Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM-1®) Displays In Vitro Activity against Some Intestinal Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Carbon Source Preferences of the Strain IM-1®
2.2.1. Growth Assays
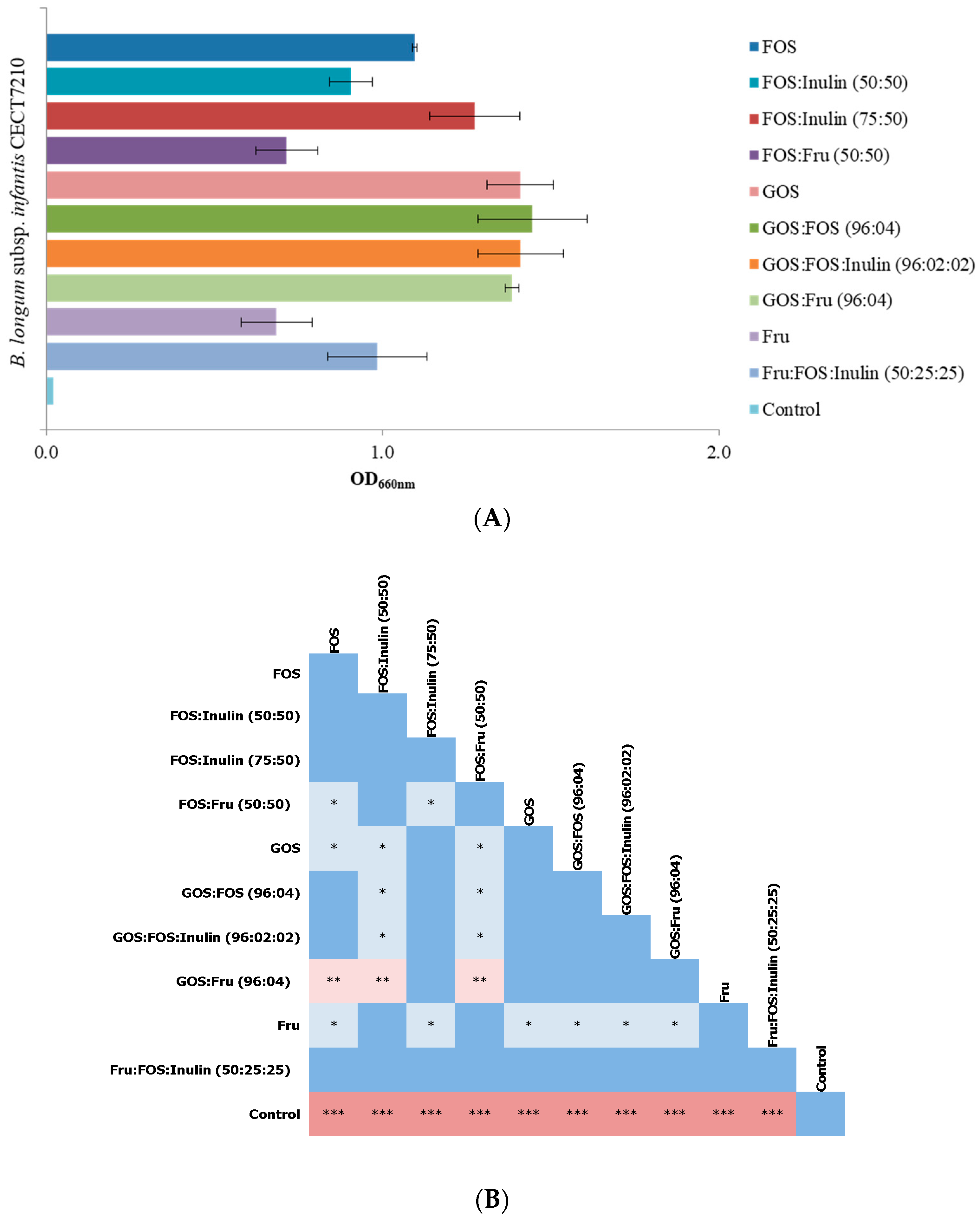
2.2.2. Determination of the Best Prebiotic Oligosaccharide Mixture
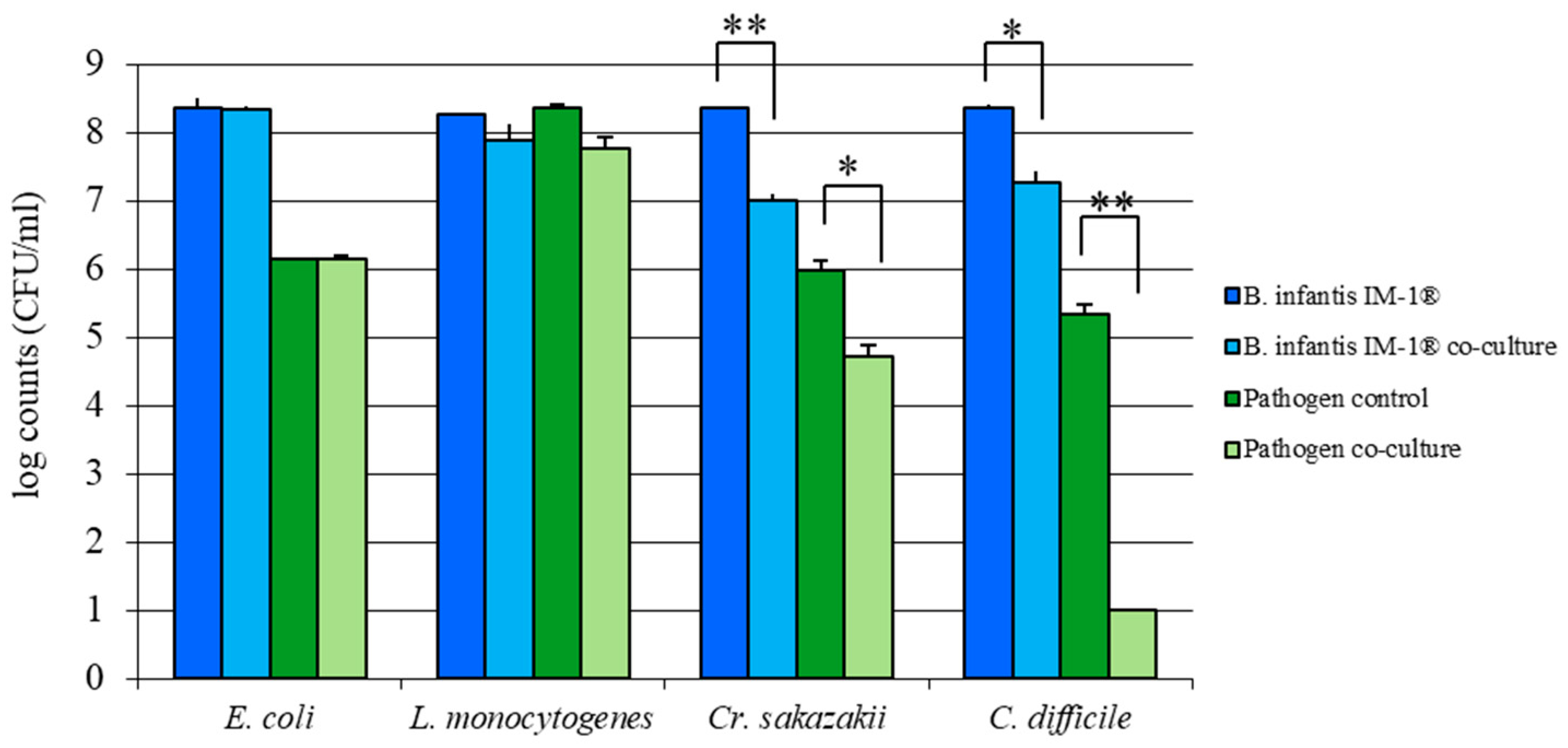
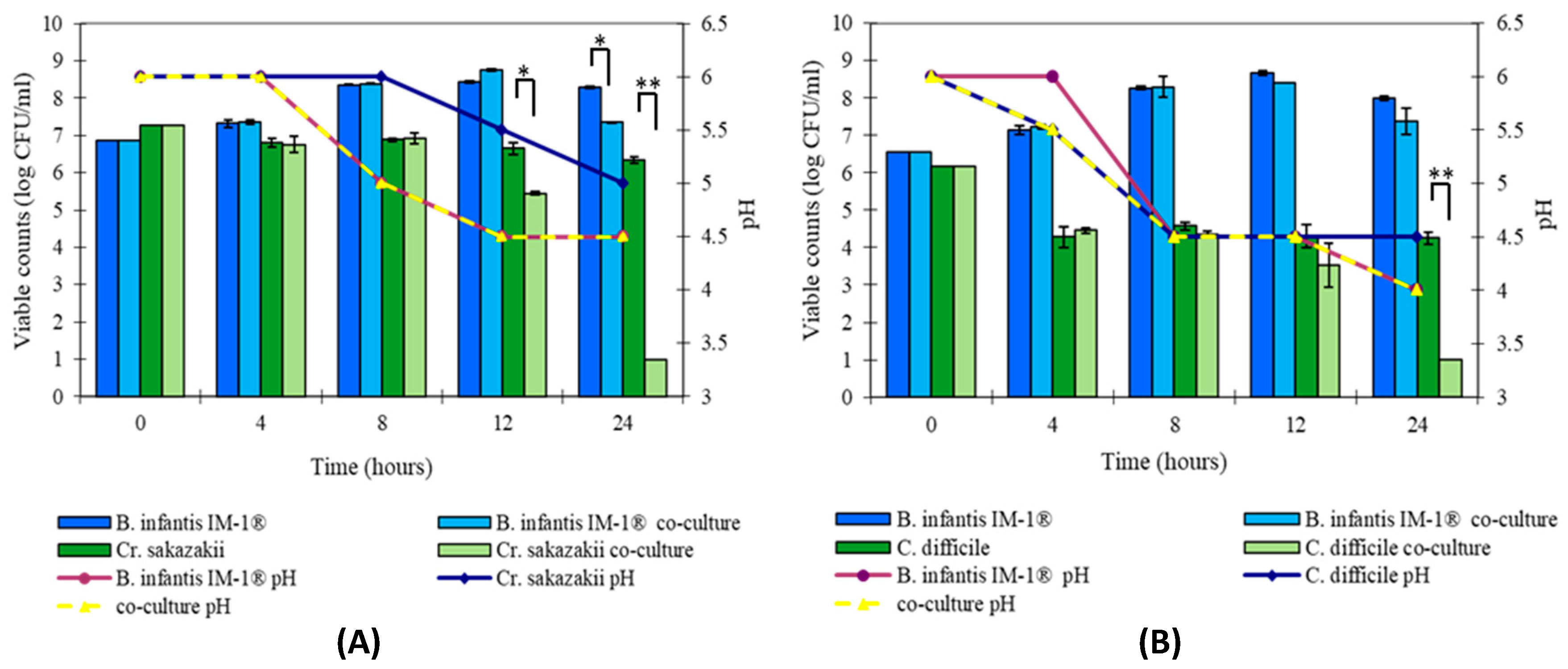
2.3. Inhibition of Pathogen Growth
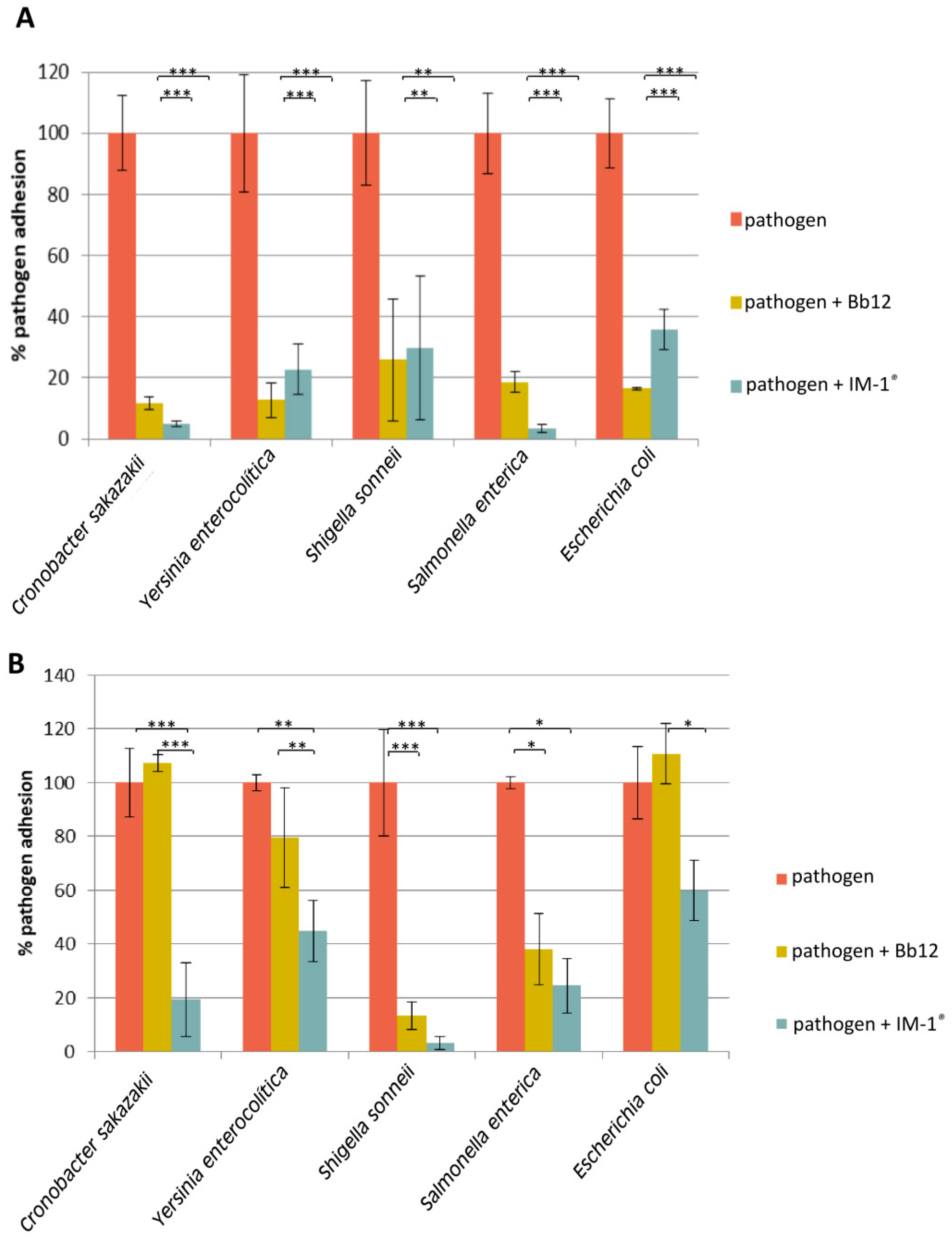
2.4. Pathogen Displacement and Prevention of Pathogen’s Adhesion to Enterocytes
2.5. Statistical Analysis
3. Results and Discussion
3.1. Selection of Prebiotic Candidates
3.2. B. infantis IM-1® Inhibits Growth of Enteropathogens In Vitro
3.3. Capability of Probiotic Strain B. Infantis IM-1® to Modify the Adhesion of Enteropathogens to HT29
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019, 83. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.; Fukuda, S. Protective effects of bifidobacteria against enteropathogens. Microb. Biotechnol. 2019, 12, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Biavati, B.; Mattarelli, P. The family Bifidobacteriaceae. In The Prokaryotes; Dworkin, M., Fallow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2001; pp. 1–70. [Google Scholar]
- Langendijk, P.S.; Schut, F.; Jansen, G.J.; Raangs, G.C.; Kamphuis, G.R.; Wilkinson, M.H.F.; Welling, G.W. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 1995, 61, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.M.; Raangs, G.C.; He, T.; Degener, J.E.; Welling, G.W. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 2002, 68, 2982–2990. [Google Scholar] [CrossRef]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Wildeboer-Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Veereman, G. Prebiotics in infant formula. Gut Microbes 2015, 5, 681–687. [Google Scholar] [CrossRef]
