Development of RisObIn.Com, a Screening Tool for Risk of Childhood Obesity in the Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of the Risk Index Tool and Scoring
2.2. Statistical Analysis
3. Results
3.1. Study Sample Characteristics
3.2. Risk Factors for Overweight (Including Obesity) with WtHr > 0.5
3.2.1. Peri-Natal Dimension
3.2.2. Anthropometric Dimension
3.2.3. Sociodemographic Dimension
3.2.4. Past Eating Habits Dimension
3.2.5. Current Eating Habits Dimension
3.2.6. Subjective Anthropometry Perception Dimension
3.2.7. Subjective Eating Habits Perception Dimension
3.2.8. Physical Activity and Sleeping Habits Dimension
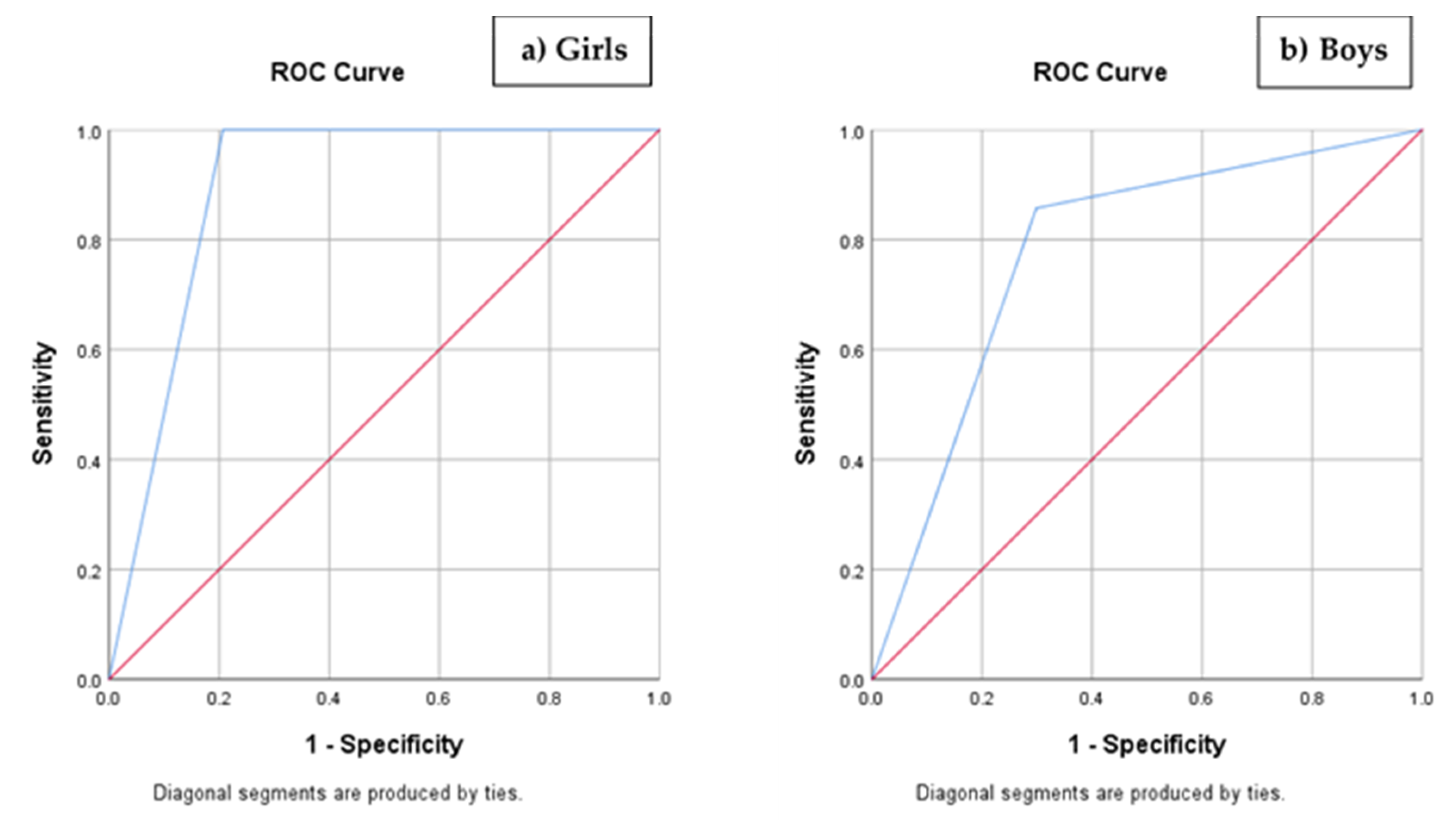
3.3. Scoring and Risk Index
4. Discussion
4.1. Peri-Natal Dimension
4.2. Anthropometric Dimension
4.3. Previous Eating Habits Dimension
4.4. Current Eating Habits Dimension
4.5. Subjective Perception of Anthropometry Dimension
4.6. Subjective Perception of Eating Habits Dimension
4.7. Physical Activity and Sleeping Habits Dimension
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Wijnhoven, T.M.A.; Van Raaij, J.M.A.; Sjöberg, A.; Eldin, N.; Yngve, A.; Kunešová, M.; Starc, G.; Rito, A.I.; Duleva, V.; Hassapidou, M.; et al. WHO European childhood obesity surveillance initiative: School nutrition environment and body mass index in primary schools. Int. J. Environ. Res. Public Health 2014, 11, 11261–11285. [Google Scholar] [CrossRef]
- Spinelli, A.; Buoncristiano, M.; Kovacs, V.A.; Yngve, A.; Spiroski, I.; Obreja, G.; Starc, G.; Pérez, N.; Rito, A.I.; Kunešová, M.; et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts 2019, 12, 244–258. [Google Scholar] [CrossRef]
- WHO Consultation on Obesity. Diseases WHOD of N World Health Organization. In Proceedings of the Programme of Nutrition F and, R.H. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva, Switzerland, 3–5 June 1997; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of obesity: An EASO position statement on a diagnostic term with clinical and scientific implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Burch, J.; Llewellyn, A.; Griffiths, C.; Yang, H.; Owen, C.; Duffy, S.; Woolacott, N. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: A systematic review and meta-analysis. Health Technol. Assess. 2015, 19, 1–336. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, A.; Sahoo, K.; Sahoo, B.; Choudhury, A.; Sufi, N.; Kumar, R. Childhood obesity: Causes and consequences. J. Fam. Med. Prim. Care 2015, 4, 187. [Google Scholar] [CrossRef]
