Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Lactic Acid Bacteria Culture
2.3. Reagents and Antibodies
2.4. Small Intestinal-Lamina Propria (SI-LP) Cells Isolation
2.5. Peyer’s Patch (PP) Cells Isolation
2.6. Splenocytes Preparation
2.7. Flow Cytometry
2.8. In Vitro and Ex Vivo Stimulation of PP Isolated Cells
2.9. Bone Marrow Derived Dendritic Cells (BMDCs) Preparation
2.10. BMDCs Stimulation Assay
2.11. Antigen Uptake Assay
2.12. Antigen Presentation Assay
2.13. Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.14. Statistics
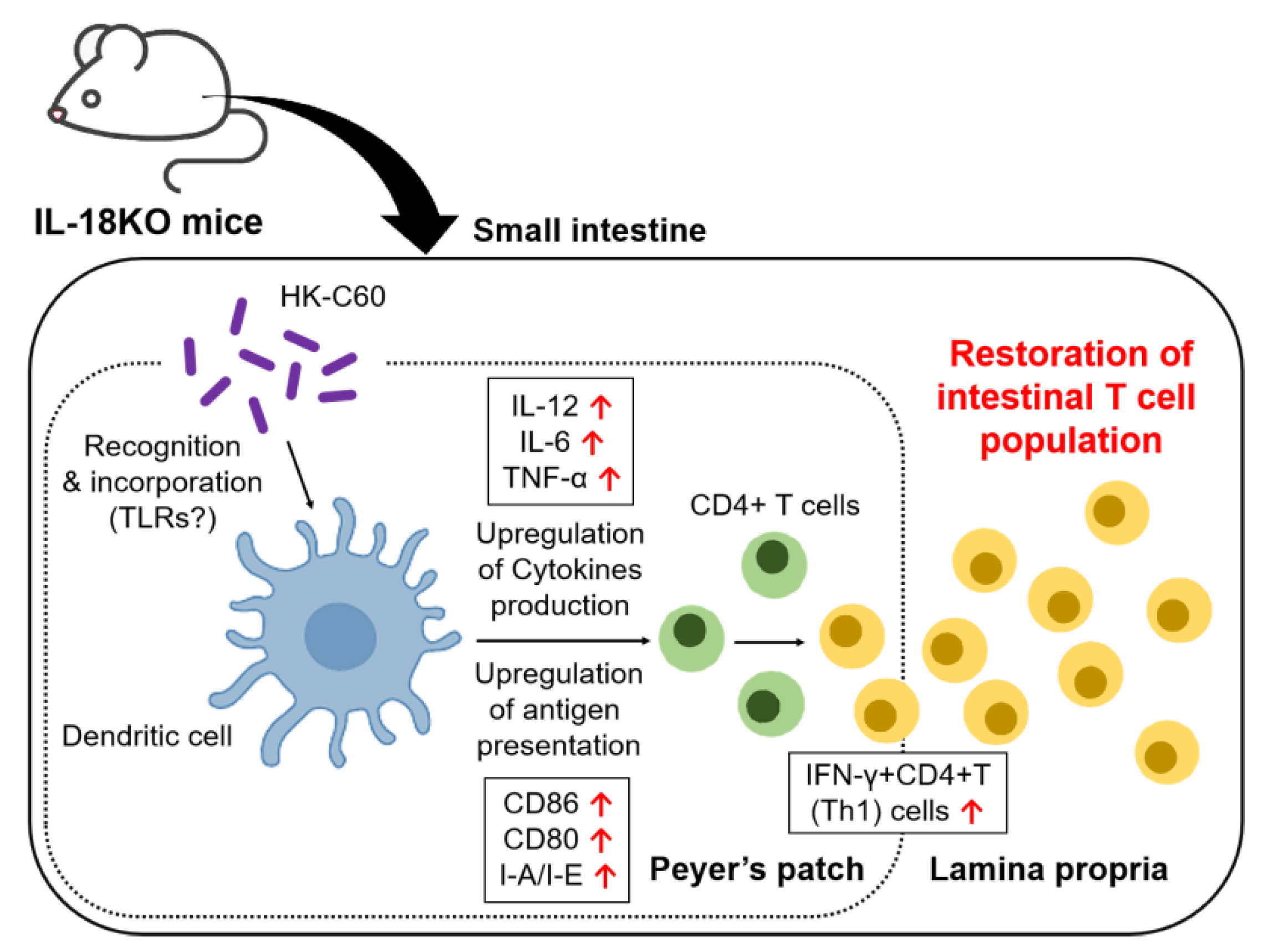
3. Results
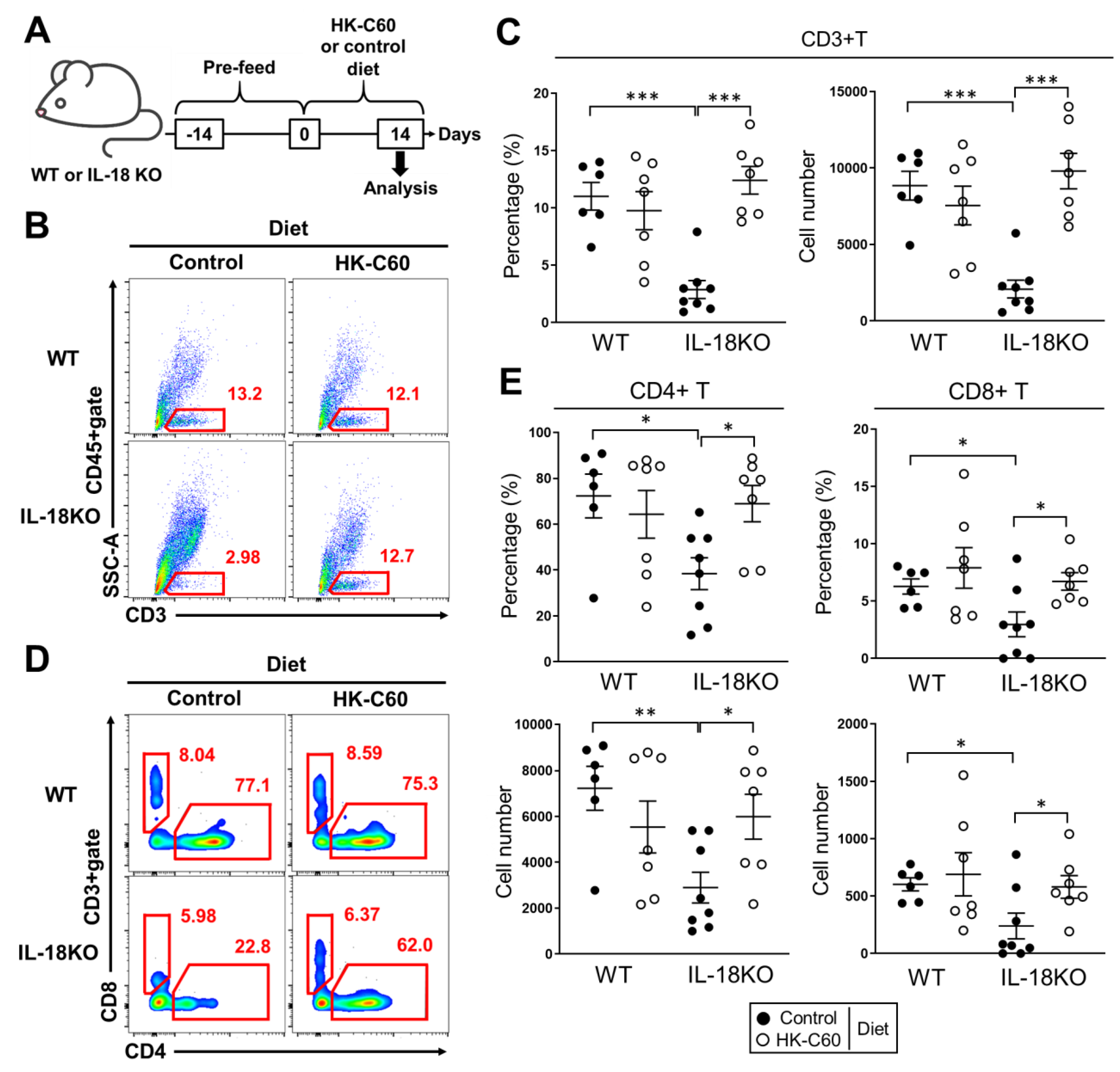
3.1. HK-C60 Diet Restores T Cell Population Which Decreased in SI-LP of Aged IL-18KO Mice
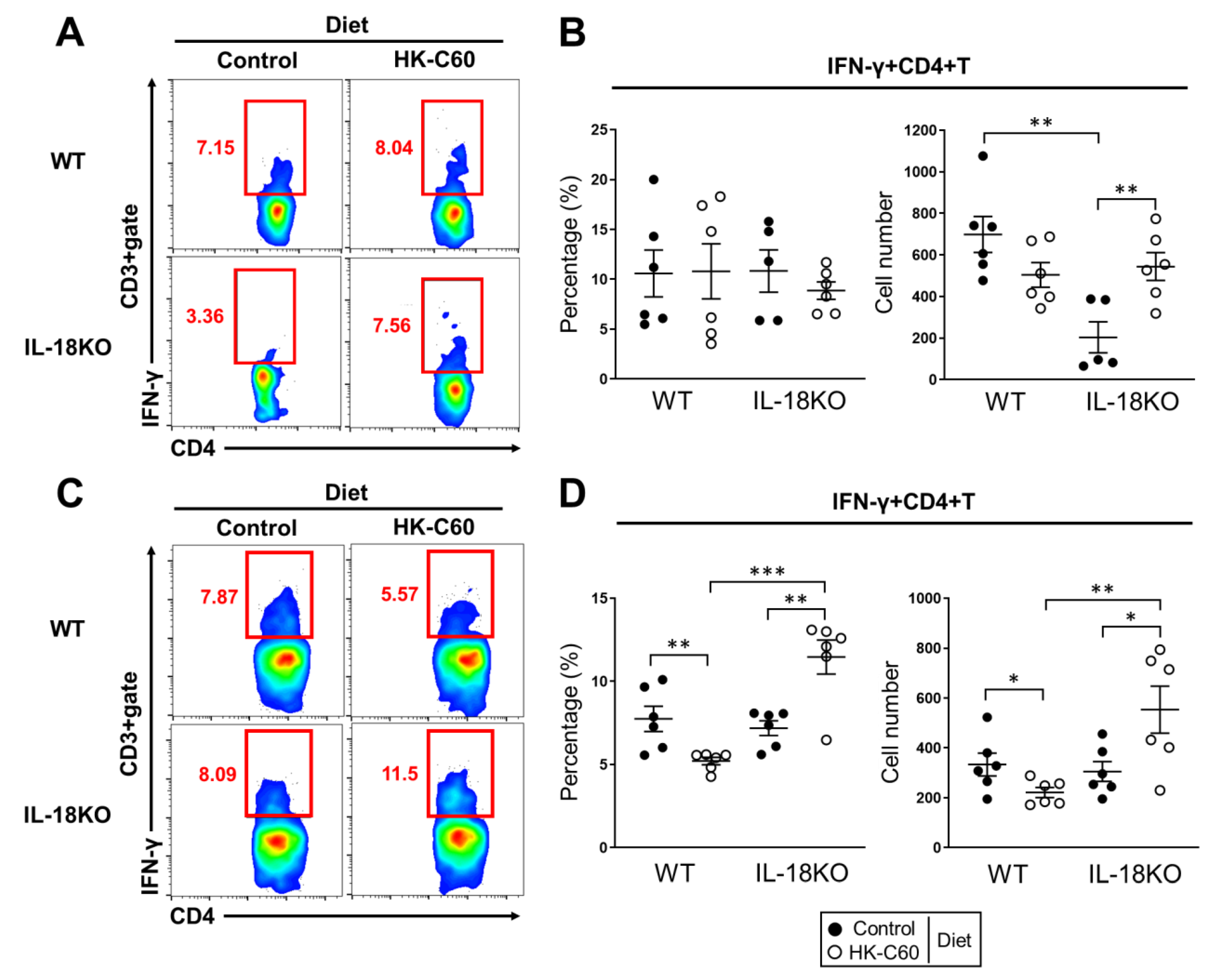
3.2. HK-C60 Diet Increases IFN-γ Producing CD4+ T Cells in Small Intestine of Aged IL-18KO Mice
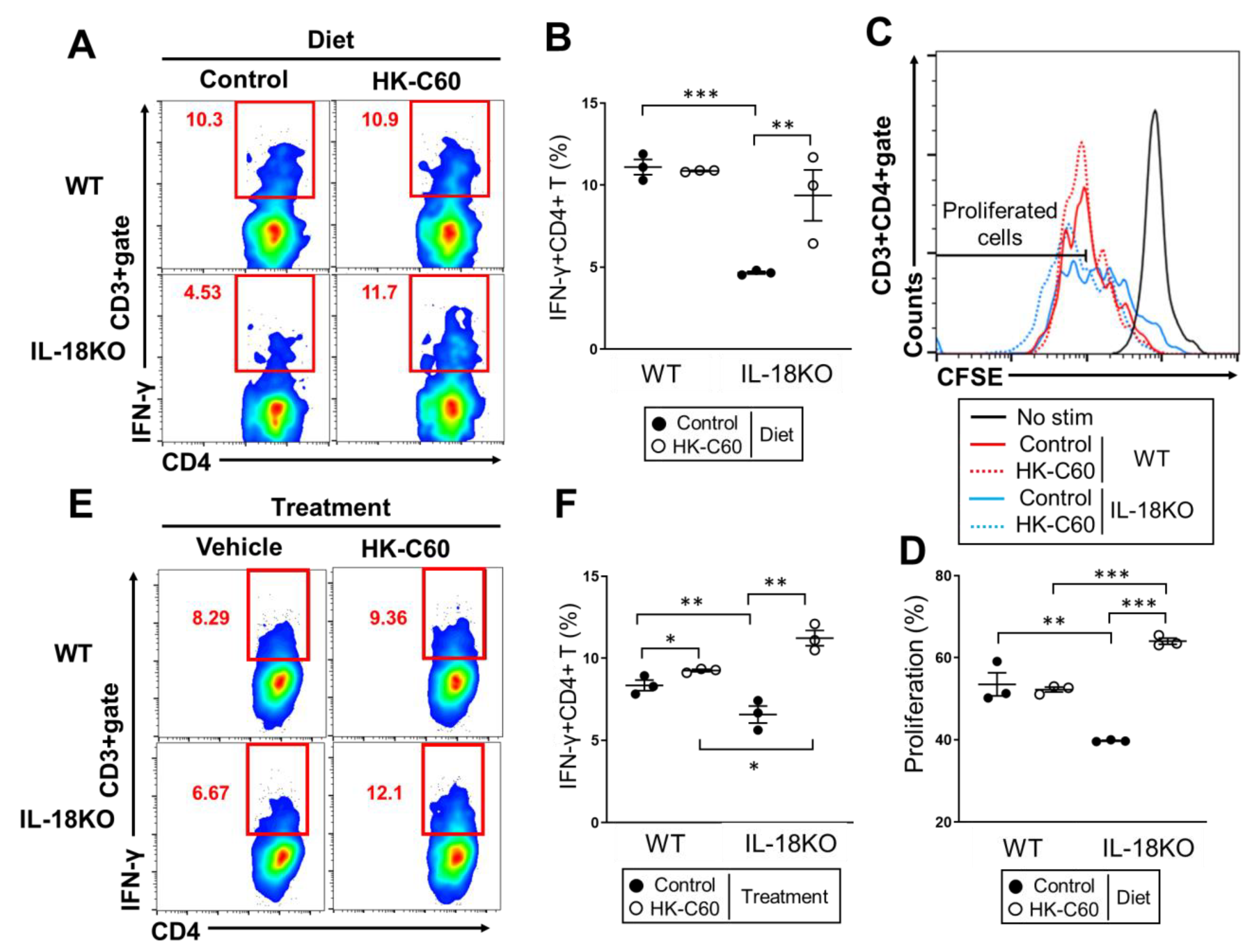
3.3. HK-C60 Enhances Cellular Activity of CD4+ T Cells in PP of IL-18KO Mice
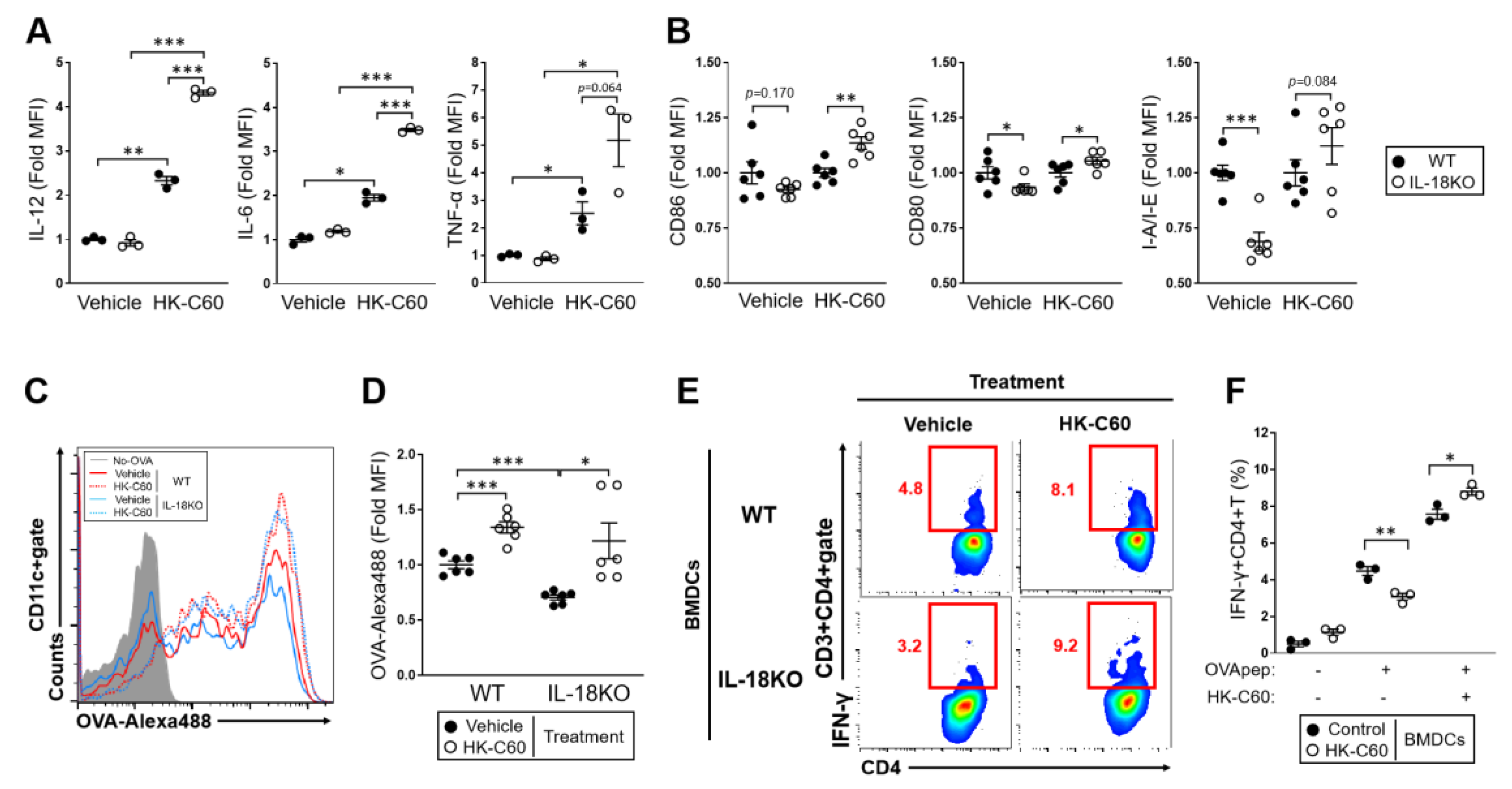
3.4. The IL-18-Deficient DCs Function Is over Activated by HK-C60 Stimulation, Which Directory Influences in Th1 Cells Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 24, 1785. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; van Tol, E.A.; Heine, H.; Braaksma, M.; Gross, G.; Overkamp, K.; Hennen, M.; Alrifai, M.; Conrad, M.L.; Renz, H.; et al. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in life. Clin. Exp. Allergy 2013, 43, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, K.; El Hadad, S.; Assaeedi, A.; Aldahlawi, A.; Khider, M.; Alhebshi, A. In vitro and in vivo evidences for innate immune stimulators lactic acid bacterial starters isolated from fermented camel dairy products. Sci. Rep. 2018, 22, 12553. [Google Scholar] [CrossRef]
- Kamiya, T.; Watanabe, Y.; Makino, S.; Kano, H.; Tsuji, N.M. Improvement of Intestinal Immune Cell Function by Lactic Acid Bacteria for Dairy Products. Microorganisms 2016, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Abeijon-Mukdsi, M.C.; Ross, R.; Medina, R.; González, S.; Gauffin-Cano, P. Specific Strains of Lactic Acid Bacteria Differentially Modulate the Profile of Adipokines In Vitro. Front. Immunol. 2017, 8, 266. [Google Scholar] [CrossRef]
- Kawano, M.; Miyoshi, M.; Miyazaki, T. Lactobacillus helveticus SBT2171 Induces A20 Expression via Toll-Like Receptor 2 Signaling and Inhibits the Lipopolysaccharide-Induced Activation of Nuclear Factor-kappa B and Mitogen-Activated Protein Kinases in Peritoneal Macrophages. Front. Immunol. 2019, 17, 845. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 34561. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, D.J.; Carreras, J.; et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Watanabe, Y.; Kubota, Y.; Yoshio, S.; Kanto, T.; Motohashi, S.; Shimojo, N.; Tsuji, N.M. Double-Stranded RNA Derived from Lactic Acid Bacteria Augments Th1 Immunity via Interferon-β from Human Dendritic Cells. Front. Immunol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Okamura, H.; Tsutsui, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Soudja, S.M.; Ruiz, A.L.; Marie, J.C.; Lauvau, G. Inflammatory monocytes activate memory CD8 (+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 2012, 37, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Gardella, S.; Andrei, C.; Costigliolo, S.; Poggi, A.; Zocchi, M.R.; Rubartelli, A. Interleukin-18 synthesis and secretion by dendritic cells are modulated by interaction with antigen-specific T cells. J. Leukoc. Biol. 1999, 66, 237–241, PMID: 10449160. [Google Scholar] [CrossRef]
- Van Der Sluijs, K.F.; Van Elden, L.J.; Arens, R.; Nijhuis, M.; Schuurman, R.; Florquin, S.; Kwakkel, J.; Akira, S.; Jansen, H.M.; Lutter, R.; et al. Enhanced viral clearance in interleukin-18 gene-deficient mice after pulmonary infection with influenza A virus. Immunology 2005, 114, 112–120. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Briken, V.; Ahlbrand, S.E.; Shah, S. Mycobacterium tuberculosis and the host cell inflammasome: A complex relationship. Front. Cell. Infect. Microbiol. 2013, 3, 62. [Google Scholar] [CrossRef]