- Moreno Villares, J.M.; Collado, M.C.; Larqué, E.; Leis Trabazo, M.R.; Sáenz De Pipaon, M.; Moreno Aznar, L.A. The first 1000 days: An opportunity to reduce the burden of noncommunicable diseases. Nutr. Hosp. 2019, 36, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93, S31–S34. [Google Scholar] [CrossRef]
- Meyer, D.; Stasse-Wolthuis, M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 2009, 63, 1277–1289. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Silva, J.P.B.; Navegantes-Lima, K.C.; de Oliveira, A.L.B.; Rodrigues, D.V.S.; Gaspar, S.L.F.; Monteiro, V.V.S.; Moura, D.P.; Monteiro, M.C. Protective mechanisms of butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2018, 24, 4154–4166. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Puolakkainen, P.A.; Rautonen, N.E. Bifidobacterium lactis sp. 420 up-regulates cyclooxygenase (Cox)-1 and down-regulates Cox-2 gene expression in a caco-2 cell culture model. Nutr. Cancer 2005, 51, 83–92. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Pogori, N.; Chen, W.; Zhang, H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: From production to their application. Int. J. Food Microbiol. 2008, 125, 215–222. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef]
- Vazquez-Gutierrez, P.; de Wouters, T.; Werder, J.; Chassard, C.; Lacroix, C. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Margolles, A.; de los Reyes-Gavilán, C.G.; Salminen, S. Competitive exclusion of enteropathogens from human intestinal mucus by Bifidobacterium strains with acquired resistance to bile—A preliminary study. Int. J. Food Microbiol. 2007, 113, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 678–701. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Izumi, H.; Ehara, T.; Sugahara, H.; Matsubara, T.; Mitsuyama, E.; Nakazato, Y.; Tsuda, M.; Shimizu, T.; Odamaki, T.; Xiao, J.-Z.; et al. The combination of Bifidobacterium breve and three prebiotic oligosaccharides modifies gut immune and endocrine functions in neonatal mice. J. Nutr. Nutr. Immunol. 2019, 149. [Google Scholar] [CrossRef]
