Citrate Supplementation Restores the Impaired Mineralisation Resulting from the Acidic Microenvironment: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Culture Media and Working Solutions of Citrate-Based Compounds
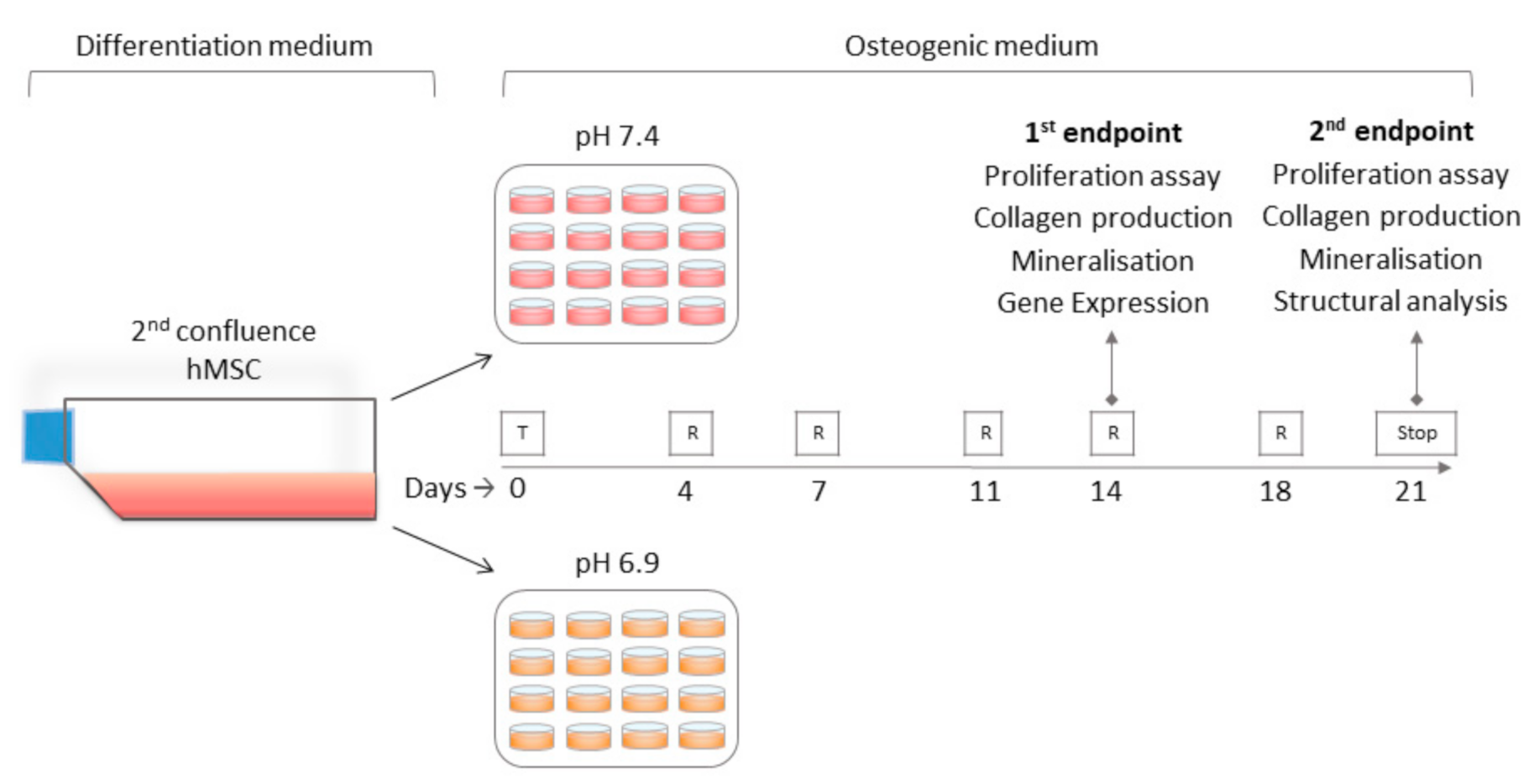
2.2. hMSC Culture and Experimental Plan
2.3. Alamar Blue Assay
2.4. Quantification of Citrate Release
2.5. Collagen Production
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Mineralisation Assay
2.8. Transmission Electron Microscopy (TEM) Analysis
2.9. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.10. Calculations and Statistical Analysis
3. Results
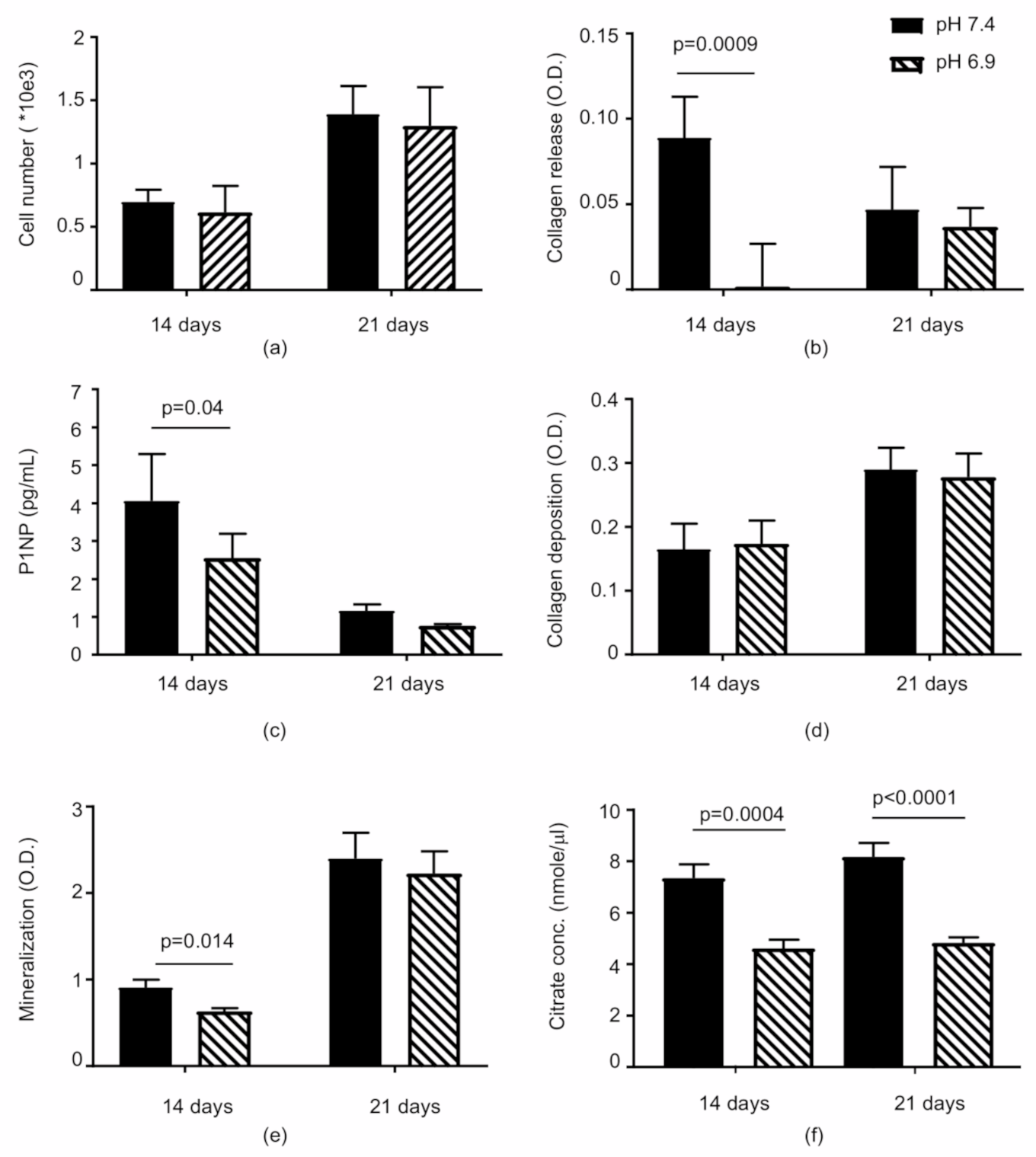
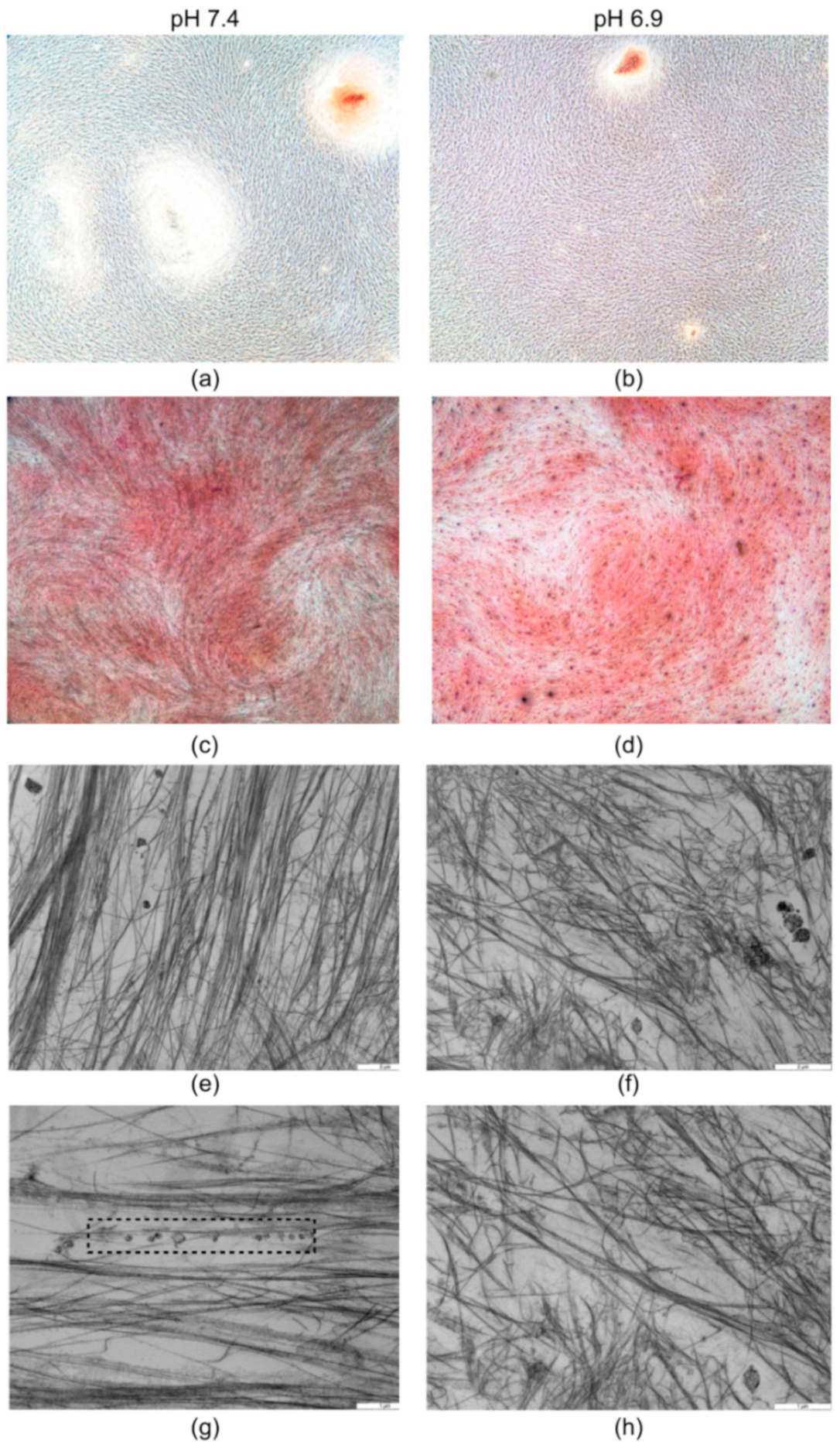
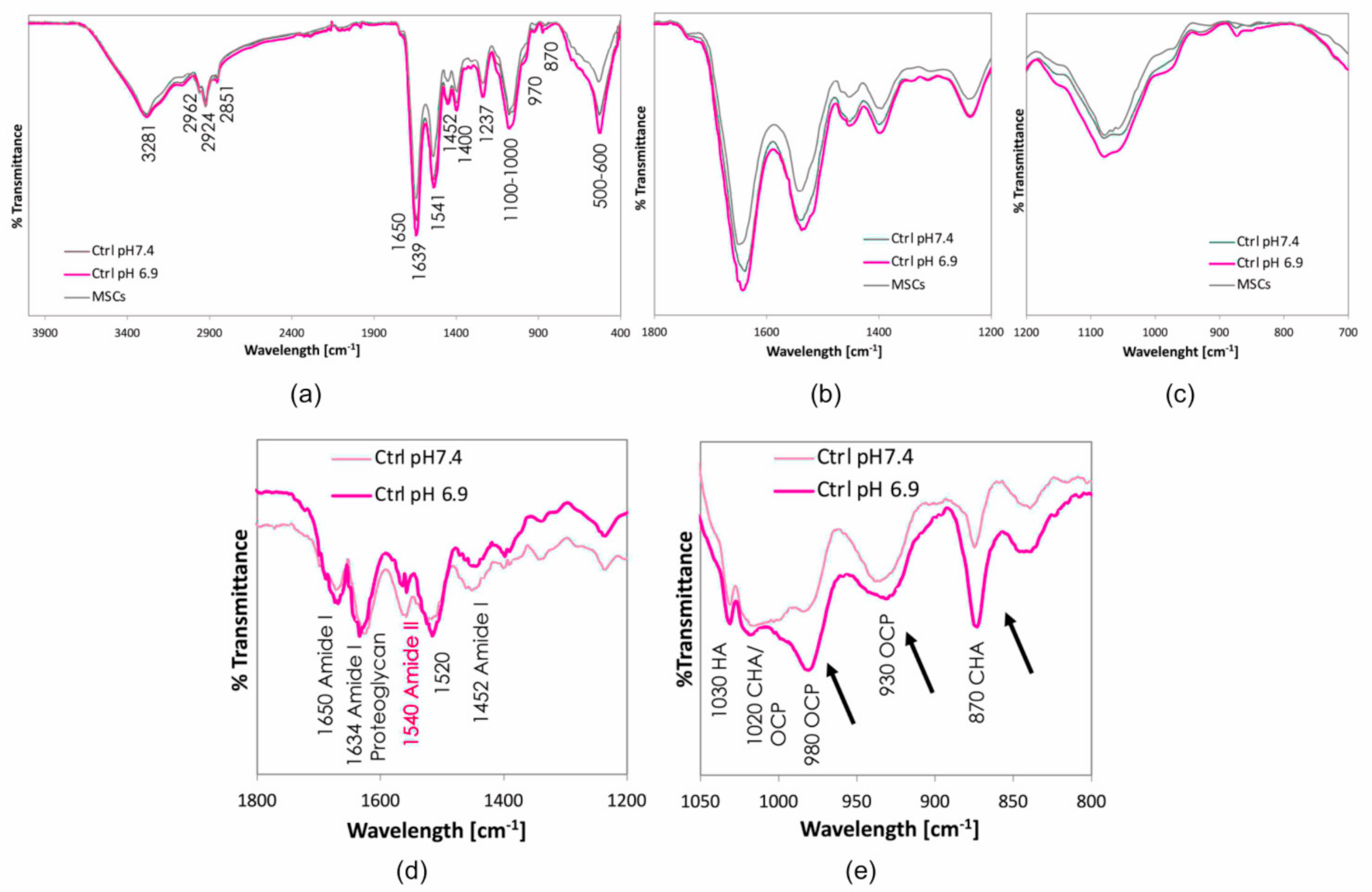
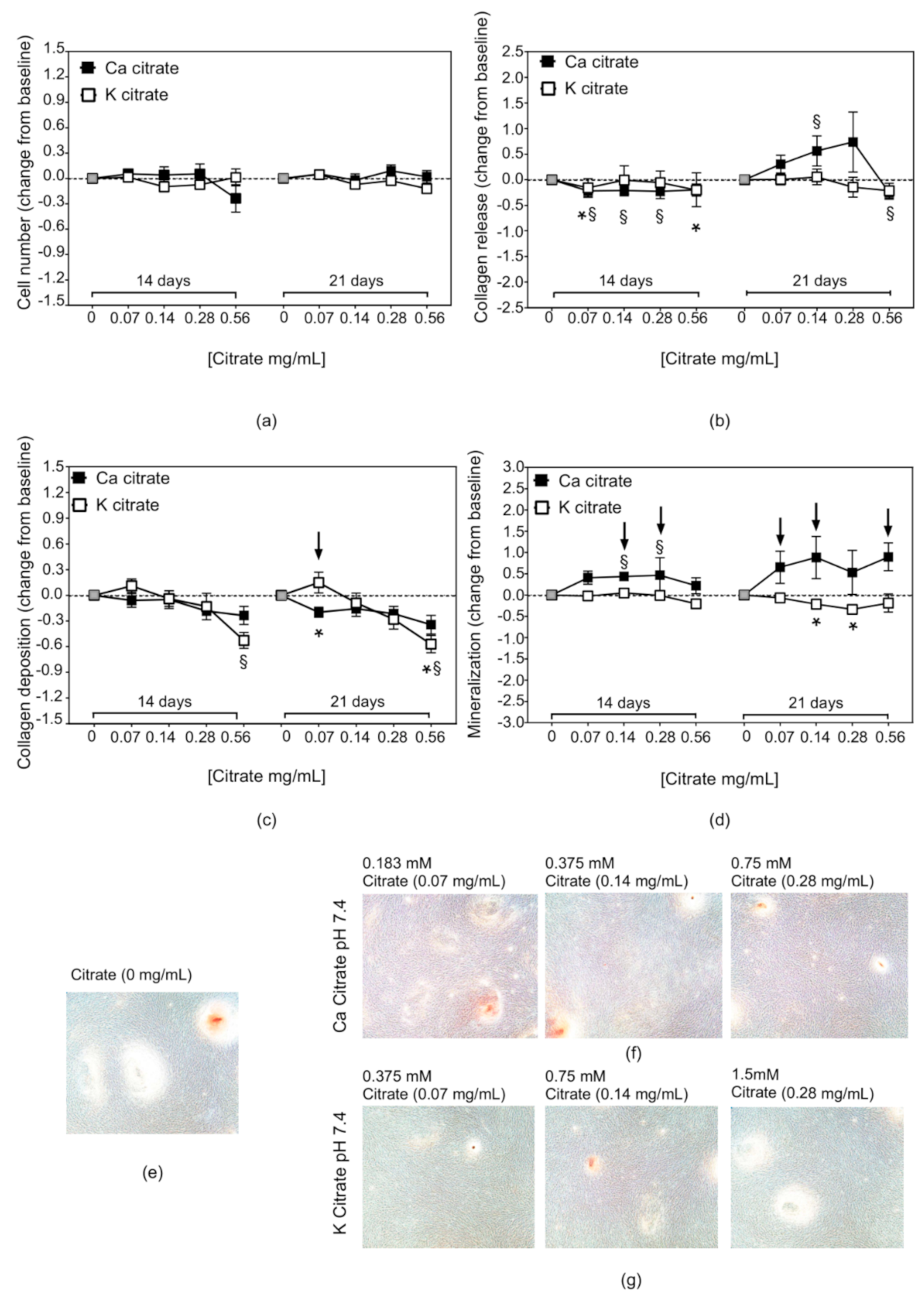
3.1. The Acidic Milieu Impairs the Osteogenic Properties of hMSC and the Extracellular Matrix Organisation
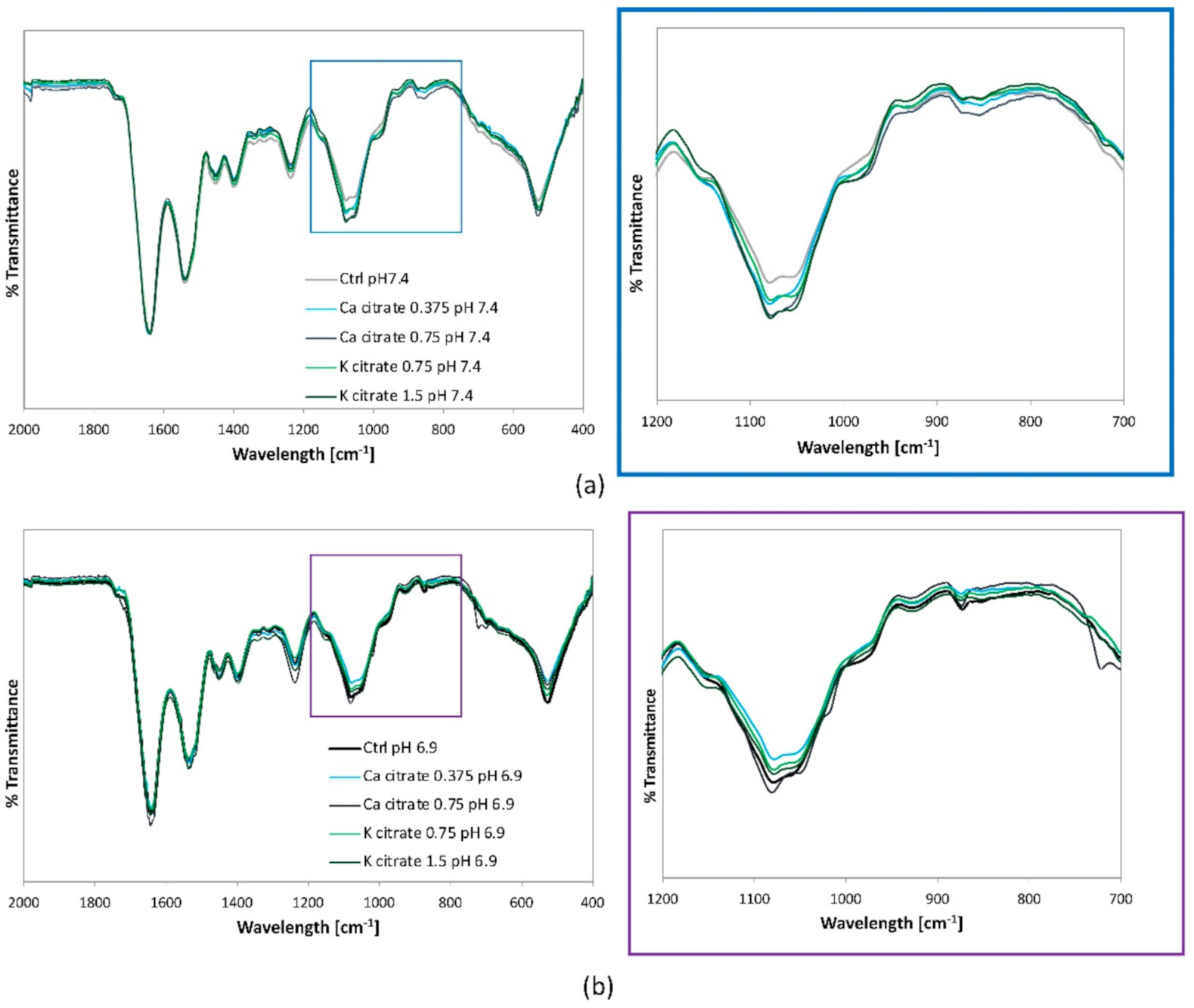
3.2. Citrate-Based Supplements Affect the Mineralisation Properties of hMSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bushinsky, D.A.; Frick, K.K. The effects of acid on bone. Curr. Opin. Nephrol. Hypertens. 2000, 9, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010, 503, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Jorgetti, V.; Drüeke, T.B.; Ott, S.M. Role of proton receptor OGR1 in bone response to metabolic acidosis? Kidney Int. 2016, 89, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R. Acid–base regulation of bone metabolism. Int. Congr. Ser. 2007, 1297, 255–267. [Google Scholar] [CrossRef]
- Granchi, D.; Baldini, N.; Ulivieri, F.M.; Caudarella, R. Role of Citrate in Pathophysiology and Medical Management of Bone Diseases. Nutrients 2019, 11, 2576. [Google Scholar] [CrossRef]
- Pizzorno, J. Acidosis: An Old Idea Validated by New Research. Integr. Med. 2015, 14, 8–12. [Google Scholar]
