Approximate Prevalence (%) | Soundness ◦ |
|---|---|---|
| Blood pressure normal (or low normal) | 100 | high |
| Blood parameters | ||
| Metabolic alkalosis (HCO3− > 26 mmol/L) | 100 | high |
| Hypokalemia (<3.5 mmol/L) | 90 | high |
| Hypochloremia (<95 mmol/L) | 80 | high |
| Hyponatremia (<135 mmol/L) | 70 | high |
| Creatinine, urea, uric acid slightly increased | 50 | moderate |
| Renin and aldosterone increased | 100 | high |
| Urinary parameters | ||
| Low urinary chloride excretion | 100 | high |
| Microhematuria | 50 | high |
| Potential for nephrocalcinosis △ | 50 | moderate |
| Absent juxtaglomerular hyperplasia | 100 | poor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorelli, G.C.; Bianchetti, M.G.; Jermini, L.M.M.; Agostoni, C.; Milani, G.P.; Simonetti, G.D.; Lava, S.A.G. Dietary Chloride Deficiency Syndrome: Pathophysiology, History, and Systematic Literature Review. Nutrients 2020, 12, 3436. https://doi.org/10.3390/nu12113436
Signorelli GC, Bianchetti MG, Jermini LMM, Agostoni C, Milani GP, Simonetti GD, Lava SAG. Dietary Chloride Deficiency Syndrome: Pathophysiology, History, and Systematic Literature Review. Nutrients. 2020; 12(11):3436. https://doi.org/10.3390/nu12113436
Chicago/Turabian StyleSignorelli, Giulia C., Mario G. Bianchetti, Luca M. M. Jermini, Carlo Agostoni, Gregorio P. Milani, Giacomo D. Simonetti, and Sebastiano A. G. Lava. 2020. "Dietary Chloride Deficiency Syndrome: Pathophysiology, History, and Systematic Literature Review" Nutrients 12, no. 11: 3436. https://doi.org/10.3390/nu12113436
APA StyleSignorelli, G. C., Bianchetti, M. G., Jermini, L. M. M., Agostoni, C., Milani, G. P., Simonetti, G. D., & Lava, S. A. G. (2020). Dietary Chloride Deficiency Syndrome: Pathophysiology, History, and Systematic Literature Review. Nutrients, 12(11), 3436. https://doi.org/10.3390/nu12113436