- Chenoll, E.; Rivero, M.; Codoñer, F.M.; Martinez-Blanch, J.F.; Ramón, D.; Genovés, S.; Muñoz, J.A.M. Complete genome sequence of Bifidobacterium longum subsp. infantis strain CECT 7210, a probiotic strain active against rotavirus infections. Genome Announc. 2016, 3. [Google Scholar] [CrossRef]
- Muñoz, J.A.M.; Chenoll, E.; Casinos, B.; Bataller, E.; Ramón, D.; Genovés, S.; Montava, R.; Ribes, J.M.; Buesa, J.; Fàbrega, J.; et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl. Environ. Microbiol. 2011, 77, 8775–8783. [Google Scholar] [CrossRef]
- Escribano, J.; Ferré, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoñer, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018, 83, 1120–1128. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Castillejos, L.; López-Colom, P.; Rivero Urgell, M.; Moreno Muñoz, J.A.; Martín-Orúe, S.M. Evaluation of the probiotic strain Bifidobacterium longum subsp. infantis CECT 7210 capacities to improve health status and fight digestive pathogens in a piglet model. Front. Microbiol. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–549. [Google Scholar] [CrossRef]
- López, P.; Monteserín, D.C.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Margolles, A.; Suárez, A.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 2012, 46, 99–107. [Google Scholar] [CrossRef]
- Mao, B.; Gu, J.; Li, D.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W. Effects of different doses of fructooligosaccharides (FOS) on the composition of mice fecal microbiota, especially the bifidobacterium composition. Nutrients 2018, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Arthur, J.C.; Jobin, C.; Keku, T.