- van de Kolk, I.; Gerards, S.M.; Harms, L.S.E.; Kremers, S.P.J.; Gubbels, J.S. The effects of a comprehensive, integrated obesity prevention intervention approach (SuperFIT) on children’s physical activity, sedentary behavior, and BMI z-score. Int. J. Environ. Res. Public Health 2019, 16, 5016. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.T.; Wang, Y.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef]
- Birch, L.L.; Ventura, A.K. Preventing childhood obesity: What works? Int. J. Obes. 2009, 33, S74–S81. [Google Scholar] [CrossRef]
- Davison, K.K.; Birch, L.L. Childhood overweight: A contextual model and recommendations for future research. Obes. Rev. 2001, 2, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Golab, B.P.; Santos, S.; Voerman, E.; Lawlor, D.A.; Jaddoe, V.W.; Gaillard, R.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: An individual participant data meta-analysis. Lancet Child Adolesc. Health 2018, 2, 812–821. [Google Scholar] [CrossRef]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; Solano, M.G.; Fijałkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative–COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Schrempft, S.; Van Jaarsveld, C.H.M.; Fisher, A.; Herle, M.; Smith, A.D.; Fildes, A.; Llewellyn, C.H. Variation in the Heritability of Child Body Mass Index by Obesogenic Home Environment. JAMA Pediatr. 2018, 172, 1153–1160. [Google Scholar] [CrossRef]
- Santos, M.; Cadieux, A.; Gray, J.; Ward, W. Pediatric Obesity in Early Childhood: A Physician Screening Tool. Clin. Pediatr. 2016, 55, 356–362. [Google Scholar] [CrossRef]
- Manios, Y.; Birbilis, M.; Moschonis, G.; Birbilis, G.; Mougios, V.; Lionis, C.; Chrousos, G.P. Childhood Obesity Risk Evaluation based on perinatal factors and family sociodemographic characteristics: CORE Index. Eur. J. Pediatr. 2013, 172, 551–555. [Google Scholar] [CrossRef]
- Simpson, J.R.; Keller, H.; Rysdale, L.; Beyers, J. Nutrition Screening Tool for Every Preschooler (NutriSTEPTM): Validation and test-retest reliability of a parent-administered questionnaire assessing nutrition risk of preschoolers. Eur. J. Clin. Nutr. 2008, 62, 770–780. [Google Scholar] [CrossRef]
- Sintra Cresce Saudável. Sintra Cresce Saudável [Internet]. Available online: http://crescesaudavel.sintra.pt/ (accessed on 26 September 2020).