- Arnett, T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003, 62, 511–520. [Google Scholar] [CrossRef]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and medical management. Rev. Urol. 2009, 11, 134–144. [Google Scholar] [CrossRef]
- Krebs, H.A.; Johnson, W.A. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett. 1980, 117 (Suppl. S1), K2–K10. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Milenkovic, V.M.; Wetzel, C.H.; Rümmele, P.; Geissler, E.K. Extracellular Citrate in Health and Disease. Curr. Mol. Med. 2015, 15, 884–891. [Google Scholar] [CrossRef]
- Franklin, R.B.; Chellaiah, M.; Zou, J.; Reynolds, M.A.; Costello, L.C. Evidence that Osteoblasts are Specialized Citrate-producing Cells that Provide the Citrate for Incorporation into the Structure of Bone. Open Bone J. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Plasma citrate homeostasis, how it is regulated, and its physiological and clinical implications. An important, but neglected, relationship in medicine. HSOA J. Hum. Endocrinol. 2016, 1, 5. [Google Scholar] [CrossRef]
- Esche, J.; Johner, S.; Shi, L.; Schönau, E.; Remer, T. Urinary Citrate, an Index of Acid-Base Status, Predicts Bone Strength in Youths and Fracture Risk in Adult Females. J. Clin. Endocrinol. Metab. 2016, 101, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Sager, L.N.; Madias, N.E.; Wesson, D.E. Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int. 2019, 95, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Caudarella, R.; Ripamonti, C.; Spinnato, P.; Bazzocchi, A.; Massa, A.; Baldini, N. Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2018, 10, 1293. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Dawson-Hughes, B. Association of Urinary Citrate with Acid-Base Status, Bone Resorption, and Calcium Excretion in Older Men and Women. J. Clin. Endocrinol. Metab. 2018, 103, 452–459. [Google Scholar] [CrossRef]