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. High purity galacto-oligosaccharides enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef. Microbes 2016, 7, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Rada, V.; Nevoral, J.; Trojanová, I.; Tománková, E.; Šmehilová, M.; Killer, J. Growth of infant faecal bifidobacteria and clostridia on prebiotic oligosaccharides in in vitro conditions. Anaerobe 2008, 14, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yu, W.K.; Heo, T.R. Identification and screening for antimicrobial activity against Clostridium difficile of Bifidobacterium and Lactobacillus species isolated from healthy infant faeces. Int. J. Antimicrob. Agents 2003, 21, 340–346. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, F.; Wu, Q.; Gao, J.; Liu, W.; Liu, C.; Guo, X.; Suwal, S.; Kou, Y.; Zhang, B.; et al. Protective effects of bifidobacterial strains against toxigenic Clostridium difficile. Front. Microbiol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Macfarlane, G.T. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl. Environ. Microbiol. 2003, 69, 1920–1927. [Google Scholar] [CrossRef]
- Valdés-Varela, L.; Hernández-Barranco, A.M.; Ruas-Madiedo, P.; Gueimonde, M. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front. Microbiol. 2016, 7, 738. [Google Scholar] [CrossRef]
- Drakoularakou, A.; Wells, A.; Robinsons, R.; Rastall, R.; Gibson, G.; Mccartney, A. Acid and bile tolerance, adhesion properties and anti-pathogenic effects of three potential probiotic strains. Int. J. Probiotics Prebiotics 2007, 2, 185–194. [Google Scholar]
- Fooks, L.J.; Gibson, G.R. Mixed culture fermentation studies on the effects of synbiotics on the human intestinal pathogens Campylobacter jejuni and Escherichia coli. Anaerobe 2003, 9, 231–242. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Ovidi, M.; Di Mattia, E.; Trovatelli, L.D.; Canganella, F. Screening of Bifidobacterium strains isolated from human faeces for antagonistic activities against potentially bacterial pathogens. Microbiol. Res. 2003, 158, 179–185. [Google Scholar] [CrossRef]
