Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome?
Abstract
1. Introduction
- -
- IBS with predominant diarrhea (IBS-D): >25% of bowel movements with Bristol stool form types 6–7;
- -
- IBS with predominant constipation (IBS-C): >25% of bowel movements with Bristol stool form types 1–2
- -
- IBS with mixed bowel habits (IBS-M): >25% of bowel movements with Bristol stool form types 1 or 2 and >25% of bowel movements with Bristol stool form types 6 or 7.
- -
- The difficulty in establishing an effective blinding. This is because over the years IBS patients continue or simply come to know many diets commonly suggested for IBS therapy. This makes it difficult to create a blind trial as the patients often recognize these different diets when they are suggested to the patients.
- -
- The unclear adherence rates, except for very expensive and complex studies, such as trials that provide patients with all the food needed for the study.
- -
- The unclear evidence about the right length of wash out period in crossover studies in order to avoid carry over effects on symptoms and also on gut microbiota.
- -
- IBS dietary trials are rarely supported by pharmaceutical companies or investors as IBS is not seen as a profitable business.
2. Gluten Free Diet
3. Low FODMAP Diet
- -
- By increasing the small bowel water content as they are osmotically active;
- -
- By increasing the production of gas through bacterial fermentation;
- -
- By increasing the production of bacterial metabolites such as Short-Chain Fatty Acids (SCFAs).
- -
- be complex and difficult to teach and learn, because it consists of several steps and requires time, motivation and the involvement of an expert in nutritional matters;
- -
- be potentially expensive, due to the choice of more expensive, and difficult to find alternative foods;
- -
- reduce the normal intake of natural prebiotics, strongly modifying the gut microbiota;
- -
- increase the risk of constipation, limiting fiber intake;
- -
- be nutritionally inadequate;
- -
- favor the onset of or precipitate an eating disorder behavior;
- -
- be ineffective in the long term.
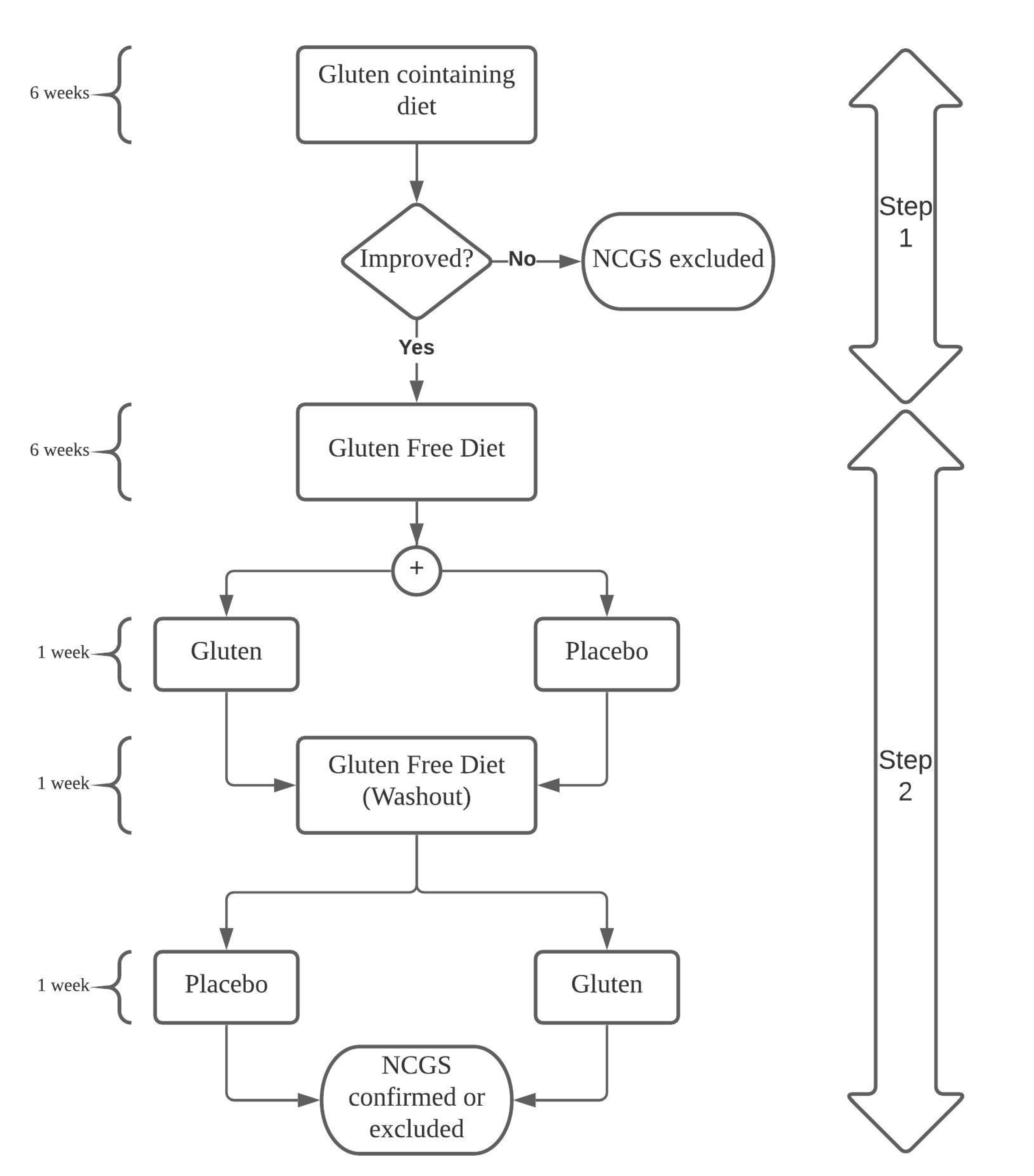
4. Non-Celiac Gluten/Wheat Sensitivity and IBS
5. Gluten Free Diet vs. Low FODMAP Diet
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [CrossRef] [PubMed]
- Cozma-Petruţ, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Werlang, M.E.; Palmer, W.C.; Lacy, B.E. Irritable Bowel Syndrome and Dietary Interventions. Gastroenterol. Hepatol. (N. Y.) 2019, 15, 16–26. [Google Scholar]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef]
- El-Salhy, M.; Seim, I.; Chopin, L.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Irritable bowel syndrome: The role of gut neuroendocrine peptides. Front. Biosci. 2012, 4, 2783–2800. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Stasi, C.; Usai Satta, P.; Milazzo, G.; Bianco, M.; Leandro, G.; Montalbano, L.M.; Muscatiello, N.; Monica, F.; Galeazzi, F.; et al. IBS clinical management in Italy: The AIGO survey. Dig. Liver Dis. 2019, 51, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Usai-Satta, P.; Bove, A.; Bocchini, R.; Galeazzi, F.; Battaglia, E.; Alduini, P.; Buscarini, E.; Bassotti, G.; ChroCoDiTE Study Group, AIGO. Chronic constipation diagnosis and treatment evaluation: The “CHRO.CO.DI.T.E.” study. BMC Gastroenterol. 