- Granchi, D.; Torreggiani, E.; Massa, A.; Caudarella, R.; Di Pompo, G.; Baldini, N. Potassium citrate prevents increased osteoclastogenesis resulting from acidic conditions: Implication for the treatment of postmenopausal bone loss. PLoS ONE 2017, 12, e0181230. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.A.; Zou, J.; Reynolds, M.A.; Franklin, R.B. In vitro BMP2 stimulation of osteoblast citrate production in concert with mineralized bone nodule formation. J. Regen. Med. Tissue Eng. 2015, 4. [Google Scholar] [CrossRef][Green Version]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef] [PubMed]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, A.; Aedo-Martín, D.; Obeso, D.; Erjavec, I.; Rodríguez-Coira, J.; Buendía, I.; Ardura, J.A.; Barbas, C.; Gortazar, A.R. Metabolomics reveals citric acid secretion in mechanically-stimulated osteocytes is inhibited by high glucose. Sci. Rep. 2019, 9, 2295. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Ochoa, G.; Leonardi, E.; Devescovi, V.; Baglìo, S.R.; Osaba, L.; Baldini, N.; Ciapetti, G. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng. Part C Methods 2010, 16, 511–524. [Google Scholar] [CrossRef]
- Ciapetti, G.; Verri, E.; Granchi, D.; Cenni, E.; Gamberini, S.; Benetti, D.; Mian, M.; Pizzoferrato, A. In vitro assessment of phagocytosis of bovine collagen by human monocytes/macrophages using a spectrophotometric method. Biomaterials 1996, 17, 1703–1707. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-Laden Biomimetically Mineralized Shark-Skin-Collagen-Based 3D Printed Hydrogels for the Engineering of Hard Tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically mineralized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110660. [Google Scholar] [CrossRef]