- Chao, Y.; Kaliaperumal, N.; Chrétien, A.-S.; Tang, S.; Lee, B.; Poidinger, B.; Fairhurst, A.-M.; Connolly, J. Human plasmacytoid dendritic cells regulate IFN-α production through activation-induced splicing of IL-18Rα. J. Leukoc. Biol. 2014, 96, 1037–1046. [Google Scholar] [CrossRef]
- Gutzmer, R.; Langer, K.; Mommert, S.; Wittmann, M.; Kapp, A.; Werfel, T. Human Dendritic Cells Express the IL-18R and Are Chemoattracted to IL-18. J. Immunol. 2003, 171, 6363–6371. [Google Scholar] [CrossRef]
- Honda, K.; Takeda, K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009, 2, 187–196. [Google Scholar] [CrossRef]
- Pott, J.; Hornef, M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012, 13, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.J.; Srinivasan, N.; Pott, J.; Schiering, C.; Krausgruber, T.; Ilott, N.E.; Maloy, K.J. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal. Immunol. 2015, 8, 1226–1236. [Google Scholar] [CrossRef]
- Parlato, M.; Yeretssian, G. NOD-Like Receptors in Intestinal Homeostasis and Epithelial Tissue Repair. Int. J. Mol. Sci. 2014, 15, 9594–9627. [Google Scholar] [CrossRef]
- Adachi, T.; Kakuta, S.; Aihara, Y.; Kamiya, T.; Watanabe, Y.; Osakabe, N.; Hazato, N.; Miyawaki, A.; Yoshikawa, S.; Usami, T.; et al. Visualization of Probiotic-Mediated Ca2+ Signaling in Intestinal Epithelial Cells In Vivo. Front. Immunol. 