2017, 17, 11. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Usai-Satta, P.; De Bortoli, N.; Bertani, L.; Marchi, S.; Stasi, C. Irritable bowel syndrome and chronic constipation: Fact and fiction. World J. Gastroenterol. 2015, 21, 11362–11370. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. (N. Y.) 2018, 14, 82–91. [Google Scholar]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Jones, A.L. The Gluten-Free Diet: Fad or Necessity? Diabetes Spectr. 2017, 30, 118–123. [Google Scholar] [CrossRef]
- Falcomer, A.L.; Santos Araújo, L.; Farage, P.; Santos Monteiro, J.; Yoshio Nakano, E.; Puppin Zandonadi, R. Gluten contamination in food services and industry: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, U.; Ullrich, R.; Riecken, E.O.; Schulzke, J.D. Celiac disease-like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology 2001, 121, 1329–1338. [Google Scholar] [CrossRef]
- Wahnschaffe, U.; Schulzke, J.D.; Zeitz, M.; Ullrich, R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2007, 5, 844–850. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 2011, 106, 508–515. [Google Scholar] [CrossRef]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911.e3. [Google Scholar] [CrossRef]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a Gluten-Free Diet in Subjects With Irritable Bowel Syndrome-Diarrhea Unaware of Their HLA-DQ2/8 Genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703.e1. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef]
- Zanwar, V.G.; Pawar, S.V.; Gambhire, P.A.; Jain, S.S.; Surude, R.G.; Shah, V.B.; Contractor, Q.Q.; Rathi, P.M. Symptomatic improvement with gluten restriction in irritable bowel syndrome: A prospective, randomized, double blinded placebo controlled trial. Intest. Res. 2016, 14, 343–350. [Google Scholar] [CrossRef]
- Barmeyer, C.; Schumann, M.; Meyer, T.; Zielinski, C.; Zuberbier, T.; Siegmund, B.; Schulzke, J.; Daum, S.; Ullrich, R. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int. J. Colorectal. Dis. 2017, 32, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; de Palma, G.; Calo, N.C.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-free Diet Reduces Symptoms, Particularly Diarrhea, in Patients with Irritable Bowel Syndrome and Anti-gliadin IgG [published online ahead of print, 2020 Aug 19]. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Gibson, P.R. How healthy is a gluten-free diet? Br. J. Nutr. 2015, 114, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Sanders, D.S. Gluten-Free Diet and Its ‘Cousins’ in Irritable Bowel Syndrome. Nutrients 2018, 10, 1727. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Shepard, S.; Gibson, P.; Chey, W.D. The Complete Low-FODMAP Diet: A Revolutionary Plan for Managing IBS and Other Digestive Disorders, 1st ed.; The Experiment LLC: New York, NY, USA, 2013. [Google Scholar]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow-Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Bazzichi, L.; Bassotti, G.; Mumolo, M.G.; Fani, B.; Costa, F.; Ricchiuti, A.; De Bortoli, N.; Mosca, M.; et al. Bioelectrical impedance vector analysis in patients with irritable bowel syndrome on a low FODMAP diet: A pilot study. Tech. Coloproctol. 2017, 21, 451–459. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Linaker, B.D. Non-coeliac gluten sensitivity? Lancet 1978, 1, 1358–1359. [Google Scholar] [CrossRef]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Bassotti, G.; Bellini, M.; Oppia, F.; Lai, M.; Cabras, F. Irritable Bowel Syndrome and Gluten-Related Disorders. Nutrients 2020, 12, 1117. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; De Giorgio, R. More Than One Culprit for Nonceliac Gluten/Wheat Sensitivity. Gastroenterology 2018, 155, 227. [Google Scholar] [CrossRef]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Arias, L.; Vivas, S.; de Morales, J.M.; Calleja, S.; de Miera, L.E.; Arroyo, P.; Casqueiro, J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 2012, 18, 649–656. [Google Scholar] [CrossRef] [PubMed]

| Allowed Foods | Forbidden Foods |
|---|---|
| Corn | Wheat |
| Potatoes | Barley |
| Rice | Rye |
| Millet | Malt |
| Buckwheat | Kamut |
| Quinoa | Spelt |
| Amaranth | Triticale |
| Teff | Bulgur |
| Oats, if free from contamination | Beer Malt |
| Patients | Methods | Evaluated Parameters | Results | |
|---|---|---|---|---|
| Wahnschaffe et al. [24] 2001 | IBS-D = 26 | 6 months GFD | Stool frequency IgA anti-gliadin IgA anti-tTG IEL count HLA DQ2 | Improved stool frequency in patients with HLA DQ2. |
| Wahnschaffe et al. [25] 2007 | IBS-D = 41 | 6 months GFD | Stool Frequency IBS symptoms questionnaire (Likert) HLA DQ2 | Stool frequency and GI symptom score returned to normal values in 60% of IBS patients who were positive and in 12% who were negative for HLA DQ2. |
| Biesiekierski et al. [26] 2011 | IBS = 34 | 6 weeks gluten or placebo containing bread with GFD | HLA DQ2/8 IBS symptoms questionnaire (VAS) | 56% having HLA DQ2/8. 68% in the gluten group reported that symptoms were not adequately controlled compared with 40% on placebo. |
| Vazquez-Roque et al. [27] 2013 | IBS-D = 45 | 4 weeks gluten containing diet or GFD | Bowel function Small bowel and colonic transit Lactulose and mannitol excretion HLA DQ2/8 | The gluten containing diet increased bowel frequency in HLA DQ2/8 patients and was associated with higher intestinal permeability. |
| Aziz et al. [28] 2015 | IBS-D = 41 | 6 weeks GFD | IBS-SSS HADS FIS SF-36 HLA DQ2/8 | GFD reduced IBS-SSS by ≥50 points in 71%. HLA DQ2/8 positive subjects had a greater reduction in depression score and increase in vitality score. |
| Shahbazkhani et al. [29] 2015 | IBS = 72 | 6 weeks GFD + 6 weeks gluten powder or placebo | IBS symptoms questionnaire (VAS) | Improvement was statistically different in the gluten containing group compared with placebo group in 25% and 83% patients, respectively. |
| Zanwar et al. [30] 2016 | IBS = 60 | 4 weeks GFD + 4 weeks washout + 4 weeks DBPC rechallenge | IBS symptoms questionnaire (VAS) | Gluten group scored significantly higher in abdominal pain, bloating and tiredness and their symptoms worsened within 1 week of the rechallenge. |
| Barmeyer et al. [31] 2017 | IBS-D/M = 35 | 4 months GFD | SGA IBS-SSS IBS-QoL EQ-5D HLA DQ2/8 | HLA DQ2/8 was not associated with wheat sensitivity. 34% of the patients reported considerably or completely relieved symptoms on the GFD. |