- Richards, D.J.; Brookes, M. Osteogenesis and the pH of the osseous circulation. Calcif. Tissue Res. 1968, 2, 93. [Google Scholar] [CrossRef]
- Caudarella, R.; Vescini, F.; Buffa, A.; Stefoni, S. Citrate and mineral metabolism, kidney stones and bone disease. Front. Biosci. 2003, 8, s1084–s1106. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Haleblian, G.E.; Leitao, V.A.; Pierre, S.A.; Robinson, M.R.; Albala, D.M.; Ribeiro, A.A.; Preminger, G.M. Assessment of citrate concentrations in citrus fruit-based juices and beverages: Implications for management of hypocitraturic nephrolithiasis. J. Endourol. 2008, 22, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Pajor, A.M. Sodium-coupled transporters for Krebs cycle intermediates. Annu. Rev. Physiol. 1999, 61, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Dickens, F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 1941, 35, 1011–1023. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.; Zou, J.; Franklin, R.B.; Reynolds, M.A. The status of citrate in the hydroxyapatite/collagen complex of bone, and Its role in bone formation. J. Regen. Med. Tissue Eng. 2014, 3, 4. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Dai, H.; Tian, X.; Cui, Z.K.; Chen, Z.; Hu, L.; Song, Q.; Liu, A.; Zhang, Z.; et al. Bone and plasma citrate is reduced in osteoporosis. Bone 2018, 114, 189–197. [Google Scholar] [CrossRef]
- Brandao-Burch, A.; Utting, J.C.; Orriss, I.R.; Arnett, T.R. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif. Tissue Int. 2005, 77, 167–174. [Google Scholar] [CrossRef]
- Gene Expression Omnibus. Accession Number GSE12267. Available online: https://www.ncbi.nlm.nih.gov/geo (accessed on 12 October 2020).