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- de Onis, M.; Lobstein, T. Defining obesity risk status in the general childhood population: Which cut-offs should we use? Int. J. Pediatr. Obes. 2010, 5, 458–460. [Google Scholar] [CrossRef]
- World Health Organization. The WHO STEPwise Approach to Noncomunicable Disease Risk Factor Surveillance (STEPS); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Taylor, R.W.; Williams, S.M.; Grant, A.M.; Taylor, B.J.; Goulding, A. Predictive Ability of Waist-to-Height in Relation to Adiposity in Children Is Not Improved With Age and Sex-Specific Values. Obesity 2011, 19, 1062–1068. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L.; Institute of Medicine (U.S.). Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; p. 854. [Google Scholar]
- World Health Organization. Training Course on Child Growth Assessment: Interpreting Growth Indicators; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Viana, V.; Sinde, S.; Saxton, J. Questionário do Comportamento Alimentar da Criança (CEBQ). In Instrumentos e Contextos de Avaliação Psicológica; Almedina: Lisbon, Portugal, 2011. [Google Scholar]
- Collins, M.E. Body figure perceptions and preferences among preadolescent children. Int. J. Eat. Disord. 1991, 10, 199–203. [Google Scholar] [CrossRef]
- Daraganova, G. Data and measurement. In The Longitudinal Study of Australian Children Annual Statistical Report 2013; Australian Institute of Family Studies: Melbourne, Australia, 2014. [Google Scholar]
- Stunkard, A.J.; Sørensen, T.I.A.; Schulsinger, F. Use of the Danish Adoption Register for the study of obesity and thinness. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1983, 60, 115–120. [Google Scholar]
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Inter-relationships among childhood, B.M.I.; childhood height, and adult obesity: The Bogalusa Heart Study. Int. J. Obes. 2004, 28, 10–16. [Google Scholar] [CrossRef]
- Hebebrand, J.; Holm, J.C.; Woodward, E.; Baker, J.L.; Blaak, E.; Durrer Schutz, D.; Farpour-Lambert, N.J.; Frühbeck, G.; Halford, J.G.C.; Lissner, L.; et al. A Proposal of the European Association for the Study of Obesity to Improve the ICD-11 Diagnostic Criteria for Obesity Based on the Three Dimensions Etiology, Degree of Adiposity and Health Risk. Obes Facts 2017, 10, 284–307. [Google Scholar] [CrossRef]
- Dean, A.; Sullivan, K.; Soe, M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3.01. Updated 2013/04/06 [Internet]. Available online: www.OpenEpi.com (accessed on 26 September 2020).