- Toure, R.; Kheadr, E.; Lacroix, C.; Moroni, O.; Fliss, I. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J. Appl. Microbiol. 2003, 95, 1058–1069. [Google Scholar] [CrossRef]
- Zinedine, A.; Faid, M. Isolation and characterization of strains of bifidobacteria with probiotic properties in vitro. World J. Dairy Food Sci. 2007, 2, 28–34. [Google Scholar]
- Coconnier, M.H.; Liévin, V.; Bernet-Camard, M.F.; Hudault, S.; Servin, A.L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 1997, 41, 1046–1052. [Google Scholar] [CrossRef]
- Colombel, J.F.; Cortot, A.; Neut, C.; Romond, C. Yoghurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet 1987, 330, 43. [Google Scholar] [CrossRef]
- Plummer, S.; Weaver, M.; Harris, J.; Dee, P.; Hunter, J. Clostridium difficile pilot study: Effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int. Microbiol. 2004, 7, 59–62. [Google Scholar]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. Fems Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef]
- Candela, M.; Seibold, G.; Vitali, B.; Lachenmaier, S.; Eikmanns, B.J.; Brigidi, P. Real-time PCR quantification of bacterial adhesion to Caco-2 cells: Competition between bifidobacteria and enteropathogens. Res. Microbiol. 2005, 156, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Grześkowiak, Ł.; Salminen, S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Moroni, O.; Kheadr, E.; Boutin, Y.; Lacroix, C.; Fliss, I. Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl. Environ. Microbiol. 2006, 72, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Stivala, A.; Furneri, P.M.; Blandino, G. Growth and Adhesion to HT-29 Cells Inhibition of Gram-Negatives by Bifidobacterium longum BB536 e Lactobacillus rhamnosus HN001 alone and in combination. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4943–4949. [Google Scholar] [PubMed]