- Hering-Smith, K.S.; Hamm, L.L. Acidosis and citrate: Provocative interactions. Ann. Transl. Med. 2018, 6, 374. [Google Scholar] [CrossRef]
- Risteli, J.; Niemi, S.; Kauppila, S.; Melkko, J.; Risteli, L. Collagen propeptides as indicators of collagen assembly. Acta Orthop. Scand. Suppl. 1995, 266, 183–188. [Google Scholar] [CrossRef]
- Frick, K.K.; Jiang, L.; Bushinsky, D.A. Acute metabolic acidosis inhibits the induction of osteoblastic egr-1 and type 1 collagen. Am. J. Physiol. 1997, 272, C1450–C1456. [Google Scholar] [CrossRef]
- Disthabanchong, S.; Radinahamed, P.; Stitchantrakul, W.; Hongeng, S.; & Rajatanavin, R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007, 71, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Montes Moraleda, B.; San Román, J.; Rodríguez-Lorenzo, L.M. Adsorption and conformational modification of fibronectin and fibrinogen adsorbed on hydroxyapatite. A QCM-D study. J. Biomed. Mater. Res. A 2016, 104, 2585–2594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.; Sone, E.D. In vitro models of collagen biomineralization. J Struct Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, E.; Malek, K.; Baranska, M. Rapid approach to analyze biochemical variation in rat organs by ATR FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 981–986. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Zaman Safi, S.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Vidal, B.; Mello, M.L. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Tao, J. FTIR and Raman studies of structure and bonding in mineral and organic-mineral composites. Methods Enzym. 2013, 532, 533–556. [Google Scholar] [CrossRef]
- Johnsson, M.S.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef]
- Fujisawa, R.; Tamura, M. Acidic bone matrix proteins and their roles in calcification. Front. Biosci. 2012, 17, 1891–1903. [Google Scholar] [CrossRef]