- Toschke, A.M.; Grote, V.; Koletzko, B.; Von Kries, R. Identifying Children at High Risk for Overweight at School Entry by Weight Gain during the First 2 Years. Arch. Pediatr. Adolesc. Med. 2004, 158, 449–452. [Google Scholar] [CrossRef]
- Avis, J.L.S.; Holt, N.L.; Maximova, K.; van Mierlo, T.; Fournier, R.; Padwal, R.; Cave, A.L.; Martz, P.; Ball, G.D.C. The Development and Refinement of an e-Health Screening, Brief Intervention, and Referral to Treatment for Parents to Prevent Childhood Obesity in Primary Care. Telemed. e-Health 2016, 22, 385–394. [Google Scholar] [CrossRef]
- Hammond, R.; Athanasiadou, R.; Curado, S.; Aphinyanaphongs, Y.; Abrams, C.; Messito, M.J.; Gross, R.; Katzow, M.; Jay, M.; Razavian, N.; et al. Predicting childhood obesity using electronic health records and publicly available data. PLoS ONE 2019, 14, e0215571. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef]
- Zhang, T.; Whelton, P.K.; Xi, B.; Krousel-Wood, M.; Bazzano, L.; He, J.; Chen, W.; Li, S. Rate of change in body mass index at different ages during childhood and adult obesity risk. Pediatr. Obes. 2019, 14, e12513. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J. Children grow and horses race: Is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004, 4, 6. [Google Scholar] [CrossRef]
- Pei, Z.; Flexeder, C.; Fuertes, E.; Thiering, E.; Koletzko, B.; Cramer, C.; Berdel, D.; Lehmann, I.; Bauer, C.-P.; Heinrich, J.; et al. Early life risk factors of being overweight at 10 years of age: Results of the German birth cohorts GINIplus and LISAplus. Eur. J. Clin. Nutr. 2013, 67, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Goulding, A. Patterns of gowth associated with the timing of adiposity rebound. Obesity 2009, 17, 335–341. [Google Scholar] [CrossRef]
- Grote, V.; Theurich, M.; Koletzko, B. Do complementary feeding practices predict the later risk of obesity? Vol. 15, Current Opinion in Clinical Nutrition and Metabolic Care. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.; Siega-Riz, A.M. Trends in food and beverage consumption among infants and toddlers: 2005–2012. Pediatr. Am. Acad. Pediatr. 2017, 139, e20163290. [Google Scholar] [CrossRef]
- Rêgo, C.; Lopes, C.; Durão, C.; Pinto, E.; Mansilha, H.; Pereira da Silva, L.; Nazareth, M.; Graça, P.; Ferreira, R.; Lima, R.M.; et al. Alimentação Saudável dos 0 aos 6 anos–Linhas De Orientação Para Profissionais E Educadores; Direção-Geral da Saúde: Lisbon, Portugal, 2019.
- Zheng, M.; Lamb, K.E.; Grimes, C.; Laws, R.; Bolton, K.; Ong, K.K.; Campbell, K. Rapid weight gain during infancy and subsequent adiposity: A systematic review and meta-analysis of evidence. Obes. Rev. 2018, 19, 321–332. [Google Scholar] [CrossRef]
- MacLean, P.S.; Blundell, J.E.; Mennella, J.A.; Batterham, R.L. Biological control of appetite: A daunting complexity. Obesity 2017, 25 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef]
- Santos, T.; Moreira, A.C. Alimentação em contexto pré-escolar: Relação com estado nutricional e local de residência. Acta Port. Nutr. 2017, 8, 34–37. [Google Scholar] [CrossRef][Green Version]
- Eagleton, S.G.; Brown, C.L.; Moses, M.J.; Skelton, J.A. Restrictive feeding and excessive hunger in young children with obesity: A case series. Clin. Case Rep. 2019, 7, 1962–1967. [Google Scholar] [CrossRef]
- Robinson, E.; Aveyard, P.; Jebb, S.A. Is plate clearing a risk factor for obesity? A cross-sectional study of self-reported data in US adults. Obesity 2015, 23, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E. Overweight but unseen: A review of the underestimation of weight status and a visual normalization theory. Obes. Rev. 2017, 18, 1200–1209. [Google Scholar] [CrossRef]