2016, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.C.; Andrusaite, A.; Shergold, A.L.; Milling, S.; Perona-Wright, G. Isolation and functional characterisation of lamina propria leukocytes from helminth-infected, murine small intestine. J. Immunol. Methods 2019, 477, 112702. [Google Scholar] [CrossRef]
- Saito, S.; Kawamura, T.; Higuchi, M.; Kobayashi, T.; Yoshita-Takahashi, M.; Yamazaki, M.; Abe, M.; Sakimura, K.; Kanda, Y.; Kawamura, H.; et al. RASAL3, a novel hematopoietic RasGAP protein, regulates the number and functions of NKT cells. Eur. J. Immunol. 2015, 45, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Cao, D.-Y.; Wu, H.-Y.; Saito, S. Immunization with a Bacterial Lipoprotein Establishes an Immuno-Protective Response with Upregulation of Effector CD4+ T Cells and Neutrophils Against Methicillin-Resistant Staphylococcus aureus Infection. Pathogens 2020, 9, 138. [Google Scholar] [CrossRef]
- Saito, S.; Quadery, A.F. Staphylococcus aureus Lipoprotein Induces Skin Inflammation, Accompanied with IFN-γ-Producing T Cell Accumulation through Dermal Dendritic Cells. Pathogens 2018, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef]
- Tsuji, N.M.; Kimoto, H. Lactic Acid Bacteria and their Cellular Components Inducing Immunoregulatory Function, and Method of Obtaining the Same. Patent EP 1538398 A2 2005, 5 January 2010. [Google Scholar]