| Paduano et al. [32] 2019 | IBS = 42 | 4 weeks LFD + 4 weeks GFD + 4 weeks Mediterranean diet | Bristol stool scale IBS-SSS IBS-QoL IBS symptom questionnaire (VAS) SF-12 | After GFD, improvement in symptoms, in particular, VAS bloating, VAS pain and IBS-SSS, with a smaller improvement in bloating compared to the low FODMAP diet, but with an adherence index of only 11%. |
| Pinto-Sanchez et al. [33] 2020 | IBS = 50 | 4 weeks GFD | GI transit Birmingham IBS questionnaire Bristol Stool Scale HADS STAI-TAY PHQ-15 PGWB Anti-gliadin | After the GFD, patients with anti-gliadin reported less diarrhea. IBS symptoms improved in 75% of the patients with anti-gliadin and in 38% without the antibodies. GI transit normalized in a higher proportion of patients with anti-gliadin. |
| Food Categories | Allowed Foods | Forbidden Foods |
|---|---|---|
| Cereals | Rice, porridge, oats, quinoa, tapioca, millet, amaranth, buckwheat, gluten-free bread and cereals, potato-flour. | Bread and bakery products, biscuits, croissants, pasta, wheat flour, Kamut, barley, rye, couscous, flour, muesli. |
| Milk and derivates | Lactose-free milk, rice milk, oat milk, soy milk and all vegetable drinks, yogurt lactose free, soy yogurt, Greek yogurt, hard cheeses, fruit sorbets. | Cow milk, goat milk, yogurt with lactose, fresh cheeses, butter, ice cream, cream. |
| Vegetables | Carrot, pumpkin, Chinese cabbage, celery, lettuce, spinach, potato, tomato, zucchini, eggplant, green bean, beets, red pepper, herbs, olives, bamboo shoot, fresh herbs. | Asparagus, cauliflower, garlic, onion, shallot, mushroom, leek, chicory, fennel, artichoke, Brussel sprout, broccoli, radish, pepper, turnips, Jerusalem artichoke. |
| Legumes | Peas, soy products. | Beans, chickpeas, lentils, soybeans. |
| Fruit | Banana, blueberry, strawberry, raspberry, grape, melon, grapefruit, kiwi, orange, lemon, limes, pineapple, passion fruit. | Apple, pear, watermelon, mango, apricot, avocado, cherry, peach, plum, persimmon, lychee, fruit juices. |
| Dried fruits | Almonds, hazelnuts, walnuts, pine nuts. | Pistachios, cashews. |
| Sweeteners | White sugar, brown sugar, maple syrup. | Agave, honey, fructose, xylitol, maltitol, mannitol, sorbitol. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, M.; Tonarelli, S.; Mumolo, M.G.; Bronzini, F.; Pancetti, A.; Bertani, L.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Marchi, S.; et al. Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome? Nutrients 2020, 12, 3368. https://doi.org/10.3390/nu12113368
Bellini M, Tonarelli S, Mumolo MG, Bronzini F, Pancetti A, Bertani L, Costa F, Ricchiuti A, de Bortoli N, Marchi S, et al. Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome? Nutrients. 2020; 12(11):3368. https://doi.org/10.3390/nu12113368
Chicago/Turabian StyleBellini, Massimo, Sara Tonarelli, Maria Gloria Mumolo, Francesco Bronzini, Andrea Pancetti, Lorenzo Bertani, Francesco Costa, Angelo Ricchiuti, Nicola de Bortoli, Santino Marchi, and et al. 2020. "Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome?" Nutrients 12, no. 11: 3368. https://doi.org/10.3390/nu12113368
APA StyleBellini, M., Tonarelli, S., Mumolo, M. G., Bronzini, F., Pancetti, A., Bertani, L., Costa, F., Ricchiuti, A., de Bortoli, N., Marchi, S., & Rossi, A. (2020). Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome? Nutrients, 12(11), 3368. https://doi.org/10.3390/nu12113368