- Klesges, R.C.; Stein, R.J.; Eck, L.H.; Isbell, T.R.; Klesges, L.M. Parental influence on food selection in young children and its relationships to childhood obesity. Am. J. Clin. Nutr. 1991, 53, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.; Wilkinson, S.; Virella, D.; Alves, M.; Calhau, C.; Coelho, R. Anxiety, family functioning and neuroendocrine biomarkers in obese children. Acta Med. Port. 2017, 30, 273–280. [Google Scholar] [CrossRef]
- Pinto, I.; Wilkinson, S.; Virella, D.; Alves, M.; Calhau, C.; Coelho, R. Attachment Strategies and Neuroendocrine Biomarkers in Obese Children. Acta Med. Port. 2016, 29, 332–339. [Google Scholar] [CrossRef][Green Version]
- Luby, J.L.; Belden, A.; Sullivan, J.; Spitznagel, E. Preschoolers’ Contribution to their Diagnosis of Depression and Anxiety: Uses and Limitations of Young Child Self-Report of Symptoms. Child Psychiatry Hum. Dev. 2007, 38, 321–338. [Google Scholar] [CrossRef]
- Fox, C.K.; Gross, A.C.; Rudser, K.D.; Foy, A.M.H.; Kelly, A.S. Depression, Anxiety, and Severity of Obesity in Adolescents. Clin. Pediatr. 2016, 55, 1120–1125. [Google Scholar] [CrossRef]
- Sheinbein, D.H.; Stein, R.I.; Hayes, J.F.; Brown, M.L.; Balantekin, K.N.; Conlon, R.P.K.; Saelens, B.E.; Perri, M.G.; Welch, R.R.; Schechtman, K.B.; et al. Factors associated with depression and anxiety symptoms among children seeking treatment for obesity: A social-ecological approach. Pediatr. Obes. 2019, 14, e12518. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.F.; Paxton, S.J.; Massey, R.; Campbell, K.J.; Wertheim, E.H.; Skouteris, H.; Gibbons, K. Maternal feeding practices predict weight gain and obesogenic eating behaviors in young children: A prospective study. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 24. [Google Scholar] [CrossRef]
- Gouveia, C.; Pereira-da-Silva, L.; Silva, P.; Virella, D.; Videira-Amaral, J. [Physical activity and sedentarism in adolescent students in Lisbon] Actividade física e sedentarismo em adolescentes escolarizados do Concelho de Lisboa. Acta Pediatr. Port. 2007, 38, 7–12. [Google Scholar]
- Li, N.; Zhao, P.; Diao, C.; Qiao, Y.; Katzmarzyk, P.T.; Chaput, J.P.; Fogelholm, M.; Kuriyan, R.; Kurpad, A.; Lambert, E.V.; et al. Joint associations between weekday and weekend physical activity or sedentary time and childhood obesity. Int. J. Obes. 2019, 43, 691–700. [Google Scholar] [CrossRef] [PubMed]
| Sample Characteristics | n | % | Girls | Boys | ||
|---|---|---|---|---|---|---|
| n | n | |||||
| Child | Age n = 300 | Median 8.0 (5.9–10.2) years old | ||||
| Sex n = 304 | Girls | 148 | 48.7 | n/a | n/a | |
| Boys | 156 | 51.3 | n/a | n/a | ||
| Body Mass Index n = 304 | Underweight | 4 | 1.3 | 1 | 3 | |
| Normal weight | 208 | 68.4 | 95 | 113 | ||
| Overweight | 63 | 20.7 | 38 | 25 | ||
| Obesity | 29 | 9.5 | 14 | 15 | ||
| Caretakers | Mother’s age n = 300 | Median 38.0 (26.0–52.0) | ||||
| Father’s age n = 280 | Median 40.0 (26.0–63.0) | |||||
| Mother’s Body Mass Index n = 288 | Underweight | 9 | 3.1 | 3 | 6 | |
| Normal weight | 165 | 57.3 | 82 | 83 | ||
| Overweight | 84 | 29.2 | 43 | 41 | ||
| Obesity | 30 | 10.4 | 14 | 16 | ||
| Father’s Body Mass Index n = 275 | Underweight | 0 | 0 | 0 | 0 | |
| Normal weight | 104 | 37.8 | 57 | 47 | ||
| Overweight | 132 | 48.0 | 55 | 77 | ||
| Obesity | 39 | 14.2 | 22 | 17 | ||