- Kimoto-Nira, H.; Suzuki, C.; Kobayashi, M.; Sasaki, K.; Kurisaki, J.-I.; Mizumachi, K. Anti-ageing effect of a lactococcal strain: Analysis using senescence-accelerated mice. Br. J. Nutr. 2007, 98, 1178–1186. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Moriya, N.; Hayakawa, S.; Kuramasu, K.; Ohmori, H.; Yamasaki, S.; Ogawa, M. Effects of rare sugar D-allulose on acid production and probiotic activities of dairy lactic acid bacteria. J. Dairy Sci. 2017, 100, 5936–5944. [Google Scholar] [CrossRef]
- Ma, H.; Tao, W.; Zhu, S. T lymphocytes in the intestinal mucosa: Defense and tolerance. Cell. Mol. Immunol. 2019, 16, 216–224. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front. Immunol. 2019, 1, 2345. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. The Probiotic Bacterium Lactobacillus casei Induces Activation of the Gut Mucosal Immune System through Innate Immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Mora, J.R.; Bono, M.R.; Manjunath, N.; Weninger, W.; Cavanagh, L.L.; Rosemblatt, M.; Von Andrian, U.H. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 2003, 424, 88–93. [Google Scholar] [CrossRef]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Di Sabatino, A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Investig. 2009, 119, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.H.; Engering, A.; Van Der Kleij, D.; De Jong, E.C.; Schipper, K.; Van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; Van Kooyk, Y. Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Kenny, D.J.; Xavier, R.J. Gut Microbiota Regulation of T Cells during Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.-D.; Zhao, L.-D.; Li, H. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Matar, C.; Perdigon, G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-Like Receptors. Clin. Diagn. Lab. Immunol. 2005, 12, 1075–1084. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial Teichoic Acids Reverse Predominant IL-12 Production Induced by Certain Lactobacillus Strains into Predominant IL-10 Production via TLR2-Dependent ERK Activation in Macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Kakizaki, N.; Okuno, A.; Maekawa, T.; Tsuji, N.M. Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice. Nutrients 2020, 12, 3287. https://doi.org/10.3390/nu12113287
Saito S, Kakizaki N, Okuno A, Maekawa T, Tsuji NM. Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice. Nutrients. 2020; 12(11):3287. https://doi.org/10.3390/nu12113287
Chicago/Turabian StyleSaito, Suguru, Nanae Kakizaki, Alato Okuno, Toshio Maekawa, and Noriko M. Tsuji. 2020. "Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice" Nutrients 12, no. 11: 3287. https://doi.org/10.3390/nu12113287
APA StyleSaito, S., Kakizaki, N., Okuno, A., Maekawa, T., & Tsuji, N. M. (2020). Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice. Nutrients, 12(11), 3287. https://doi.org/10.3390/nu12113287