- Xie, B.; Nancollas, G.H. How to control the size and morphology of apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22369–22370. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef]
- Davies, E.; Müller, K.H.; Wong, W.C.; Pickard, C.J.; Reid, D.G.; Skepper, J.N.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B.; Reynolds, M.A.; Chellaiah, M. The Important Role of Osteoblasts and Citrate Production in Bone Formation: “Osteoblast Citration” as a New Concept for an Old Relationship. Open Bone J. 2012, 4. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Lin, Z.; Zhou, P.; Zhang, X.; Zhang, W.; Chen, Q.; Kou, D.; Ying, X.; Shen, Y.; et al. Effects of extracellular calcium on viability and osteogenic differentiation of bone marrow stromal cells in vitro. Hum. Cell 2013, 26, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Tang, Q.; Li, X.; Zhang, Y.; Liu, W.; Gao, Z.; Yang, H.; Zhao, R.C. In Vitro Survival of Human Mesenchymal Stem Cells is Enhanced in Artificial Endolymph with Moderately High Concentrations of Potassium. Stem. Cells Dev. 2018, 27, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Heredia, M.; Ferrer-Luque, C.M.; González-Rodríguez, M.P.; Martín-Peinado, F.J.; González-López, S. Decalcifying effect of 15% EDTA, 15% citric acid, 5% phosphoric acid and 2.5% sodium hypochlorite on root canal dentine. Int. Endod. J. 2008, 41, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchetti, A. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metabol. Disord. 2019, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Frassetto, L.; Moore, J.B.; Torgerson, D.; Gannon, R.; Burckhardt, P.; Lanham-New, S. The effect of supplementation with alkaline potassium salts on bone metabolism: A meta-analysis. Osteoporos. Int. 2015, 26, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Dallal, G.E.; Krall, E.A.; Sadowski, L.; Sahyoun, N.; Tannenbaum, S. A Controlled Trial of the Effect of Calcium Supplementation on Bone Density in Postmenopausal Women. N. Engl. J. Med. 1990, 323, 878–883. [Google Scholar] [CrossRef]
- Kenny, A.M.; Prestwood, K.M.; Biskup, B.; Robbins, B.; Zayas, E.; Kleppinger, A.; Burleson, J.A.; Raisz, L.G. Comparison of the Effects of Calcium Loading with Calcium Citrate or Calcium Carbonate on Bone Turnover in Postmenopausal Women. Osteoporos. Int. 2004, 15, 290–294. [Google Scholar] [CrossRef]
- Thomas, S.D.; Need, A.G.; Tucker, G.; Slobodian, P.; O’Loughlin, P.D.; Nordin, B.E. Suppression of parathyroid hormone and bone resorption by calcium carbonate and calcium citrate in postmenopausal women. Calcif. Tissue Int. 2008, 83, 81–84. [Google Scholar] [CrossRef]
- Sellmeyer, D.E.; Schloetter, M.; Sebastian, A. Potassium Citrate Prevents Increased Urine Calcium Excretion and Bone Resorption Induced by a High Sodium Chloride Diet. J. Clin. Endocrinol. Metab. 2002, 87, 2008–2012. [Google Scholar] [CrossRef]
- Marangella, M.; Di Stefano, M.; Casalis, S.; Berutti, S.; D’Amelio, P.; Isaia, G.C. Effects of potassium citrate supplementation on bone metabolism. Calcif. Tissue Int. 2004, 74, 330–335. [Google Scholar] [CrossRef]
- Jehle, S.; Zanetti, A.; Muser, J.; Hulter, H.N.; Krapf, R. Partial Neutralization of the Acidogenic Western Diet with Potassium Citrate Increases Bone Mass in Postmenopausal Women with Osteopenia. J. Am. Soc. Nephrol. 2006, 17, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Hulter, H.N.; Krapf, R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Moseley, K.F.; Weaver, C.M.; Appel, L.; Sebastian, A.; Sellmeyer, D.E. Potassium Citrate Supplementation Results in Sustained Improvement in Calcium