| Mother’s nationality n = 290 | Portuguese | 270 | 93.1 | 137 | 133 | |
| Non-Portuguese | 20 | 6.9 | 9 | 11 | ||
| Father’s nationality n = 277 | Portuguese | 265 | 95.7 | 131 | 134 | |
| Non-Portuguese | 12 | 4.3 | 6 | 6 | ||
| Mother’s education n = 297 | Basic education or lower | 62 | 20.9 | 32 | 30 | |
| Higher secondary education or a professional course | 119 | 40.1 | 68 | 51 | ||
| Graduation or bachelor’s degree | 99 | 33.3 | 44 | 55 | ||
| Master or doctoral degree | 17 | 5.7 | 3 | 14 | ||
| Father’s education n = 280 | Basic education or lower | 91 | 32.5 | 48 | 43 | |
| Higher secondary education or a professional course | 129 | 46.1 | 59 | 70 | ||
| Graduation or bachelor’s degree | 46 | 16.4 | 22 | 24 | ||
| Master or doctoral degree | 14 | 5.0 | 6 | 8 | ||
| Professional situation n = 263 | Both parents are employed | 209 | 79.5 | 108 | 101 | |
| Only one parent is employed | 44 | 16.7 | 21 | 23 | ||
| Both parents are jobless | 10 | 3.8 | 3 | 7 | ||
| Mean monthly household income n = 271 | Less than €500 | 9 | 3.32 | 4 | 5 | |
| €500–€1000 | 80 | 29.52 | 40 | 40 | ||
| €1000–€1500 | 80 | 29.52 | 36 | 44 | ||
| €1500–€2000 | 52 | 19.18 | 28 | 24 | ||
| €2000–€3000 | 41 | 15.12 | 17 | 24 | ||
| Above €3000 | 9 | 3.32 | 3 | 6 | ||
| Total | Girls | Boys | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% Confidence Interval | n | % | 95% Confidence Interval | n | % | 95% Confidence Interval | |||||
| Lower Level | Upper Level | Lower Level | Upper Level | Lower Level | Upper Level | ||||||||
| Body Mass Index z-Score | Median 0.36 (−2.77–3.89) | ||||||||||||
| Body Mass Index Class | Underweight | 4 | 1.3 | 0.5 | 3.3 | 1 | 0.68 | 0.1 | 3.7 | 3 | 1.92 | 0.7 | 5.5 |
| Normal Weight | 208 | 68.4 | 62.9 | 73.4 | 95 | 64.19 | 56.2 | 71.5 | 113 | 72.44 | 64.9 | 78.8 | |
| Overweight | 63 | 20.7 | 16.6 | 25.6 | 38 | 25.68 | 19.3 | 33.3 | 25 | 16.02 | 11.1 | 22.6 | |
| Obesity | 29 | 9.5 | 6.7 | 13.4 | 14 | 9.45 | 5.7 | 15.3 | 15 | 9.62 | 5.9 | 15.3 | |
| n Total | 304 | 100.0 | - | - | 148 | 100.0 | - | - | 156 | 100.0 | - | - | |
| Waist Circumference | Median 58.5 (37.5–91.5) | ||||||||||||
| Waist-to-Height ratio | Median 0.46 (0.34–0.66) | ||||||||||||
| ≤0.5 | 244 | 80.8 | 75.9 | 84.8 | 110 | 74.8 | 67.2 | 81.2 | 134 | 86.5 | 80.2 | 90.9 | |
| >0.5 | 58 | 19.2 | 15.5 | 24.4 | 37 | 25.2 | 18.9 | 32.8 | 21 | 13.5 | 9.0 | 19.8 | |
| n Total | 302 | 100.0 | - | - | 147 | 100.0 | - | - | 155 | 100.0 | - | - | |
| Overweight (Including Obesity) with Waist-to-Height ratio | >0.5 | 51 | 16.8 | 13.0 | 21.4 | 31 | 20.9 | 15.3 | 28.4 | 20 | 12.8 | 8.5 | 18.9 |
| n Total | 304 | - | - | - | 148 | - | - | - | 156 | - | - | - | |
| Dimension | Variables | Exp(β) (95% Confidence Interval) | p-Value | ||
|---|---|---|---|---|---|
| Peri-natal | Pre-pregnancy Body Mass Index | Pre-pregnancy Body Mass Index of Overweight | 2.591 (1.164–5.766) | 0.020 | |
| Pre-pregnancy Body Mass Index of Obesity | 4.145 (0.925–8.570) | 0.063 | |||
| Body Mass Index z-score 5-years-old | 4.159 (2.404–8.497) | 0.000 | |||
| Paternal Body Mass Index | Paternal Body Mass Index of Overweight | 0.772 (0.252–2.364) | 0.650 | ||
| Paternal Body Mass Index of Obesity | 4.041 (1.271–12.844) | 0.018 | |||
| Type of meal introduced in solid food introduction | Soup | 0.401 (0.176–0.914) | 0.030 | ||
| Current eating habits | Child’s appetite | Would eat only with insistence or frequently would not eat in totality; Would eat all and be satisfied | 0.174 (0.050–0.603) | 0.006 | |
| Caretaker’s perception of child’s intake through image | 1.489 (0.985–2.249) | 0.059 | |||