- Serafini, F.; Strati, F.; Ruas-Madiedo, P.; Turroni, F.; Foroni, E.; Duranti, S.; Milano, F.; Perotti, A.; Viappiani, A.; Guglielmetti, S.; et al. Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe 2013, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.; Cooney, S.; O′Brien, S.; Iversen, C.; Whyte, P.; Nally, J.; Callanan, J.J.; Fanning, S. Cronobacter (Enterobacter sakazakii): An opportunistic foodborne pathogen. Foodborne Pathog. Dis. 2010, 7, 339–350. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, L.; Flórez, A.B.; Sánchez, B.; Moreno-Muñoz, J.A.; Rodriguez-Palmero, M.; Jiménez, J.; Gavilán, C.G.d.l.R.; Gueimonde, M.; Ruas-Madiedo, P.; Margolles, A. Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM-1®) Displays In Vitro Activity against Some Intestinal Pathogens. Nutrients 2020, 12, 3259. https://doi.org/10.3390/nu12113259
Ruiz L, Flórez AB, Sánchez B, Moreno-Muñoz JA, Rodriguez-Palmero M, Jiménez J, Gavilán CGdlR, Gueimonde M, Ruas-Madiedo P, Margolles A. Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM-1®) Displays In Vitro Activity against Some Intestinal Pathogens. Nutrients. 2020; 12(11):3259. https://doi.org/10.3390/nu12113259
Chicago/Turabian StyleRuiz, Lorena, Ana Belén Flórez, Borja Sánchez, José Antonio Moreno-Muñoz, Maria Rodriguez-Palmero, Jesús Jiménez, Clara G. de los Reyes Gavilán, Miguel Gueimonde, Patricia Ruas-Madiedo, and Abelardo Margolles. 2020. "Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM-1®) Displays In Vitro Activity against Some Intestinal Pathogens" Nutrients 12, no. 11: 3259. https://doi.org/10.3390/nu12113259
APA StyleRuiz, L., Flórez, A. B., Sánchez, B., Moreno-Muñoz, J. A., Rodriguez-Palmero, M., Jiménez, J., Gavilán, C. G. d. l. R., Gueimonde, M., Ruas-Madiedo, P., & Margolles, A. (2020). Bifidobacterium longum subsp. infantis CECT7210 (B. infantis IM-1®) Displays In Vitro Activity against Some Intestinal Pathogens. Nutrients, 12(11), 3259. https://doi.org/10.3390/nu12113259