Balance in Older Men and Women. J. Bone Miner. Res. 2013, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Kumar, R.; Stein, E.M.; Alexander, E.; Christos, P.; Bockman, R.S.; Rodman, J.S. Potassium Citrate Decreases Bone Resorption in Postmenopausal Women with Osteopenia: A Randomized, Double-Blind Clinical Trial. Endocr. Pract. 2015, 21, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Maalouf, N.M.; Abrams, S.A.; Pak, C.Y. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Karp, H.J.; Ketola, M.E.; Lamberg-Allardt, C.J. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br. J. Nutr. 2009, 102, 1341–1347. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perut, F.; Graziani, G.; Columbaro, M.; Caudarella, R.; Baldini, N.; Granchi, D. Citrate Supplementation Restores the Impaired Mineralisation Resulting from the Acidic Microenvironment: An In Vitro Study. Nutrients 2020, 12, 3779. https://doi.org/10.3390/nu12123779
Perut F, Graziani G, Columbaro M, Caudarella R, Baldini N, Granchi D. Citrate Supplementation Restores the Impaired Mineralisation Resulting from the Acidic Microenvironment: An In Vitro Study. Nutrients. 2020; 12(12):3779. https://doi.org/10.3390/nu12123779
Chicago/Turabian StylePerut, Francesca, Gabriela Graziani, Marta Columbaro, Renata Caudarella, Nicola Baldini, and Donatella Granchi. 2020. "Citrate Supplementation Restores the Impaired Mineralisation Resulting from the Acidic Microenvironment: An In Vitro Study" Nutrients 12, no. 12: 3779. https://doi.org/10.3390/nu12123779
APA StylePerut, F., Graziani, G., Columbaro, M., Caudarella, R., Baldini, N., & Granchi, D. (2020). Citrate Supplementation Restores the Impaired Mineralisation Resulting from the Acidic Microenvironment: An In Vitro Study. Nutrients, 12(12), 3779. https://doi.org/10.3390/nu12123779