| Family Mediterranean pattern (PREDIMED) | How many vegetable servings do you consume per day? | 0.624 (0.389–0.999) | 0.050 | ||
| How many servings of butter, margarine, or cream do you consume per day? | 1.535 (0.976–2.413) | 0.063 | |||
| Subjective anthropometry perception | Adequacy of father’s body image perception vs. actual Body Mass Index | Relatively correct | 4.902 (1.116–21.536) | 0.035 | |
| Correct | 2.597 (0.634–10.643) | 0.185 | |||
| Adequacy of the caretaker’s opinion on the child’s nutritional status vs. child’s Body Mass Index | Relatively correct | 3.483 (0.882–13.753) | 0.075 | ||
| Correct | 31.605 (6.055–164.951) | 0.000 | |||
| Subjective eating habits perception | Caretaker’s perception regarding child’s intake when anxious | No | 0.260 (0.056–1.204) | 0.085 | |
| Caretaker’s perception of the adequacy of the child’s food intake for age | Inferior or adequate | 0.083 (0.024–0.286) | 0.000 | ||
| Physical activity and hours of sleep | Child’s participation in programmed sport activity | Yes | 0.194 (0.052–0.724) | 0.015 | |
| Dimension | Item | Response Options and Scoring | Scoring |
|---|---|---|---|
| Anthropometric | Body Mass Index at 5 years old | 0—Overweight | −2.3 (p = 0.031) |
| 1—Underweight, Normal weight | |||
| Peri-natal | Mother’s pre-pregnancy Body Mass Index | 0—Obesity | −1.0 (p = 0.063) |
| 1—Underweight, Normal weight, Overweight | |||
| Current eating habits | Child’s appetite | 0—Would eat only with insistence or frequently would not eat in totality; Would eat all and be satisfied | 1.5 (p = 0.015) |
| 1—Would eat more than what is offered | |||
| Previous eating habits | Type of meal introduced in solid food introduction | 0—Soup | −0.9 (p = 0.030) |
| 1—Infant cereal | |||
| Subjective eating habits perception | Caretaker’s perception of the adequacy of the child’s food intake for age | 0—Less than adequate; Adequate | 2.5 (p = 0.000) |
| 1—More than adequate | |||
| Caretaker’s perception regarding the child’s higher intake when worried, irritated, or anxious | 0—No | 1.4 (p = 0.085) | |
| 1—Yes | |||
| Subjective anthropometry perception | Adequacy of the caretaker’s opinion on the child’s nutritional status vs. child’s Body Mass Index | 0—Correct | 3.4 (p = 0.000) |
| 1—Incorrect | |||
| Physical activity and hours of sleep | Child’s participation in programmed sport activity | 0—Yes | 1.6 (p = 0.015) |
| 1—No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.C.; Almeida Oliveira, P.; Borrego, R.; Nogueira, T.; Ferreira, R.; Virella, D. Development of RisObIn.Com, a Screening Tool for Risk of Childhood Obesity in the Community. Nutrients 2020, 12, 3288. https://doi.org/10.3390/nu12113288
Moreira AC, Almeida Oliveira P, Borrego R, Nogueira T, Ferreira R, Virella D. Development of RisObIn.Com, a Screening Tool for Risk of Childhood Obesity in the Community. Nutrients. 2020; 12(11):3288. https://doi.org/10.3390/nu12113288
Chicago/Turabian StyleMoreira, Ana Catarina, Patrícia Almeida Oliveira, Rute Borrego, Telma Nogueira, Raquel Ferreira, and Daniel Virella. 2020. "Development of RisObIn.Com, a Screening Tool for Risk of Childhood Obesity in the Community" Nutrients 12, no. 11: 3288. https://doi.org/10.3390/nu12113288
APA StyleMoreira, A. C., Almeida Oliveira, P., Borrego, R., Nogueira, T., Ferreira, R., & Virella, D. (2020). Development of RisObIn.Com, a Screening Tool for Risk of Childhood Obesity in the Community. Nutrients, 12(11), 3288. https://doi.org/10.3390/nu12113288







